Abstract
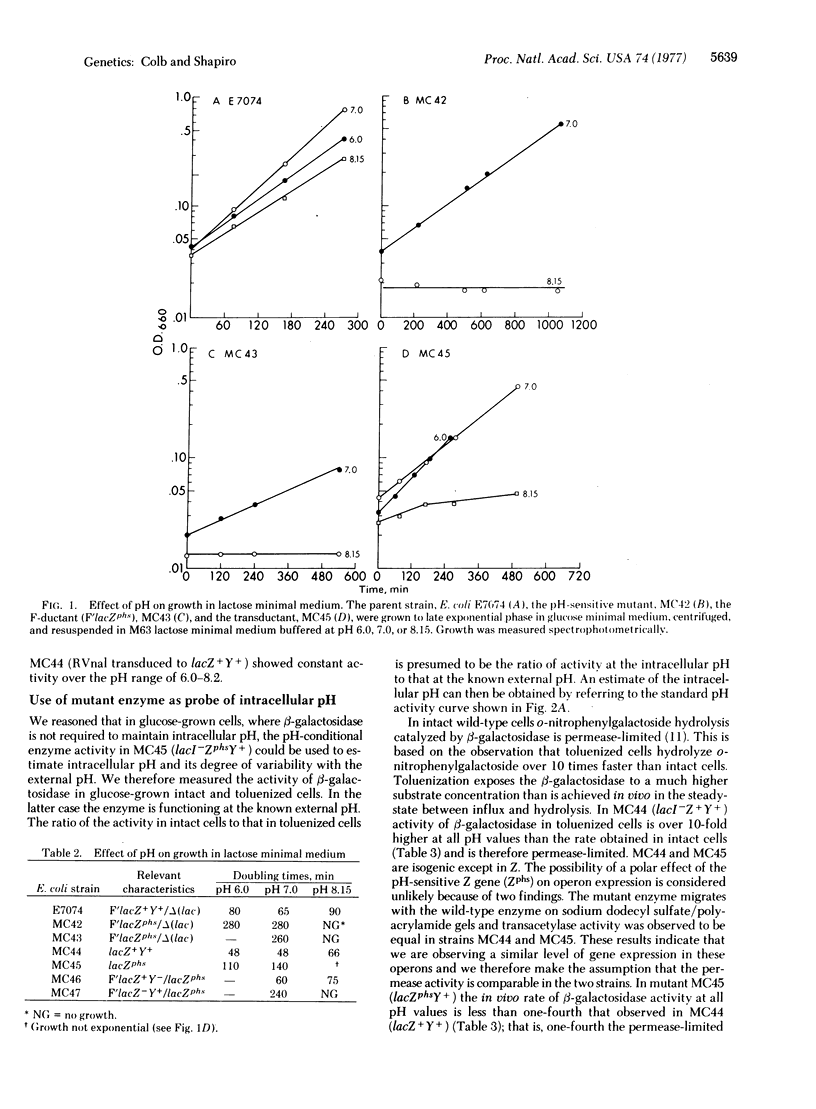
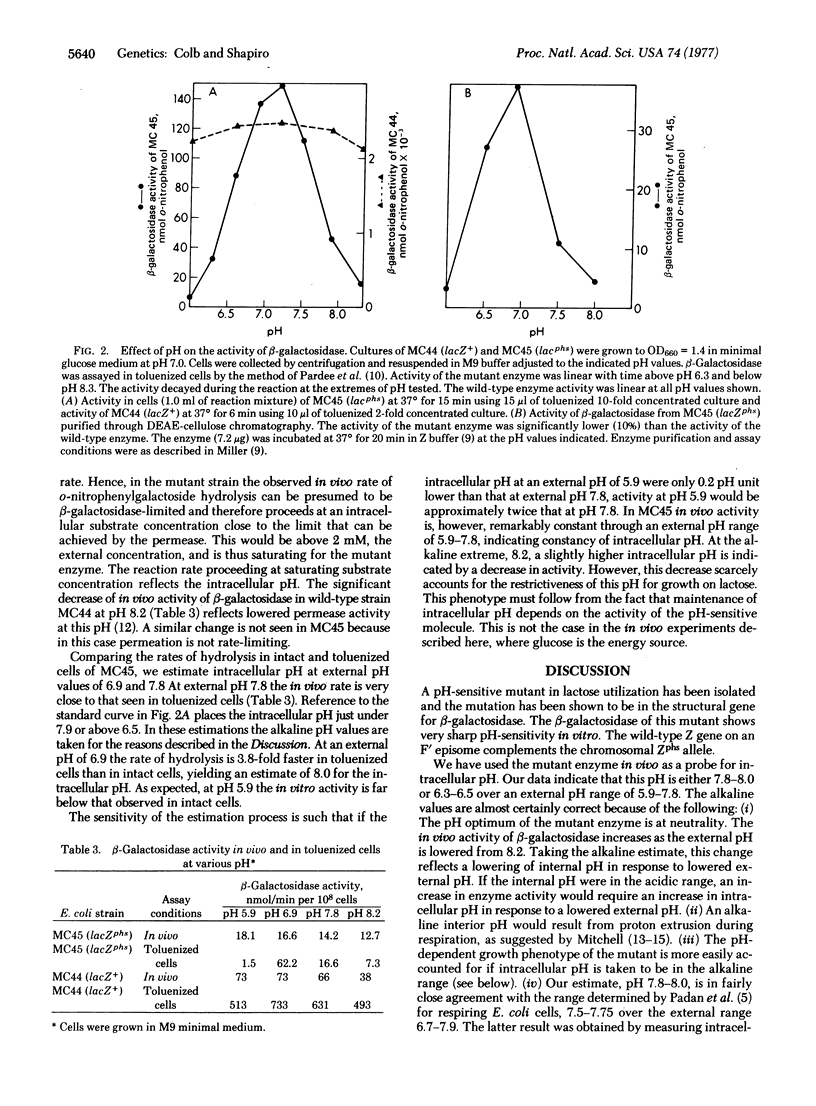
Mutants of Escherichia coli have been isolated that are able to grow on lactose at pH 7.0 but not at pH 8.1. One of these mutants was analyzed and shown to map in the Z region of the lactose operon. beta-Galactosidase (beta-D-galactoside galactohydrolase; EC 3.2.1.23) activity in toluenized mutant cells at pH 8.0 was one-tenth that at pH 7.0. Enzyme purified to near homogeneity from the pH-conditional mutant similarly exhibited pH-conditional activity under conditions where wild-type enzyme was unaffected over a pH range of 6.0-8.0. The pH-conditional beta-galactosidase was used in vivo as a probe for intracellular pH. We show that an internal pH of approximately 7.8-8.0 is maintained through an external pH range of 5.9-7.8. The phenotype of pH-conditional mutants was defined on medium with lactose as the sole carbon source. Under such conditions the gene product itself, beta-galactosidase, is required to maintain intracellular pH, since such maintenance is clearly energy-dependent. Therefore, we were able to recover a pH-conditional mutant in a cytoplasmic gene product. We predict that with any phenotype independent of energy production, however, pH-sensitive mutants will be recovered only in surface elements.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTTIN G., COHEN G. N., MONOD J., RICKENBERG H. V. La galactoside-perméase d'Escherichia coli. Ann Inst Pasteur (Paris) 1956 Dec;91(6):829–857. [PubMed] [Google Scholar]
- Brett M., Chambers G. K., Holder A. A., Fincham J. R., Wootton J. C. Mutational amino acid replacements in Neurospora crassa NADP-specific glutamate dehydrogenase. J Mol Biol. 1976 Sep 5;106(1):1–22. doi: 10.1016/0022-2836(76)90297-7. [DOI] [PubMed] [Google Scholar]
- Kent C., Lennarz W. J. An approach to the selection of membrane mutants of Staphylococcus aureus based on pH sensitivity. Biochim Biophys Acta. 1972 Oct 23;288(1):225–230. doi: 10.1016/0005-2736(72)90241-6. [DOI] [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Manson M. D., Tedesco P., Berg H. C., Harold F. M., Van der Drift C. A protonmotive force drives bacterial flagella. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3060–3064. doi: 10.1073/pnas.74.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Performance and conservation of osmotic work by proton-coupled solute porter systems. J Bioenerg. 1973 Jan;4(1):63–91. doi: 10.1007/BF01516051. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Rottenberg H. The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem. 1976 Apr 1;63(2):533–541. doi: 10.1111/j.1432-1033.1976.tb10257.x. [DOI] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The electrochemical gradient of protons and its relationship to active transport in Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1892–1896. doi: 10.1073/pnas.73.6.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick G., Kaback H. R., Weil R. Photoinactivation of the beta-galactoside transport system in Escherichia coli membrane vesicles with 2-nitro-4-azidophenyl-1-thio-beta-D-galactopyranoside. J Biol Chem. 1975 Feb 25;250(4):1371–1375. [PubMed] [Google Scholar]
- Schuldiner S., Kerwar G. K., Kaback H. R., Weil R. Energy-dependent binding of dansylgalactosides to the beta-galactoside carrier protein. J Biol Chem. 1975 Feb 25;250(4):1361–1370. [PubMed] [Google Scholar]
- Wilson T. H., Kusch M. A mutant of Escherichia coli K 12 energy-uncoupled for lactose transport. Biochim Biophys Acta. 1972 Mar 17;255(3):786–797. doi: 10.1016/0005-2736(72)90391-4. [DOI] [PubMed] [Google Scholar]



