Abstract
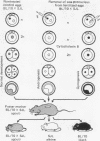
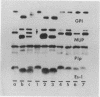
Shortly after fertilization, either the male or the female pronucleus was microsurgically removed from 202 F1 hybrid eggs derived from crosses of two inbred strains. Subsequent incubation of these haploid eggs in medium containing cytochalasin B, which inhibits cytokinesis but not nuclear division, enabled the remaining pronucleus to become diploid. After nuclear diploidization and transfer to regular culture medium, cleavage commenced normally, and a total of 135 successfully manipulated eggs continued in development and yielded 93 morulae and blastocysts. These embryos were surgically transferred to the uteri of pseudopregnant foster mothers who gave birth to seven live female offspring. Five of the females were derived from the maternal genome (gynogenesis) and the remaining two mice inherited only the paternal genes (androgenesis), depending on whether the female or male pronucleus had been retained in the egg, respectively. Homozygosity for a number of genetic loci positioned on different chromosomes and effecting the coat color phenotype and strain-specific allelic variants of several enzymes, urinary and plasma proteins, and hemoglobins could be demonstrated unequivocally in all instances. Chromosomal analysis revealed a normal diploid karyotype including two X chromosomes. Thus far, six of the seven homozygous-diploid (isogeneic) females have proved to be fertile and have given birth to progeny corresponding only to the pronuclear genotype of the mother.
Keywords: androgenesis, gynogenesis, removal of pronucleus, cytochalasin B, isogeneic mice
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter S. B. Effects of cytochalasins on mammalian cells. Nature. 1967 Jan 21;213(5073):261–264. doi: 10.1038/213261a0. [DOI] [PubMed] [Google Scholar]
- Champlin A. K., Dorr D. L., Gates A. H. Determining the stage of the estrous cycle in the mouse by the appearance of the vagina. Biol Reprod. 1973 May;8(4):491–494. doi: 10.1093/biolreprod/8.4.491. [DOI] [PubMed] [Google Scholar]
- Eicher E. M., Stern R. H., Womack J. E., Davisson M. T., Roderick T. H., Reynolds S. C. Evolution of mammalian carbonic anhydrase loci by tanden duplication: close linkage of Car-1 and Car-2 to the centromere region of chromosome 3 of the mouse. Biochem Genet. 1976 Aug;14(7-8):651–660. doi: 10.1007/BF00485843. [DOI] [PubMed] [Google Scholar]
- Eppig J. J., Kozak L. P., Eicher E. M., Stevens L. C. Ovarian teratomas in mice are derived from oocytes that have completed the first meiotic division. Nature. 1977 Oct 6;269(5628):517–518. doi: 10.1038/269517a0. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H., Richardson C. R. An improved banding technique exemplified in the karyotype analysis of two strains of rat. Chromosoma. 1973;41(3):259–263. doi: 10.1007/BF00344020. [DOI] [PubMed] [Google Scholar]
- Graham C. F. The production of parthenogenetic mammalian embryos and their use in biological research. Biol Rev Camb Philos Soc. 1974 Aug;49(3):399–424. doi: 10.1111/j.1469-185x.1974.tb01085.x. [DOI] [PubMed] [Google Scholar]
- Hoppe P. C., Pitts S. Fertilization in vitro and development of mouse ova. Biol Reprod. 1973 May;8(4):420–426. doi: 10.1093/biolreprod/8.4.420. [DOI] [PubMed] [Google Scholar]
- Markert C. L., Petters R. M. Homozygous mouse embryos produced by microsurgery. J Exp Zool. 1977 Aug;201(2):295–302. doi: 10.1002/jez.1402010213. [DOI] [PubMed] [Google Scholar]
- Modliński J. A. Haploid mouse embryos obtained by microsurgical removal of one pronucleus. J Embryol Exp Morphol. 1975 Jul;33(4):897–905. [PubMed] [Google Scholar]
- Mullen R. J., Carter S. C. Efficiency of transplanting normal, zona-free, and chimeric embryos to one and both uterine horns of inbred and hybrid mice. Biol Reprod. 1973 Sep;9(2):111–115. doi: 10.1093/biolreprod/9.2.111. [DOI] [PubMed] [Google Scholar]
- Ruddle F. H., Shows T. B., Roderick T. H. Esterase genetics in Mus musculus: expression, linkage, and polymorphism of locus Es-2. Genetics. 1969 Jun;62(2):393–399. doi: 10.1093/genetics/62.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow M. H. Tetraploid mouse embryos produced by cytochalasin B during cleavage. Nature. 1973 Aug 24;244(5417):513–515. doi: 10.1038/244513a0. [DOI] [PubMed] [Google Scholar]
- Tarkowki A. K. In vitro development of haploid mouse embryos produced by bisection of one-cell fertilized eggs. J Embryol Exp Morphol. 1977 Apr;38:187–202. [PubMed] [Google Scholar]
- Triman K. L., Davisson M. T., Roderick T. H. A method for preparing chromosomes from peripheral blood in the mouse. Cytogenet Cell Genet. 1975;15(3):166–176. doi: 10.1159/000130515. [DOI] [PubMed] [Google Scholar]
- Wilcox F. H. Simplified procedure for electrophoresis of the major urinary protein of Mus musculus. Biochem Genet. 1975 Apr;13(3-4):243–245. doi: 10.1007/BF00486018. [DOI] [PubMed] [Google Scholar]