Abstract
The goal of surgical treatment for gastrointestinal stromal tumor (GIST) is the complete resection of the tumor. A 62-year-old male had a clearly distinguishable mass having a smooth surface at the right side of the lower esophagus by computed tomography. Thoracoscopic resection of the tumor was performed. Immunohistochemical analysis showed that the tumor was positive for c-KIT and CD34 without mitosis, and diagnosed to be a low-risk GIST. At 6 years after surgery, the patient survived without recurrence. This study described the long-term surviving patient without the recurrence of tumor after the thoracoscopic resection of an esophageal GIST.
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) in the esophagus are extremely rare. Reliable surgical therapy for GIST is the complete tumor resection. In principle, the resection of involved organ has been recommended for preserving physiological functions, and lymph-node dissection is unnecessary. This study described a patient who underwent the thoracoscopic enucleation of an esophageal GIST and survived without the recurrence of tumor for 6 years after surgery.
CASE REPORT
A 62-year-old male was found to have a well-demarcated mass with a smooth surface at the right side of the lower esophagus by chest computed tomography (CT) upon medical check-up (Fig. 1). Routine laboratory data and the serum levels of tumor markers were within normal limits. Positron emission tomography (PET) revealed the accumulation of 18F-fluoro-2-deoxyglucose (FDG) at the tumor. The maximum standardized uptake value was 4.0, increasing to 4.8 in the late phase. Upper gastrointestinal endoscopy revealed no distinct mucosal abnormalities or no evidence of tumors. Endoscopic ultrasonography showed a mass ∼2 cm in diameter arising in the third layer 40 cm from the incisor teeth (Fig. 2a and b). These findings diagnosed the lesion as an esophageal submucosal tumor with suspecting an esophageal leiomyoma or GIST. Being under general endotracheal double-lumen anesthesia with split-lung ventilation, the patient was allowed to lie on the healthy side (left) and posture lateral decubitus. A 6-cm long access incision was made at the posterior axillary line in the 10th intercostal space. Two 10.5-mm ports were made at the seventh intercostal space in the anterior axillary line and other at the same intercostal space in the posterior axillary line. After no adhesion in the thoracic cavity was observed, the pulmonary ligament was dissected and the lower lobe of the right lung was moved anteriorly. A tumefactive lesion was found in the lower esophagus. An incision was made in the mediastinal pleura, and the mass was confirmed at the outer longitudinal muscle of the lower esophagus. The tumor was easily removed from the surrounding tissue by a tumor enucleation procedure. No esophageal mucosa was injured intraoperatively. The operation time was 118 min, and the blood loss was 11 g (Fig. 3). Macroscopic examination found that the removed white parenchymal mass was 29 × 20 × 14 mm in size. Histopathological examination showed that the specimens stained with hematoxylin and eosin-contained tumor cells, which had elongated oval nuclei and eosinophilic and spindle-like reticulum, were arranged in fascicles without mitosis. Immunohistochemical results were positive for c-KIT, S-100 protein, CD34 and vimentin, and the specimen was diagnosed as a low-risk GIST (Fig. 4a and b). The patient took an uneventful postoperative course, was allowed to remove the surgical drain after confirming the clinical status by esophagography on postoperative day 9, and discharged on postoperative day 13. At 6 years after surgery, the patient was found to be healthy without tumor recurrence.
Figure 1:
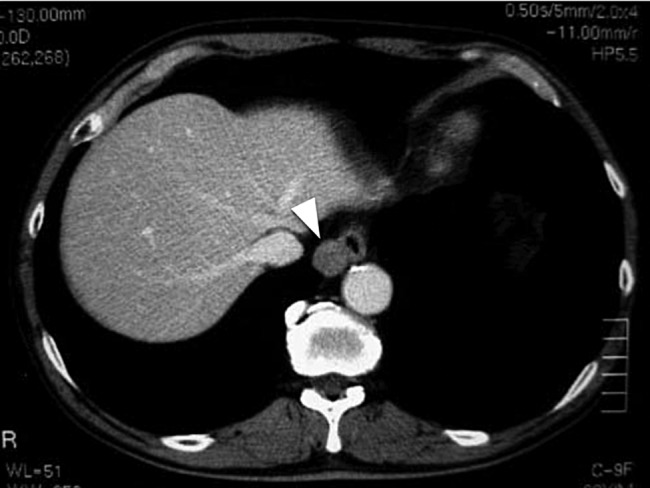
Chest CT of a 62-year-old male. The white arrow head indicates a clearly distinguishable mass having a smooth surface at the right side of the lower esophagus.
Figure 2:
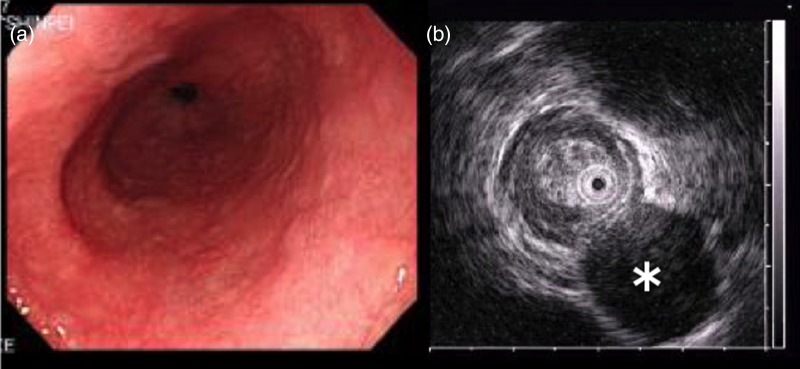
Endoscopic examination and endoscopic ultrasonography. (a) Tumor with a normally appearing mucosa was located 40 cm from the incisor teeth. (b) Hypoechoic submucosal tumor (asterisk) with annular localization, arising from the submucosal layer.
Figure 3:
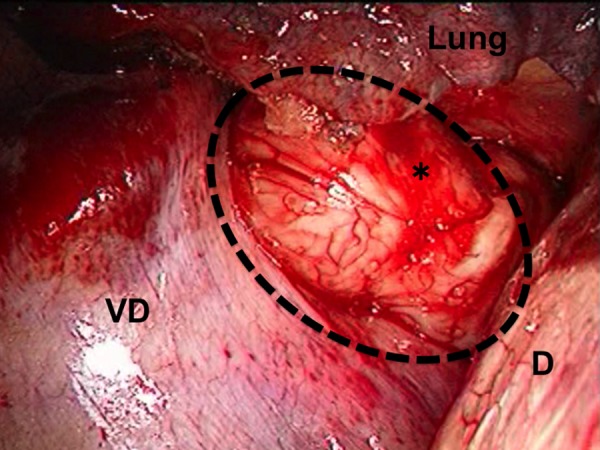
Thoracoscopic view of the tumor. The image shows bulging through the right thoracic cavity. The black dash line and asterisk indicate the GIST; D, the diaphragm; VD, the vertebral body.
Figure 4:
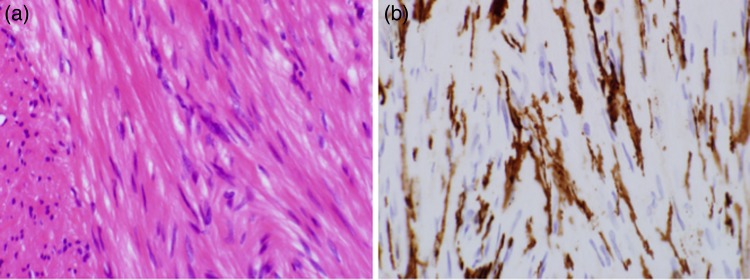
Histological specimens prepared from the removed GIST. The left and right microphotographs were taken at a magnification of ×40. (a) Hematoxylin and eosin-stained specimen showed spindle cells. (b) Immunohistochemical-stained specimen showed that the cells were positive for c-KIT.
DISCUSSION
In recent years, guidelines for managing GISTs are prepared by the National Comprehensive Cancer Network (NCCN) and issued in 2004 [1]. GISTs are known to consist of spindle and epithelioid cells. Among gastrointestinal mesenchymal tumors, GISTs are stained positively for c-KIT or CD34, and the diagnostic rates of the tumors are <1% of all gastrointestinal tumors. Gastrointestinal GISTs often appear in the stomach and small intestine. Esophageal GISTs are extremely rare, accounting for only 1% of all GISTs [2, 3]. Esophagography, ultrasonography and CT are used to diagnose GIST. Although the results of FDG-PET have been reported to correlate with the degree of malignancy of GIST, the definitive diagnosis is difficult to be performed. On the other hand, FDG-PET is effective for evaluating postoperative recurrence and the response of GIST to chemotherapy [4]. For treating GIST, if the tumor is resectable, the partial resection of affected organ is the treatment of choice with considering the preservation of organ functions. In patients with unresectable tumors, recurrence or metastasis, chemotherapy with imatinib mesylate (Gleevec®) is recommended [1, 2]. Esophagectomy including partial resection, as a conventional treatment for esophageal GIST, is more invasive than the resection of other parts of the gastrointestinal tract and remarkably reduces the quality of life of patients after surgery. Recently, thoracoscopic enucleation has been used for treating small and low-risk GISTs confirmed histologically [5]. Previous case reports on esophageal GISTs have described only small numbers of patients, and long-term outcomes after enucleation have rarely been reported [6]. However, GISTs are difficult to be diagnosed preoperatively, and esophageal submucosal tumors such as leiomyoma are often initially diagnosed, followed by the diagnosis of GIST on postoperative pathological examination [7]. The patient in this report was being followed up 6 years after surgery, and there was no evidence of recurrence. In patients with small tumors, diagnosis is frequently challenging. Minimally invasive thoracoscopic surgery could particularly be useful for the diagnosis and treatment of GIST appearing in the esophagus. This report described a patient who survived for a long term without tumor recurrence after the thoracoscopic enucleation of an esophageal GIST, considered a rare tumor. Being a lower aggressive procedure, the thoracoscopic enucleation could allow patients to survive for a long term and their quality of life to be enhanced.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Demetri GD, Benjamin RS, Blanke CD, Choi H, Corless C, DeMatteo RP, et al. NCCN task force report: optimal management of patients with gastrointestinal stromal tumor (GIST): expansion and update of NCCN clinical practice guidelines. J Natl Compr Canc Netw. 2004;Suppl 1:S1–26.. [PubMed] [Google Scholar]
- 2.Gupta P, Tewari M, Shukla HS. Gastrointestinal stromal tumor. Surg Oncol. 2008;17:129–38. doi: 10.1016/j.suronc.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Miettinen M, Lasota J. Gastrointestinal stromal tumors. Gastroenterol Clin North Am. 2013;42:399–415. doi: 10.1016/j.gtc.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park JW, Cho CH, Jeong DS, Chae HD. Role of F-fluoro-2-deoxyglucose positron emission tomography in gastric GIST: predicting malignant potential pre-operatively. J Gastric Cancer. 2011;11:173–9. doi: 10.5230/jgc.2011.11.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koide N, Kishimoto K, Komatsu O, Yoshizawa A, Sugiyama A, Miyagawa S. Thoracoscopic enucleation of esophageal stromal tumor. Dis Esophagus. 2004;17:104–8. doi: 10.1111/j.1442-2050.2004.00364.x. [DOI] [PubMed] [Google Scholar]
- 6.Koyanagi K, Nakagawa M, Ozawa S, Nagase T, Seishima R, Kanai T. Thoracoscopic enucleation for small-sized gastrointestinal stromal tumor of the esophagus: report of two cases. Esophagus. 2010;7:219–24. [Google Scholar]
- 7.Nishimura K, Tanaka T, Tanaka Y, Matono S, Murata K, Naito Y, et al. Esophageal gastrointestinal stromal tumor-incidence and prognosis after enucleation. Jpn J Gastroenterol Surg. 2009;42:1551–6. [Google Scholar]


