Abstract
Cancers can be addicted to continued and relatively high expression of nuclear oncoproteins. This is evident in colorectal cancer (CRC) where the oncoprotein and transcription factor MYB is over expressed and essential to continued proliferation and tumour cell survival. Historically, targeting transcription factors in the context of cancer has been very challenging. Nevertheless, we formulated a DNA vaccine to generate a MYB-specific immune response in the belief MYB peptides might be aberrantly presented on the cell surface of CRC cells. MYB, like many tumour antigens, is weakly immunogenic as it is a ‘self' antigen and is subject to tolerance. To break tolerance, a fusion vaccine was generated comprising a full-length MYB complementary DNA (cDNA) flanked by two potent CD4-epitopes derived from tetanus toxoid. Vaccination was achieved against tumours initiated by two distinct highly aggressive, syngeneic cancer cell lines (CT26 and MC38) that express MYB. This was done in BALB/c and C57BL/6 mouse strains respectively. We introduced multiple inactivating mutations into the oncogene sequence for safety and sub-cloned the cDNA into a Food and Drug Administration (FDA)-compliant vector. We used low dose cyclophosphamide (CY) to overcome T-regulatory cell immune suppression, and anti-program cell death receptor 1 (anti-PD-1) antibodies to block T-cell exhaustion. Anti-PD-1 administered alone slightly delayed tumour growth in MC38 and more effectively in CT26 bearing mice, while CY treatment alone did not. We found that therapeutic vaccination elicits protection when MC38 tumour burden is low, mounts tumour-specific cell killing and affords enhanced protection when MC38 and CT26 tumour burden is higher but only in combination with anti-PD-1 antibody or low dose CY, respectively.
Epithelial cancers like colorectal cancer (CRC) are managed most effectively by surgery followed by adjuvant chemo-radiotherapy to remove residual disease and prevent metastases. Nevertheless, 10–50% of patients will still relapse with rebounding disease. This is a genuine unresolved clinical issue leading to the demise of 3982 Australian CRC patients in 2010 (Cancer Australia Data). There is now compelling evidence that the failure of the patient's immune response, as measured by the absence of lymphocytes within the tumour at the time of excision, is a major predictor of relapse.1, 2 It is thus evident that initiating either, de novo or reawakening a latent, immune response in CRC patients may improve patient outcomes.
Transcription factors, when aberrantly activated and highly expressed, can have an oncogenic function to which cancer cells are addicted.3, 4 Perhaps more importantly, transcription factors are at the hub of multiple pro-oncogenic pathways and therefore represent alluring therapeutic targets. The transcription factor MYB is responsible for a broad range of pro-tumourigenic functions, most recently for suppressing anti-tumour immune responses via expression of VEGF-A (unpublished data) and GRP78/BIP.5, 6 High MYB expression is also an excellent predictor of poor patient outcome.7 However, targeting nuclear transcription factors pharmacologically is very challenging. Here we have found an effective approach to achieve this in an enduring and clinically acceptable manner, through the utilization of the immune system.
MYB drives leukaemia, breast cancer and adenoid cystic carcinoma, and in CRC patients with highest MYB have the worst outcome.7, 8 Indeed, it would appear that CRC cells are oncogene addicted to MYB, as are leukaemia cells,9 ERα-positive and HER2-dependent mammary tumours.10 Through the generation of DNA vaccines, we have explored this novel approach to targeting cells that aberrantly present MYB peptides, or other transcription factor peptides such as MYCN as in the case of neuroblastoma.11 This therapy does not require long-term drug treatment and in theory has a capacity to kill residual cancer cells over an extended period of time. The generation of our DNA vaccine involved the ligation of DNA sequences that encode two immunodominant, MHC class II-restricted tetanus peptides that flank the target sequences encoding the amino and carboxyl termini of the full length mouse MYB cDNA. These tetanus peptides (P2 and P30) have been characterised extensively to show that they can enhance both Th1 and Th2 immune responses when coupled to HER2 peptides.12 Although most Westerners have been vaccinated against tetanus toxoid, these two peptides are not detrimental to the induction of immune responses to co-expressed proteins.13 This allows for the generation of a potent cytotoxic T-cell response to be achieved, even in individuals with no tetanus vaccination history.13 These tetanus peptides are MHC class II promiscuous, eliciting immunological responses in most individuals and have a well-established safety profile. Finally, the full length cDNA allows for whole antigen presentation, thus avoiding MHC-restriction issues commonly associated with peptide-based vaccines. Thus our vaccine has the potential to induce an immune response in the majority of CRC patients.
To date the MYB vaccine has been used in a pre-clinical prophylactic setting, providing protection to mice in both CRC transgenic and cell line based models.11, 14 Here we have advanced its application to a therapeutic context in two highly aggressive CRC pre-clinical models. Significant single agent protection is evident when the tumour burden is low. When tumour burden is higher, protection can also be achieved through the combination with other immune modulators. The potential to harness the immune system to remove residual disease and provide protection over extended periods of time is an appealing quality of vaccination approaches. This study indicates this might be achievable in CRC.
Results
MYB targeting vector
A DNA vaccine was generated with the full-length MYB coding region coupled to tetanus peptides, an amino-terminal P2 and a carboxyl-terminal P30.14 We have previously characterized an enhanced green fluorescent protein control vaccine with the same tetanus peptides and a FLAG-tagged MYB vector demonstrating a P2-MYB-P30-dependent prophylactic vaccine response without any evidence of auto-immunity in tissues where MYB is normally expressed.14
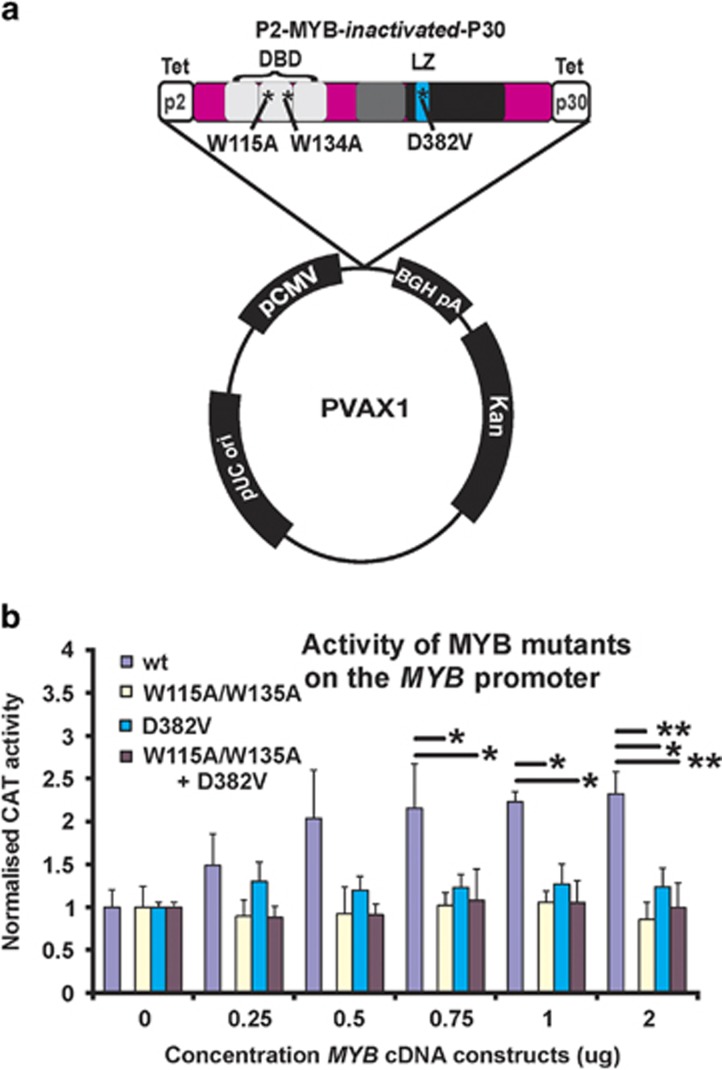
As the delivery of a potential oncogene to a patient presents genuine ethical issues, we introduced two disabling mutations into the DNA binding domain (W115A and W134A) and confirmed that these mutations abolished its transcriptional activation capacity.11 As MYB under some circumstances is also able to influence gene expression in the absence of a functional DNA binding domain,15 an additional inactivating mutation (D382V) that has been extensively characterized11, 16 was also introduced (Figure 1a). This is important as there is a remote, but plausible possibility, that single or close-by mutations could be aligned with the endogenous MYB exonic sequences to serve as templates, allowing gene conversion events returning the sequence to wild type. By employing different regions of the MYB gene to target mutations, the prospect of conversion of all three mutations would be highly improbable. The combined topography of the MYB DNA vaccine sequence within the FDA-compliant plasmid vector pVAX is depicted (Figure 1a). When the activity of MYB with all three mutations was evaluated in chloramphenicol acetyltransferase reporter assays, it is clear that the transfected triple mutant MYB is unable to transactivate a MYB-responsive reporter, in this instance the MYB promoter itself17, 18 (Figure 1b).
Figure 1.
MYB vaccine vector structure and evidence of engineered mutations rendering the encoded protein inactive as a transcription factor. (a) We have developed a MYB DNA vaccine that employs tetanus peptides, P2 and P30 that flank the MYB cDNA with three incorporated functionally inactivating mutations in the DNA binding domain (DBD) and leucine rich domain (LZ). This sequence in subcloned into a FDA-compliant vector pVAX1. (b) Confirmation that the MYB mutations render the protein functionally inactive when used to activate a MYB-responsive CAT reporter, in this instance its own promoter. (*P<0.05; **P<0.01: analysis of variance multiple comparisons, Data based on three independent experiments.
MYB vaccination in a therapeutic setting
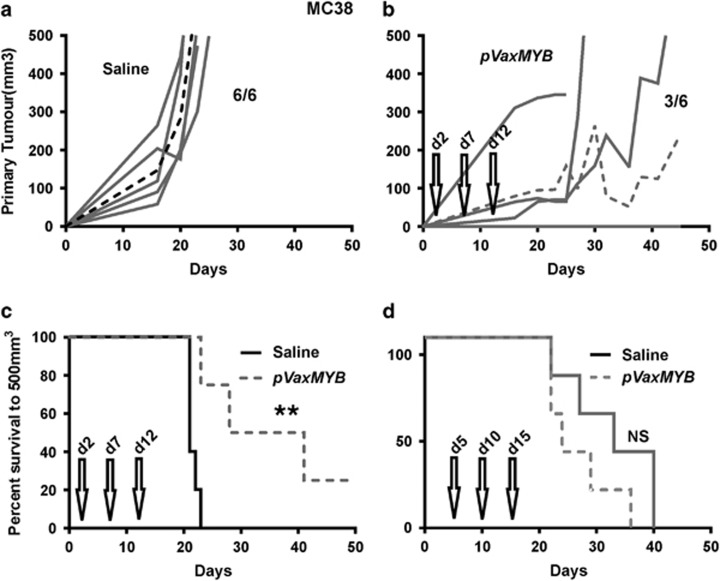
Previously, we successfully used the MYB vaccine in prophylactic settings and compared it to vaccination with enhanced green fluorescent protein in the same backbone which by contrast was not protective.14 However, to advance a vaccination therapy in the context of CRC, evidence of activity against established tumour burden is required. Therefore, we explored vaccination strategies in a therapeutic setting by injecting 5 × 105 MC38 cells into the flanks of C57BL/6 mice and initiating vaccination with the DNA construct or with saline at day 2 post-tumour cell inoculation, boosting on days 7 and 12. Under these conditions, the vaccine afforded significant protection in all mice by controlling tumour growth (Figures 2a and b) compared to saline-treated mice. Indeed, in three out of six vaccinated animals, tumour growth was essentially suppressed (Figure 2b), leading to a highly significant (P=0.0041; Log-rank, Mantel–Cox test) survival benefit (Figure 2c). However, when mice were vaccinated at day 5, boosting on days 10 and 15, no survival benefit was observed (Figure 2d). These data indicate that the pVAXMYB DNA vaccine affords protection only when the tumour burden is low and perhaps while the immune system is tumour naïve, with tumour-mediated editing of the immune system potentially working against its efficacy.
Figure 2.
The MYB vaccine affords protection from MC38 CRC tumour growth. C57Bl/6 mice were inoculated with 5 × 105 MC38 cells subcutaneously before treatment with either saline or 15 μg pVaxMYB in 50 μl intramuscularly. Growth curves are those of mice injected with saline (a) or vaccinated (b) on day 2, with further vaccine boosts on days 7 and 12 (n=6 per cohort). Dashed line represents mean response. (c) Kaplan–Meier survival analysis for mice vaccinated on day 2 shows significant protection (**P=0.004, Log-rank, Mantel–Cox test; n=6 per cohort). (d) By contrast survival of mice vaccinated on day 5, with boost shots administered on days 10 and 15 show no survival benefit (n=6 mice per cohort).
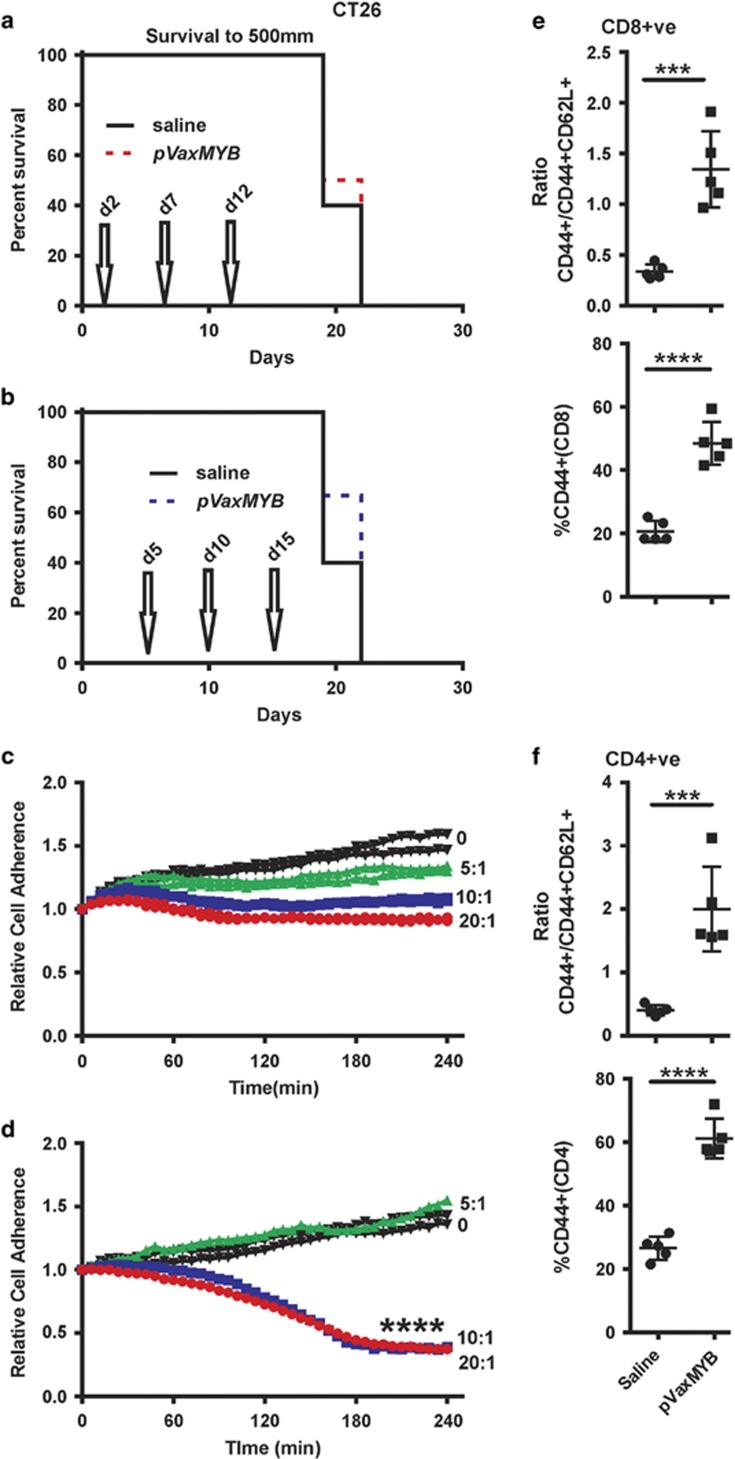
MC38 cells generate very aggressive tumours in C57BL/6 mice, such that delaying DNA vaccination from day 2 to day 5 post-tumour cell inoculation removed the survival benefit. To determine whether the same response occurs in another CRC cell line in a different strain of mice, CT26 cells were inoculated into BALB/c animals and vaccinated on day 2, boosting on days 7 and 12 without any evidence of protection (Figure 3a). Similarly, vaccination on day 5 and boosting on days 10 and 15 afforded no apparent protection (Figure 3b).
Figure 3.
No protection against CRC was observed in BALB/c mice despite evidence of T-cell activation. (a, b) BALB/c mice were inoculated with 5 × 105 CT26 cells subcutaneously before treatment with either saline or 15 μg pVaxMYB in 50 μl intramuscularly. Survival curves for mice vaccinated starting day 2 or 5, with boosts on days (7) 10 and (12) 15 shows no survival benefit. Splenocytes were activated in vitro from either naïve (c) or from mice 3 months post vaccination (d). Irradiated CT26 cells were used as targets for T-cell activation in medium containing anti-CD28 and IL-2. After 7 days, live CT26 cells were subject to killing assays by the cultured T cells using the Xcelligence system. Splenocytes to CRC cell ratios shown, (****P<0.0001; two tailed t-test comparing 20:1 ratios depicted in b, c). (d) Spleens from CT26 tumour-bearing animals were interrogated for T-cell activation markers (CD62L and CD44) at 7 days after vaccination in TCRβ+CD8+ and (e) TCRβ+CD4+ T cells showing significant activation (***P<0.001; two-sided t-test).
Generation of a memory response in MYB DNA-vaccinated mice
While prophylactic vaccination has been shown to provide protection, the formation of long term memory T cells has not been fully explored. Splenocytes were harvested from non-tumour bearing BALB/c mice that had been vaccinated 3 months previously or from naïve controls. These splenocytes were activated and added into a killing assay that measures changes in cancer cell attachment (Xcelligence, ACEA Biosciences Inc, San Diego, CA, USA) (Figures 3c and d). While a dose-dependent drop in resistance (cell growth stasis) was observed from the naïve activated T cells (Figure 3b) it was only the vaccinated cell ratios of splenocytes to CT26 CRC cells of 20:1 and 10:1 that showed evidence of killing (Figure 3c). Thus MYB DNA vaccination is able to create a sustained memory population of T cells, which enhance tumour target lysis over de novo T-cell activation.
T-cell activation by the MYB DNA vaccine
Having previously shown that T-cell activation by the MYB vaccine was dependent on both the MYB sequences plus the tetanus peptides, p2 and p30 when in a standard laboratory vector, pCDNA3,14 it was considered important that with the transfer of this construct to a new vector plus the introduction of an additional mutation, to show that T-cell activation was still effective. To test this, CT26 tumour-bearing BALB/c mice were injected intramuscularly with the new vector at day 5. Splenocytes were harvested on day 12 and analysed for T-cell activation markers. Increased expression of CD44 was observed in both CD4 and CD8 T-cell subpopulations (Figures 3e and f). Analysis of the activation states in both the CD4 and CD8 subpopulations showed that a significant shift from CD44+CD62L+ to CD44+ has occurred, indicating an increased activated state of the adaptive immune system (Figures 3e and f).
Collectively the data indicate that even under conditions where tumour control in vivo is not achieved, the MYB DNA vaccine retains the capacity for T-cell activation. It would therefore appear that the presence of established tumours impedes the capacity of the MYB-vaccinated mice to control tumour growth. Consequently, we embarked upon a series of studies to define the basis for ineffective control to inform possible combined therapies.
Exploring immune suppression in the context of MYB vaccination
The efficacy of immune therapies has been shown frequently to be influenced by the presence of T-regulatory cell (T-regs).19 Indeed the presence and relative abundance of T-regs is predictive of CRC patient outcome.20 One of the most robust markers of T-regs in CD4+ cells is the expression of the transcription factor FOXP3.21 With loss of MYB vaccine efficacy at day 5 compared with day 2 post-tumour inoculations in the C57BL/6 studies as well as evidence of in vitro cell killing activity in the BALB/c vaccinated mice it was postulated that T-regs may be in part responsible for this suppression of anti-tumour T-cell function.
T-regs in both patients and mice are sensitive to low dose cyclophosphamide (CY) treatment.22, 23 To determine if CY might modify T-regs in both BALB/c and C57Bl/6 mice, without altering the CD4 to CD8 ratio, non-tumour bearing mice were treated with one dose of CY at 25 or 50 mg kg−1 and evaluated for the percentage of CD4+/FoxP3+ cells 2 days later. If effective, then CY might be employed in concert with the MYB vaccine. Treatment of BALB/c, but not C57BL/6 mice, with CY led to a reduced proportion of T-regs (Supplementary Figures 1a and c). Interestingly, while no effect on the CD4+FoxP3+ population was observed in C57Bl/6 mice, CY did modestly affect the CD4/CD8 T-cell ratio, but this was not observed in BALB/c mice (Supplementary Figures 1b and d). These data demonstrate that CY at the doses used can affect T-regs in BALB/c mice, as previously published.22, 23 Furthermore, CY treatment may provide a window of T-reg suppression within which the expansion of the immune system by the MYB vaccine could occur.
Assessing the PD-1 status of TILs in vaccinated mice
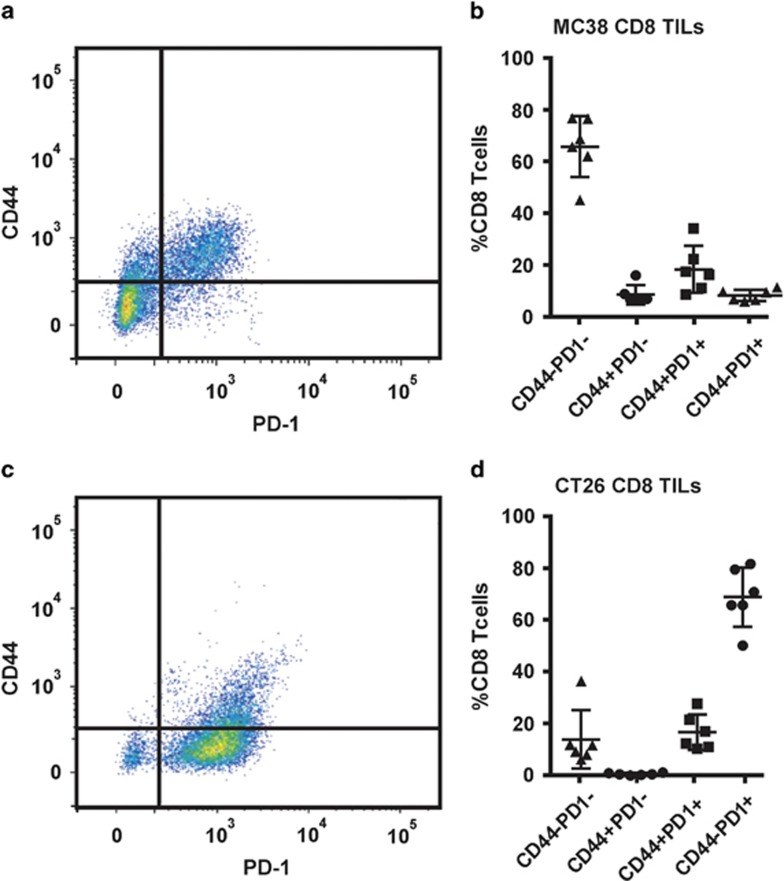
Increasing evidence shows that solid epithelial cancers can upregulate the immune suppressive protein program cell death ligand 1 (PD-L1) on their surface.24 Previously published results have also shown increased efficacy of therapeutic DNA vaccination when combined with immune modulation through the use of anti-PD1 antibodies that break this axis of T-cell inhibition.25, 26 Both CRC tumour lines express PD-L1 (data not shown). To determine whether tumour infiltrating lymphocytes (TILs) increase their expression of program cell death receptor 1 (PD-1) in mice bearing MC38 and CT26 tumours, CD8+ TILs were analysed for activation status (CD44) and PD-1 expression in unvaccinated animals (Figure 4). Unexpectedly no increase in the proportion of PD-1+;CD8+ TILs was observed in either model following vaccination at day 2 and 5 (data not shown), accordingly only the saline injected control data are presented. In non-vaccinated mice, CD8+ TILs in MC38 tumours were predominantly in an inactivated state, with ~70% of CD8+ TILs being CD44−PD1− (Figures 4a and b). Two minor populations of CD44+ CD8+ TILs were observed, with ~20% having elevated PD-1 expression (Figure 4b). These data indicate that CD8+ TILs migrating to the primary tumour are inactivated perhaps by the tumour, potentially through PD-L1 engagement. Thus, inhibition of this interaction using anti-PD-1 antibodies may increase the activation state of CD8+ TILs, leading to increased tumour cell killing, as these data indicate that such TILs may have trafficked to the primary tumour site.
Figure 4.
Evidence of differential immune check point control antigen expression by tumour infiltrating lymphocytes (TILs) isolated from different strains of CRC-bearing mice. CT26 and MC38 tumour-bearing animals were culled with average tumour weights of ~0.3 g and primary tumours were analyzed for TILs. Activation markers CD44 and PD1 were evaluated for MC38 (a, b) and CT26 (c, d) as a percentage of CD8+ cells.
Similarly, when CT26 tumours were analysed for CD8+ TILs, few CD44+ cells (~20%) were observed (Figures 4c and d). However, the remaining population of the CD8+ TILs in the CT26-tumour bearing mice were markedly different in that they did not appear to downregulate their expression of PD-1, with a large proportion (~70%) of the CD44− population still expressing PD-1 (Figure 4d). In the MC38-tumour bearing mice, this TIL population was <10%. These results indicate that either the CD8+ TILs are unable to downregulate PD-1 or perhaps that we have captured a time point at which they have not been fully inactivated. In both models, the observed data indicate that if inhibition of the PD-1/PD-L1 axis can be achieved, CD8+ TILs may be able to sustain an activated state, resulting in increased tumour cell killing.
Restoration of MYB vaccine efficacy by CY and/or anti-PD-1 therapy
To investigate approaches that might restore MYB vaccine efficacy in day 5 established CT26 and MC38 CRC tumours, we employed CY and/or anti-PD-1 antibody treatment in combination with the MYB vaccine. The rational for CY use was to reduce T-regs before vaccination, by treating at day 5, with vaccination beginning on day 6 and boosts on days 11 and 16. In addition, anti-PD-1 antibody was administered on days 6, 11 and 16 to increase activation, expansion and infiltration of TILs through the inhibition of suppression/exhaustion of CD8+ T cells.
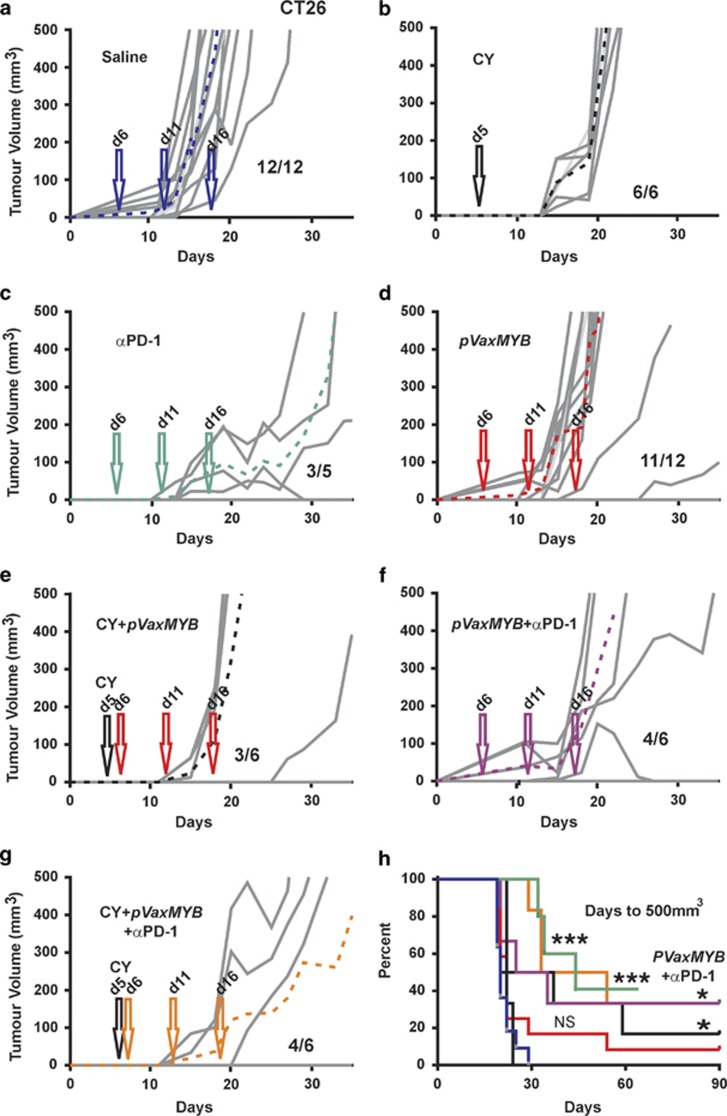
This vaccination strategy was first applied in BALB/c mice challenged with CT26 cells. Saline-treated mice showed the characteristic tumour outgrowth observed previously (Figure 5a), with all 12 mice developing tumours by day 20. CY treatment alone delayed tumour development such that mice were palpable-tumour-free up to day 14 (Figure 5b). Similarly, anti-PD-1 therapy alone delayed tumour growth and led to one regression (Figure 5c). MYB vaccination alone on average delayed tumour growth by 5 days but at 30 days post CT26 cell inoculation 11/12 mice had palpable tumours (Figure 5d). CY treatment in combination with the vaccine resulted in responders (three out of six) and non-responders (three out of six) (Figure 5e), with responders having their tumour growth significantly delayed or completely suppressed. The combination of anti-PD-1 antibody and MYB vaccination provided two complete regressions (two out of six), with four out of six progressing (Figure 5f). When the vaccine was combined with both the CY and anti-PD-1 antibody treatments, enduring tumour control was achieved in the same proportion of mice (four out of six; Figure 5g). Such tumour control translated into significantly improved survival in vaccine combination therapy groups (Figure 5h). However, in terms of days to 500 mm3 tumour burden the data indicate that αPD-1 therapy alone provides comparable protection to other combinations. It is noteworthy that CY plus vaccine affords protection that was superior to each when used alone and that the addition of αPD-1 did not add any further benefit to this dual combination.
Figure 5.
Tumour control in BALB/c mice using combinations of immunotherapies. BALB/c mice were inoculated with 5 × 105 CT26 cells subcutaneously before treatment with saline (a). A single 50 mg kg−1 dose CY was administered at day 5 (b) or anti-PD-1 antibody at 250 μg on days 6, 11 and 16 (c). At days 6, 11 and 16, 15 μg pVaxMYB in 50 μl was delivered intramuscularly (d). At days 6, 11 and 16, 15 μg pVaxMYB plus 50 mg kg−1 dose CY at day 5 were injected (e). Similarly, 15 mg pVaxMYB with 250 μg anti-PD-1 antibody at days 6, 11 and 16 were administered (f). Finally, a combination of 50 mg kg−1 dose CY with 15 μg pVaxMYB plus 250 μg anti-PD-1 or saline at days 6, 11 and 16 were employed. Growth is shown for three mice with the additional three mice developing palpable tumours past 35 days and therefore not evident on graph. (g) Dashed lines represent mean response to each treatment. Data for all treatments are represented as survival plots based upon tumours reaching 500 mm3 in volume (h). (***P<0.001; *P<0.05, Log-rank, Mantel–Cox test; n=6–12 mice per cohort). Fractions represent tumour-bearing mice after 90 days.
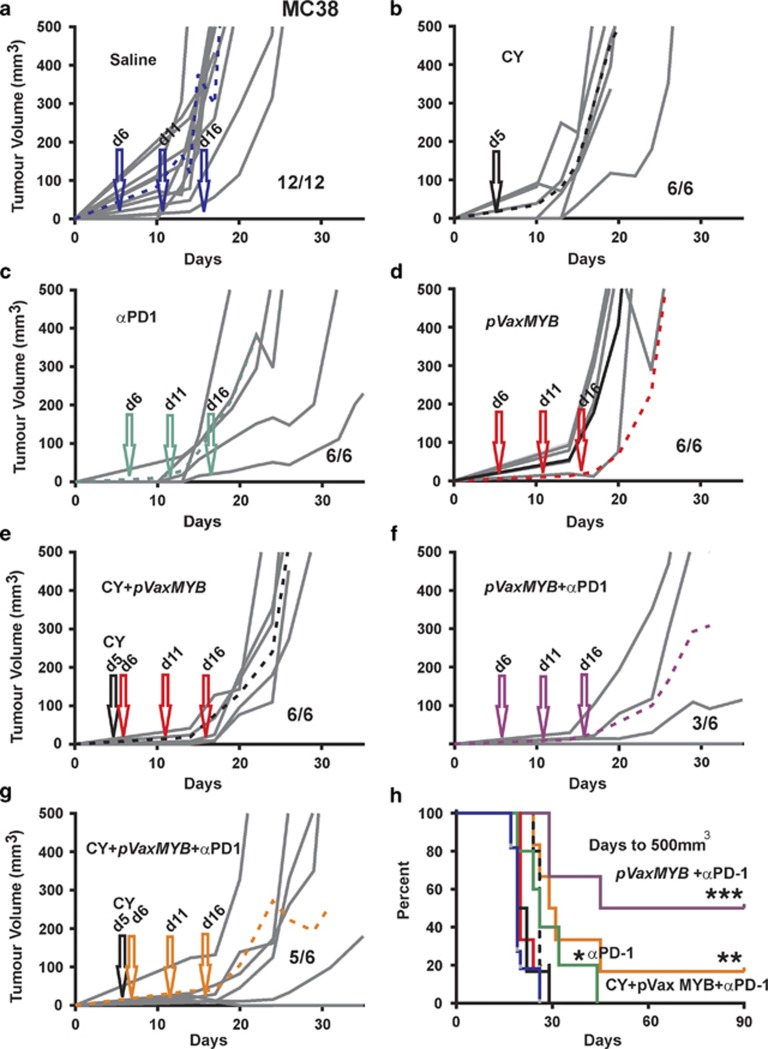
The same therapeutic approach was taken to C57BL/6 mice challenged with MC38 tumour cells. Stand-alone CY, anti-PD-1 and the MYB vaccine treatments all delayed tumour development (Figures 6b–d). As anticipated, MYB vaccination in concert with CY delayed tumour growth but provided no significant benefit in terms of tumour-free survival when compared with the saline control, with all (six out of six) mice developing tumours (Figure 6e). However, when the MYB vaccine was combined with PD-1 antibody therapy it led to protection in half of the mice (three out of six) (Figure 6f). This protection was not enhanced with the addition of CY (five out of six). Indeed the addition of CY appeared to be detrimental when combined with anti-PD-1 (Figure 6g). Thus the most significant survival benefit for the MC38/C57BL/6 model was found in the MYB vaccine plus PD-1 antibody group (Figure 6f).
Figure 6.
Tumour control in C57BL/6 mice using combinations of immunotherapies. C57Bl/6 mice were inoculated with 5 × 105 CT26 cells subcutaneously before treatment with saline (a). A single 50 mg kg−1 dose CY was administered at day 5 (b) or anti-PD-1 antibody at 250 μg on days 6, 11 and 16 (c). At days 6, 11 and 16, 15 μg pVaxMYB in 50 μl was delivered intramuscularly (d). At days 6, 11 and 16, 15 μg pVaxMYB plus 50 mg kg−1 dose CY at day 5 were injected (e). Similarly, 15 mg pVaxMYB with 250 μg anti-PD-1 antibody at days 6, 11 and 16 were administered (f). Finally, a combination of 50 mg kg−1 dose CY with 15 μg pVaxMYB plus 250 μg anti-PD-1 or saline at days 6, 11 and 16 were employed (g). Dashed lines represent mean response to each treatment. Data for all treatments are represented as survival plots based upon tumours reaching 500 mm3 in volume (h). (***P<0.001; **P<0.01;*P<0.05, Log-rank, Mantel–Cox test; n=6–12 mice per cohort). Fractions represent tumour-bearing mice after 90 days.
These data collectively demonstrate that the MYB DNA vaccine is effective at day 2 post-tumour cell challenge in mice bearing MC38 tumours but not at day 5 for this model or the CT26/BALB/c model. To restore tumour control, treatment with anti-PD-1 therapy was required for MC38, and in the case of CT26 tumours CY was required. The different responses between the strains of mice also highlighted the variation that might be expected in patients.
Discussion
We have investigated the relevance of high MYB expression in CRC, finding that is it essential for tumour cell growth, inhibition of differentiation and protection from apoptosis.27 Collectively these observations make a compelling case for exploring ways to inhibit MYB function or to target the cells that express aberrant levels of MYB. Knowing that all cellular proteins are presented on the cell surface as peptides in concert with MHC, suggests that these proteins have the potential to be recognized by T-cells should tolerance be broken. We have found that this is achievable through the targeting of MYB, without any detectable short- or long-term damage to normal cells that express physiological levels of MYB.11, 14 However to date, effective tumour control had been reported only in a prophylactic setting.11, 14 Here we show that therapeutic protection can be achieved in two pre-clinical CRC mouse models, across two different strains of mice.
The use of immune check point inhibitors that target PD-1 and CTLA-4 in cancer treatment is emerging as being most effective in malignant melanoma,28, 29, 30 which have high TILs compared with most other cancers.31, 32 By contrast, there is to date only a single patient who responded to check point inhibition as a stand-alone therapy in CRC patients.29, 33, 34 Our mouse studies suggest that anti-PD-1 therapy does produce a measureable delay in tumour progression only in the CT26 model. Such a lack of enduring therapeutic benefit in the MC38 model indicates that this is like most patients with sporadic CRC who seem to be insufficiently, immunologically activated, to benefit from stand-alone anti-PD-1. However, as the use of the vaccine with CY seems to improve CT26 tumour control, it would be interesting to determine if there is lasting protection in the context of rechallenge in the vaccinated mice compared with the anti-PD-1 protected mice. Thus, the provision of a stimulus to create an immunologically active environment to antigens that are either unique or aberrantly expressed in CRC underpins the approach we have employed here. Accordingly, there seems to be a need to additionally modulate the immune environment particularly when the tumour burden increases.
By targeting of an essential protein for tumour cell survival, we were able to elicit an effective immunological response. Protection was only evident in the MC38/C57BL/6 model when vaccinated at 2 days post tumour cell inoculation with half of the mice remaining tumour free. By contrast, the CT26/BALB/c model showed no enduring protection even though evidence of immune activation was established using a range of in vivo and in vitro assays. The negative microenvironment established by these tumours appears to inhibit the activity of this response through the induction and expansion of T-regs or the expression of PD-L1 on the tumour cell surface.
Our data suggest that in the MC38/C57BL/6 model anti-PD-1 therapy essentially rescues the vaccine efficacy presumably by increasing the activation of CD8+ MC38-TILs as defined by high CD44 expression. Intriguingly, in the case of the CT26/BALB/c model CD8+, TILs were mostly deactivated with respect to CD44 expression while being predominantly PD-1+. However, in this instance anti-PD-1 therapy was efficacious as a stand-alone therapy presumably working through the smaller fraction of CD44+(PD-1+) TILs allowing them to exert CT26 tumour control.
The use of low dose CY was investigated in response to our finding that in the BALB/c mouse a single treatment of CY reduced the percentage of FOXP3+ CD4 T cells although this was not observed in C57BL/6 mice. Accordingly, the use of CY in BALB/c mice inoculated with CT26 cells delayed tumour progression by 10 days, while CY had a negligible effect, if any, in the MC38/C57BL/6 setting. Although, as single low dose CY preferentially kills T-regs, repeated cycles of low dose CY progressively eliminates a broader spectrum of T cells,22 which was also our experience, thus this therapeutic strategy has its limitations. Nevertheless, further investigation of combining metronomic CY treatments along with the MYB vaccine are warranted.
Modulation of anti-tumour inhibitory mechanisms provides a situation in which activation of the immune system via vaccination can have therapeutic benefit both in controlling tumour growth and in some cases removing tumours. At present, the MYB vaccine when applied to minimal disease burden and in combination with anti-PD-1 or CY therapy extends survival of tumour bearing mice. Indeed, in some instances tumours were eliminated. We have taken several steps to engineer safety features into our vaccine rendering it incapable to encode an active transcription factor, with the observation that a number of mice were rendered tumour free up to 90 days post vaccination is in accord with our earlier safety studies.11, 14 While we are aware of the limitations of pre-clinical mouse models, the ability of our vaccine to work across tumour strains is most promising.
Currently there exists a window between the times when a patient is declared as having entered remission and when they may or may not relapse. Increasing evidence indicates that this dichotomy is dictated by immune activation, both in CRC1 and other epithelial cancers.35 Our vaccine offers promise as an actionable therapy that can be administered to either prevent tumour recurrence and/or slow down the growth of relapse-associated tumours. Patient selection on the basis of MHC should not in theory be necessary. Proof-of-principle has also been provided for the vaccine to be combined with immune check point inhibitors. If this study translates to CRC patients, it may precipitate a change from passive and reactionary therapeutic treatment of CRC patients to an actionable and possible preventative approach.
Methods
Mice and cell lines
C57Bl/6 and BALB/c mice were purchased from WEHI (Melbourne, VIC, Australia). All mouse experiments were conducted with 6–8-week-old female mice in accordance with the Animal Ethics Committee of Peter MacCallum Cancer Centre (Melbourne, VIC, Australia). The MC38 (C57Bl/6) and CT26 (BALB/c) are described elsewhere.36 Tumour volumes were calculated using the following formulae—(width × width)/2 × length.
Construction of DNA fusion vaccines
The generation of the P2-Myb-P30 construct has been described previously.14 The P2-Myb-P30 construct was cloned into pVAX1 from Invitrogen, Mulgrave, VIC, Australia. Mutagenesis was achieved using Invitrogen GENEART Site-Directed Mutagenesis System and primers; forward 5′-ACCAGGGCACCATTCTGGTCAATGTTAAGAACCTCTT-3′ and reverse 5′-AAGAGGTTCTTAACATTGACCAGAATGGTGCCCTGGT-3′. Amplicons were subjected to DNA sequencing and endotoxin-free plasmids were purified with Qiagen Maxi prep columns (Qiagen, Doncaster, Australia).
In vivo vaccination, tumour models and CY treatment
Vaccination targeting MC38 and CT26 cells was achieved via intra-muscular injection into the hind leg using 15 μg of DNA vaccine in 50 μl phosphate-buffered saline. Subcutaneous tumour models were generated using 5 × 105 cells, as described previously.14 CY (Endoxan) was administered by intraperitoneal injection at 25 or 50 mg kg−1 in 100 μl phosphate-buffered saline. Monoclonal Anti-PD-1 antibody 250 μg was delivered IP (clone RMP-1-14-anti-PD-1mAb), were purchased from BioXcell (West Lebanon, NH, USA).
In vitro T-cell activation and Xcelligence killing assays
The Xcelligence system was utilized, whereby changes in attached target CRC cells maintain electrical resistance while attached to the surface substrate. The loss of attachment is then measured in real time. As the target cells are lysed by effectors they lift off and a drop in resistance becomes evident.
Spleens were taken from mice that have been vaccinated 3 months prior to harvest or from age-matched naïve animals. They were homogenised through 50 μm sieve and plated on γ-irradiated (30 Gray) CT26 cells and grown in complete T-cell media (RMPI-1640 with 10% foetal calf serum, 1 mmol l−1 sodium pyruvate, 2 mmol l−1 glutamine, 0.1 mmol l−1 non-essential amino acids, 100 U ml−1 penicillin/streptomycin (Life Technologies, Mulgrave, VIC, Australia)) supplemented with 30 ng ml−1 anti-CD28 (BD Biosciences, North Ryde, NSW, Australia) and 100 U ml−1 IL-2 (NIH, Bethesda, MD, USA). Twenty-four hours later the splenocytes were removed from co-culture with tumour cells and grown for 48 h before Ficoll-Paque purification and cultured in complete T-cell media containing 100 μ ml−1 IL-2. CT26 (104) cells were allowed to adhere over night to Xcelligence E-plates, before the addition of 7 day post activation T cells. The E-plates were read every 2 min over 4 h. Normalization to starting resistance was applied before generation of graphs in Graphpad Prism.
Flow cytometry staining
Cells were stained in FACS buffer (phosphate-buffered saline; Ca/Mg-free; 2% foetal calf serum, 2mM ethylenediaminetetraacetic acid) with antibodies CD44 (BV785—Biolegend, Karrinyup, WA, Australia), CD62L (BV510—Biolegend), CD8a (BV711—Biolegend), CD4 (APC-Cy7 BD Biosciences), TRCb (BV421 BD Biosciences), FOXP3 (fluorescein isothiocyanate BD Biosciences) and propidium iodide. All flow cytometry was completed using the LSR II (BD Biosciences).
Chloramphenicol acetyltransferase assays
Chloramphenicol acetyltransferase assays using the MYB-responsive reporter construct have been described in detail elsewhere.16 Wild-type and Mutant MYB cDNAs were subcloned into pCDNA3.1/nV5-DEST.
Acknowledgments
We would like to thank the Peter Mac flow-cytometry unit for excellent assistance and advice. RGR and PKD are supported by NHMRC Fellowships and RLA is supported by an NBCF Fellowship. Research support was provided in part by the CASS foundation and the NHMRC, Australia. Also thanks to Assistant Professor Michael Kewshaw and Professor Joseph Trapani for helpful discussions and advice and the Peter Mac animal facility staff.
Footnotes
The Supplementary Information that accompanies this paper is available on the Clinical and Translational Immunology website (http://www.nature.com/cti)
Supplementary Material
References
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- Galon J, Fridman WH, Pages F. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res. 2007;67:1883–1886. doi: 10.1158/0008-5472.CAN-06-4806. [DOI] [PubMed] [Google Scholar]
- Felsher DW.Oncogene addiction versus oncogene amnesia: perhaps more than just a bad habit Cancer Res 2008683081–3086.discussion 3086. [DOI] [PubMed] [Google Scholar]
- Weinstein IB, Joe AK. Mechanisms of disease: Oncogene addiction—a rationale for molecular targeting in cancer therapy. Nat Clin Pract Oncol. 2006;3:448–457. doi: 10.1038/ncponc0558. [DOI] [PubMed] [Google Scholar]
- Ramsay RG, Ciznadija D, Mantamadiotis T, Anderson R, Pearson R. Expression of stress response protein glucose regulated protein-78 mediated by c-Myb. Int J Biochem Cell Biol. 2005;37:1254–1268. doi: 10.1016/j.biocel.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Corrigall VM, Vittecoq O, Panayi GS. Binding immunoglobulin protein-treated peripheral blood monocyte-derived dendritic cells are refractory to maturation and induce regulatory T-cell development. Immunology. 2009;128:218–226. doi: 10.1111/j.1365-2567.2009.03103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biroccio A, Benassi B, D'Agnano I, D'Angelo C, Buglioni S, Mottolese M, et al. c-Myb and Bcl-x overexpression predicts poor prognosis in colorectal cancer: clinical and experimental findings. Am J Pathol. 2001;158:1289–1299. doi: 10.1016/S0002-9440(10)64080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay RG, Thompson MA, Hayman JA, Reid G, Gonda TJ, Whitehead RH. Myb expression is higher in malignant human colonic carcinoma and premalignant adenomatous polyps than in normal mucosa. Cell Growth Differ. 1992;3:723–730. [PubMed] [Google Scholar]
- Zuber J, Rappaport AR, Luo W, Wang E, Chen C, Vaseva AV, et al. An integrated approach to dissecting oncogene addiction implicates a Myb-coordinated self-renewal program as essential for leukemia maintenance. Genes Dev. 2011;25:1628–1640. doi: 10.1101/gad.17269211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao RY, Drabsch Y, Cross RS, Cheasley D, Carpinteri S, Pereira L, et al. MYB is essential for mammary tumorigenesis. Cancer Res. 2011;71:7029–7037. doi: 10.1158/0008-5472.CAN-11-1015. [DOI] [PubMed] [Google Scholar]
- Carpinteri S, Williams BB, Miao YuR, Cullinane C, Malaterre J, Norris MD, et al. Optimizing DNA vaccines against nuclear oncogenes. Immunogastroenterology. 2012;1:108–118. [Google Scholar]
- Renard V, Sonderbye L, Ebbehoj K, Rasmussen PB, Gregorius K, Gottschalk T, et al. HER-2 DNA and protein vaccines containing potent Th cell epitopes induce distinct protective and therapeutic antitumor responses in HER-2 transgenic mice. J Immunol. 2003;171:1588–1595. doi: 10.4049/jimmunol.171.3.1588. [DOI] [PubMed] [Google Scholar]
- Fryauff DJ, Mouzin E, Church LW, Ratiwayanto S, Hadiputranto H, Sutamihardja MA, et al. Lymphocyte response to tetanus toxin T-cell epitopes: effects of tetanus vaccination and concurrent malaria prophylaxis. Vaccine. 1999;17:59–63. doi: 10.1016/s0264-410x(98)00144-3. [DOI] [PubMed] [Google Scholar]
- Williams BB, Wall M, Miao RY, Williams B, Bertoncello I, Kershaw MH, et al. Induction of T cell-mediated immunity using a c-Myb DNA vaccine in a mouse model of colon cancer. Cancer Immunol Immunother. 2008;57:1635–1645. doi: 10.1007/s00262-008-0497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanei-Ishii C, Yasukawa T, Morimoto RI, Ishii S. c-Myb-induced trans-activation mediated by heat shock elements without sequence-specific DNA binding of c-Myb. J Biol Chem. 1994;269:15768–15775. [PubMed] [Google Scholar]
- Cheasley D, Pereira L, Lightowler S, Vincan E, Malaterre J, Ramsay RG. Myb controls intestinal stem cell genes and self-renewal. Stem Cells. 2011;29:2042–2050. doi: 10.1002/stem.761. [DOI] [PubMed] [Google Scholar]
- Ciznadija D, Tothill R, Waterman ML, Zhao L, Huynh D, Yu RM, et al. Intestinal adenoma formation and MYC activation are regulated by cooperation between MYB and Wnt signaling. Cell Death Differ. 2009;16:1530–1538. doi: 10.1038/cdd.2009.94. [DOI] [PubMed] [Google Scholar]
- Nicolaides NC, Gualdi R, Casadevall C, Manzella L, Calabretta B. Positive autoregulation of c-myb expression via Myb binding sites in the 5' flanking region of the human c-myb gene. Mol Cell Biol. 1991;11:6166–6176. doi: 10.1128/mcb.11.12.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H, Daniel C. Therapeutic opportunities for manipulating T(Reg) cells in autoimmunity and cancer. Nat Rev Drug Discov. 2013;12:51–63. doi: 10.1038/nrd3683. [DOI] [PubMed] [Google Scholar]
- Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- Le DT, Jaffee EM. Regulatory T-cell modulation using cyclophosphamide in vaccine approaches: a current perspective. Cancer Res. 2012;72:3439–3444. doi: 10.1158/0008-5472.CAN-11-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistigu A, Viaud S, Chaput N, Bracci L, Proietti E, Zitvogel L. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin Immunopathol. 2011;33:369–383. doi: 10.1007/s00281-011-0245-0. [DOI] [PubMed] [Google Scholar]
- Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KA, Tam VL, Wong RM, Pagarigan RR, Meisenburg BL, Joea DK, et al. Enhancing DNA vaccination by sequential injection of lymph nodes with plasmid vectors and peptides. Vaccine. 2009;27:2603–2615. doi: 10.1016/j.vaccine.2009.02.038. [DOI] [PubMed] [Google Scholar]
- Song MY, Park SH, Nam HJ, Choi DH, Sung YC. Enhancement of vaccine-induced primary and memory CD8(+ T-cell responses by soluble PD-1. J Immunother. 2011;34:297–306. doi: 10.1097/CJI.0b013e318210ed0e. [DOI] [PubMed] [Google Scholar]
- Ramsay RG, Gonda TJ. MYB function in normal and cancer cells. Nat Rev Cancer. 2008;8:523–534. doi: 10.1038/nrc2439. [DOI] [PubMed] [Google Scholar]
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anichini A, Molla A, Vegetti C, Bersani I, Zappasodi R, Arienti F, et al. Tumor-reactive CD8+ early effector T cells identified at tumor site in primary and metastatic melanoma. Cancer Res. 2010;70:8378–8387. doi: 10.1158/0008-5472.CAN-10-2028. [DOI] [PubMed] [Google Scholar]
- Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson EJ, Sharfman WH, Drake CG, Wollner I, Taube JM, Anders RA, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19:462–468. doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte S, Rosenberg SA. Immunotherapy for metastatic solid cancers. Adv Surg. 2011;45:341–360. doi: 10.1016/j.yasu.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett TH, Griswold DP, Jr, Roberts BJ, Peckham JC, Schabel FM., Jr Tumor induction relationships in development of transplantable cancers of the colon in mice for chemotherapy assays, with a note on carcinogen structure. Cancer Res. 1975;35:2434–2439. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.