Abstract
Host defense mechanisms are impaired in patients with congenital neutrophil (polymorphonuclear neutrophils (PMN)) defects. Impaired PMN chemotaxis is observed in localized aggressive periodontitis (LAP), a familial disorder characterized by destruction of the supporting structures of dentition. In the present studies, we sought evidence for molecular events underlying this aberrant human PMN phenotype. To this end, PMN transendothelial migration and superoxide anion generation were assessed with LAP patients and asymptomatic family members, as well as patients with other chronic mucosal inflammation. PMN from LAP patients showed decreased transmigration across vascular endothelial monolayers (18 ± 12% of control, n = 4) and increased superoxide anion generation (358 ± 37%, p = 0.003). Gene expression was analyzed using oligonucleotide microarrays and fluorescence-based kinetic PCR. cDNA microarray and kinetic-PCR analysis revealed diminished RNA expression of leukocyte-type diacylglycerol (DAG) kinase α in PMN from LAP patients (4.6 ± 1.7 relative units, n = 6, p = 0.007) compared with asymptomatic individuals (51 ± 27 relative units, n = 7). DAG kinase activity was monitored by DAG phosphorylation and individual DAG molecular species were quantified using liquid chromatography and tandem mass spectrometry-based lipidomics. DAG kinase activity was also significantly decreased (73 ± 2%, p = 0.007) and correlated with increased accumulation of 1,2-diacyl-sn-3-glycerol substrates (p = 0.01). These results implicate defects in both PMN transendothelial migration and PMN DAG kinase α signaling as disordered functions in LAP. Moreover, they identify a potential molecular lesion in PMN signal transduction that may account for their aberrant responses and tissue destruction in this disease.
Orchestrated activation and recruitment of neutrophils (polymorphonuclear neutrophils (PMN)5) are essential to remove pathogenic bacteria for host defense (1). The role of PMN in innate immunity is underscored by congenital defects such as chronic granulomatous disease, Chediak-Higashi syndrome, and leukocyte adhesion deficiency syndrome (2). These illnesses are characterized by genetic abnormalities that alter PMN functional responses, leading to recurrent microbial infection and severe periodontal disease (2). In this regard, localized aggressive periodontitis (LAP) is a destructive form of periodontal disease associated with infection by specific Gram-negative bacteria and impaired PMN chemotaxis (3). LAP is clinically characterized by 1) circumpubertal onset of periodontitis, 2) bleeding on probing at these local sites, 3) alveolar bone loss localized around the first permanent molars and incisors, 4) high levels of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in the periodontal pockets, and 5) familial background. Patients with LAP are otherwise in good general health and apparently not predisposed to extra-oral microbial infections (for recent reviews, see Refs. 3 and 4). The oral cavity contains a microflora equal in mass and complexity to the lower gastrointestinal tract. The presence of teeth traversing the mucosal tissue into a bacterial-rich external cavity provides a unique environment that appears to unmask a specific PMN functional defect in LAP. To date, neither genetic nor molecular abnormalities are assigned to this familial disease.
Impaired chemotaxis toward a formylated peptide (fMLP) and several endogenous ligands such as C5a are well-recognized PMN defects in LAP (3, 5, 6). They persist following aggressive treatment and are observed in siblings before the development of clinical symptoms (3). Defects in PMN functions predispose to the development of LAP in teenage years, and specific binding of labeled chemotactic peptide as well as phagocytosis are reduced (3, 6). In addition, in PMN from LAP, pathways for the formation of temporally regulated and rapid local-acting lipid-derived signals such as the eicosanoids leukotriene B4 (LTB4) and the anti-inflammatory autacoid lipoxin A4 as well as the second messengers appear to be altered (7, 8). Other PMN responses vital to host defense, such as the respiratory burst, are elevated in an apparently agonist-specific fashion (3). In view of these altered functional responses in PMN from patients with LAP, namely, increased respiratory burst yet diminished chemotaxis, recent investigations focused on signaling pathways that might underlie these differences (3, 7, 8). Evidence at the molecular level is lacking; it remains of interest to define the disparate responses of PMN in patients with LAP. To this end, PMN from patients with LAP were examined and compared with those of other diseases and from nondisease subjects using a panel of functional, genetic, biochemical, and molecular analyses to identify potential altered metabolic pathways or other abnormalities specific to LAP.
Materials and Methods
Human polymorphonuclear leukocytes: isolation and incubations
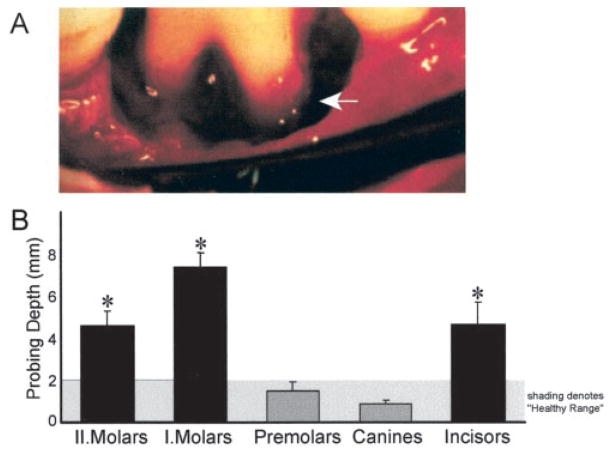
Patients with LAP (n = 11), family members without LAP (n = 4), and patients with chronic periodontitis (CP, n = 6) were recruited from Boston University Medical Center. Asymptomatic subjects and patients with LAP or CP denied systemic disease by medical history and did not report the use of tobacco products. Patients were diagnosed according to clinical and radiographic criteria including alveolar bone loss around the first permanent molars and incisors (see Fig. 1). Patients with LAP were African American (three males and eight females) and were 18 to 29 years of age. Blood samples from patients with asthma (n = 5) were obtained from the Asthma Research Center at Brigham and Women’s Hospital. Asymptomatic blood donors who were recruited randomly denied taking medications 2 wk before (n = 22).
FIGURE 1.
LAP is characterized by alveolar bone loss localized to first molars and incisors. A, Clinical picture of a first molar of a LAP patient at the time of periodontal surgery. Arrow denotes pocket from loss of alveolar bone from the roots of the tooth and the intact alveolar bone on adjacent teeth. B, Clinical values from patients with LAP. Probing depth is a parameter indicative of bone loss and tissue destruction and was measured in patients with LAP (n = 7). Shaded area signifies physiological distance of 1–2 mm from gingival margin which is considered as “healthy.”*, Probing depth for tooth groups that were significantly different from healthy subjects (ANOVA, p = 0.005).
Neutrophils (PMN) were isolated from fresh heparinized venous blood (7) and preparations were >98% PMN with >96% viability. Superoxide anion generation (9), transendothelial migration (10, 11), and diacylglycerol kinase (DAGK) activity (12) were separately determined.
RNA analysis
Total RNA was isolated from PMN using TRIzol reagent (Life Technologies, Grand Island, NY), and RNA integrity was verified by agarose gel electrophoresis and quantitated by UV. Total neutrophil RNA (~10–20 μg) from an age-, gender-, and race-matched asymptomatic subject or a characteristic patient with LAP was submitted to the Brigham and Women’s Hospital gene array Technology Center that is equipped with a GeneChip Fluidic Station 400 (Affymetrix, Santa Clara, CA) Hewlett-Packard GeneArray Scanner (Palo Alto, CA), and GeneChip Hybridization Oven 320. Abundance of specific mRNA transcripts was analyzed using HuGeneFL (7070 genes) and/or HG-U95Av2 (12626 genes which includes 7070 genes presented on HuGeneFL) Affymetrix GeneChip probe arrays. Acquisition and processing of GeneChip data was performed with Affymetrix Microarray Suite software (MAS, 5.0). Abundance of each transcript was labeled as either present, absent, or marginal based on an algorithm that identifies stray hybridization and combines results from probes that interrogated different fragments of the transcript. Statistical significance of detection for each transcript was determined. Transcripts that demonstrated statistically significant detection and were labeled as present by MAS analyses were assigned a “+,” whereas transcripts labeled as absent were assigned a “−”.
Fluorescence-based kinetic PCR
RNA from asymptomatic donors and patients with LAP was reverse transcribed (Superscript II reverse transcriptase; Life Technologies). Real-time PCR was performed with Platinum Quantitative PCR Supermix-UDG (In-vitrogen Life, Carlsbad, CA) containing SYBR Green 1 nucleic acid gel stain (Roche, Indianapolis, IN) and fluorescein calibration dye (Bio-Rad, Richmond, CA) using a Bio-Rad iCyclerQ Multicolor Real-Time PCR Detection System and software (Bio-Rad). Specific primers were selected to amplify conserved domains in the 3′ end of DAGKα corresponding to the region probed with an Affymetrix GeneChip: DAGK-3′ sense 5′-CCAAAGGGAAACACAAGTCATT-3′ and antisense 5′-CACACCCCAAAACACATACATT-3′ (95°C for 30 s, 60°C for 30 s, and 72°C for 30 s; 40 cycles). β-Actin was used as reference and amplified with the sense primer 5′-GGTGGCTTTTAGGATGGCAAG-3′ and antisense primer 5′-ACTGGAACGGTGAAGGTGACAG-3′. Isolated cDNA (~125 ng) was added as PCR template. PCR efficiency for each primer pair was determined by quantitating amplification with increasing concentrations of template cDNA (15.6, 31.2, 62.5, 125.0, 250.0, and 500.0 ng) and specific amplification was initially verified by complete nucleotide sequencing of the isolated PCR products and subsequent analysis of melt curve profiles for each amplification. RNA expression of DAGKα was calculated (13). Expression of the target gene DAGKα was calculated based on the real-time PCR efficiency (E) and the threshold crossing point (TCP) and is expressed in comparison to the reference gene β-actin. Relative DAGKα gene expression in LAP patients and asymptomatic individuals was calculated using 2−ΔTCP, where ΔTCP = (TCP DAGKα − TCPactin). Regression analysis and ANOVA were performed with STATISTICA (version 6.1, 2002; StatSoft, Tulsa, OK).
Lipidomics
DAG species were identified using matching criteria (i.e., liquid chromatography (LC) retention time, mass spectrum) and quantitated by LC/mass spectrometry (MS)/MS. Lipids from PMN were extracted by liquid/liquid extraction (14) followed by solid-phase extraction (Oasis HLB cartridges; Waters, Milford, MA) with an average recovery of ~78% as reported in detail (A. Kantarci, C. B. Clish, K. Oyaizu, H. Hasturk, C. N. Serhan, and T. E. Van Dyke, manuscript in preparation). Materials in chloroform fractions were taken to dryness under a gentle stream of nitrogen, suspended in mobile phase, and subjected to LC/MS/MS with an LCQ (Finnigan-MAT, San Jose, CA) quadrupole ion-trap mass spectrometer equipped with an electrospray ionization interface. Briefly, each isolated fraction was injected into a HPLC consisting of a SpectraSystem P4000 (Thermo Separation Products, San Jose, CA) quaternary gradient pump, Luna C18-2 (150 × 2 mm, 5 μM) column equipped with rapid spectra scanning SpectraSystem UV 2000 (Thermo Separation Products) UV-VIS absorbance detector (7). The column was eluted with acetonitrile/isopropanol (80:20, v/v) at 0.2 ml/min into the electrospray probe.
Results
A critical event in host defense is transmigration of PMN across vascular endothelial cells (4). To evaluate the dynamics of these interactions in LAP, we assessed transmigration across vascular endothelial monolayers. LTB4 was selected as a chemoattractant since this mediator is generated in early stages of inflammation in the periodontium (7, 15, 16) and is among the more potent chemoattractants (17). PMN from LAP patients gave diminished (18 ± 12% of control, n = 4, p < 0.05) migration across endothelial monolayers (Fig. 2A). This reduction was selective with LTB4 as the agonist, since differences were not obtained with a chemotactic peptide (fMLP). Also, PMN from patients with LAP did not display enhanced adhesion to endothelial cells (Fig. 2A). PMN from these patients gave increased superoxide anion generation, a finding consistent with earlier observations with chemotactic peptides (3). Superoxide anion generation initiated by LTB4 (Fig. 2B), IL-8 (Fig. 2B), fMLP (data not shown), or a nonselective agonist phorbol ester (PMA; data not shown) was similarly increased: 487 ± 75%, 348 ± 19%, 333 ± 24%, and 382 ± 39%, respectively. All values obtained with PMN from LAP patients were significantly higher than asymptomatic age-, gender-, and race-matched control subjects (p = 0.0002, n = 11), CP patients (p = 0.0002, n = 6), and family members without LAP (p = 0.003, n = 4). Unlike LAP, PMN from patients with asthma, which is characterized by chronic mucosal inflammation of the lower respiratory tract, did not give enhanced superoxide anion generation (n = 5; data not shown). PMN from family members without LAP exhibited a 71 ± 14% increase (p = 0.003) with IL-8 stimulation (Fig. 2B).
FIGURE 2.
Disparate functional alterations in PMN from LAP. A, Transmigration of PMN from four separate LAP patients and matched asymptomatic controls across vascular endothelial monolayers. Chemotaxis was initiated with LTB4 (10 nM). PMN that completely traversed (
 and ■) or were associated with the endothelial monolayer (□) were quantitated 60 min after addition of the endogenous chemoattractant. Results are the mean ± SD of three determinations. B, Superoxide anion generation in PMN from patients with LAP (n = 4), CP (n = 6), family members without LAP (n = 4), and asymptomatic individuals (n = 10). PMN were exposed to indicated ligands and superoxide dismutase-inhibitable cyto-chrome c reduction monitored in a Vmax kinetic microplate reader. Superoxide production is expressed in nanomoles O2− per 106 PMN. Differences between patient or donor groups are indicated (* and #, p < 0.05, ANOVA, Newman-Keuls test).
and ■) or were associated with the endothelial monolayer (□) were quantitated 60 min after addition of the endogenous chemoattractant. Results are the mean ± SD of three determinations. B, Superoxide anion generation in PMN from patients with LAP (n = 4), CP (n = 6), family members without LAP (n = 4), and asymptomatic individuals (n = 10). PMN were exposed to indicated ligands and superoxide dismutase-inhibitable cyto-chrome c reduction monitored in a Vmax kinetic microplate reader. Superoxide production is expressed in nanomoles O2− per 106 PMN. Differences between patient or donor groups are indicated (* and #, p < 0.05, ANOVA, Newman-Keuls test).
Gene expression was assessed using Affymetrix GeneChip probe arrays (18–20) with PMN from a characteristic LAP and age-, gender-, and race-matched asymptomatic subjects to survey potential alterations in signal transduction pathways. The human genome GeneChip probe arrays used in the analysis of HG-U95av2 (12,626 genes) and HuGeneFL (7,070 genes) interrogate 7,070 common human genes and detected expression of 3,349 of 12,626 genes (26.5%) in a LAP patient and 1,630 of 7,070 genes (23%) in an asymptomatic individual. The LAP PMN displayed genes characteristic for human leukocytes including 5-lipoxygen-ase, IL-1β, phosphatidylinositol 3-kinase, and the integrin MAC-1 (CD11b) (Table I). Of interest, the expression of the leukocyte-type DAGK, DAGKα (21–23), was not detected in PMN from LAP patients, whereas its expression was present in PMN isolated from asymptomatic individuals (vide infra and Table I). To confirm and extend these findings, oligonucleotide primers were designed for fluorescence-based kinetic PCR (13, 24) to quantitate DAGKα expression (Fig. 3). PMN from patients with LAP gave ~10-fold lower DAGKα RNA relative expression when directly compared with asymptomatic subjects (asymptomatic, 51 ± 27 relative units; LAP, 4.6 ± 1.7 relative units, n = 6, p < 0.008). In separate analyses, individual LAP patients (n = 7) were compared with individual asymptomatic subjects (n = 5) or family members (n = 2). LAP patients consistently displayed decreased expression of DAGKα RNA (p = 0.002, data not shown).
Table I.
Microarray analysis of gene expression in PMN from a patient with LAP a
| Gene | Asymptomatic | LAP |
|---|---|---|
| Eicosanoid Formation | ||
| 5-Lipoxygenase | + | + |
| 15-Lipoxygenase | − | − |
| Cyclooxygenase-2 | + | + |
| PG dehydrogenase | − | − |
| LTA4 hydrolase | + | + |
| LTC4 synthase | − | − |
| Cytokine/chemokine | ||
| IL-8 | + | + |
| IL-1β | + | + |
| IL-4 | − | − |
| TGF-β | − | − |
| Signal Transduction | ||
| DAGKγ | − | − |
| DAGKθ | − | − |
| DAGKε | − | − |
| Leukocyte type DAGKα | + | − |
| CREB | + | + |
| NF-κB (p65) | + | + |
| c-FoS | + | + |
| Phosphatidylinositol 3-kinase | + | + |
| Phosphatidylinositol 4-kinase | + | + |
| TNFR (p75) | + | + |
| TNFR (p55) | + | + |
| Leukocyte markers | ||
| Leukocyte AG (CD37) | + | + |
| MAC-1 | + | + |
Listed is the presence (+) or absence (−) of selected expressed genes in PMN from peripheral blood of a representative patient with LAP and an asymptomatic subject using Affymetrix Human GeneChip probe arrays (see Materials and Methods).
FIGURE 3.
Deficient DAGKα RNA expression in LAP. Fluorescence-based kinetic PCR analysis of DAGKα RNA in PMN from patients with LAP and asymptomatic donors. A, Representative real-time PCR amplification of DAGKα and reference gene β-actin in PMN from a LAP patient and asymptomatic individual. B, Relative expression of DAGKα in PMN from patients with LAP (n = 6) and asymptomatic individuals (n = 7), ANOVA, Newman-Keuls test, p = 0.008 (*).
DAGK activity in PMN from patients with LAP was also examined to determine whether the decrements in RNA expression were related to diminished activity of this enzyme and/or formation of its product (Fig. 4). PMN from LAP gave a dramatic reduction (p = 0.007) in their ability to convert substrate 1,2-DAG to phosphatidic acid (0.18 ± 0.02 pmol, n = 8) compared with PMN from asymptomatic individuals (0.68 ± 0.04 pmol, n = 11), family members without LAP (0.60 ± 0.06 pmol, n = 4), patients with CP (0.66 ± 0.10 pmol, n = 6), or asthmatic individuals (0.75 ± 0.14 pmol, n = 5). To assess whether inhibition of DAGK leads to abnormal functional responses, we exposed PMN from asymptomatic individuals to a DAGK inhibitor, R59022 (10 μM) (25, 26), and evaluated fMLP-induced superoxide anion generation as a hallmark PMN functional response. DAGK inhibition induced a dramatic and significant amplification (>500%, n = 5, p = 0.007) of fMLP-induced superoxide anion generation (9.54 ± 1.72 nmol/min/105 PMN) when directly compared with PMN that were exposed to fMLP alone (1.72 ± 0.17 nmol/min/105 PMN).
FIGURE 4.
Impaired DAGK activity in PMN from LAP patients. Total DAGK activity was measured in lysate as the conversion of external 1,2-dipalmitoyl-sn-glycerol to 32P-labeled phosphatidic acid. Phospholipids were separated by TLC and [32P]phosphatidic acid quantitated by scintillation counting. Results represent the mean ± SEM of patient groups with LAP (n = 6), asthma (n = 6), or CP as well as asymptomatic individuals (n = 10) and family members without LAP (n = 4). *, Significant difference among donor or patient groups (ANOVA, Newman-Keuls test, p < 0.05).
Thus, to further profile this and related pathway(s) as well as establish whether substrate levels and/or molecular species were altered in these patients, LC/MS/MS-based lipidomics was developed to identify (Fig. 5A) and quantitate individual species of 1,2-diacyl-sn-3-glycerol(s). DAG molecular species were identified by their physical properties including molecular ion, specific daughter ions, and coelution with authentic standards for each of the seven molecular species. PMN from LAP displayed an increase in the sum of 1,2-diacyl-sn-3-glycerol species identified and present in PMN (values for PMN of asymptomatic individuals were 190.6 ± 40.7 ng vs 269.1 ± 38.4 ng for PMN from LAP, p < 0.05, Fig. 5B). Specifically, 1,2-dipalmitoyl-sn-glycerol (denoted C16: 0, C16:0-DAG) was sharply increased (156 ± 65%, p = 0.01) in LAP when directly compared with values obtained with PMN from asymptomatic individuals (Fig. 5C, n = 6).
FIGURE 5.
Elevated DAG levels in PMN from LAP patients. A, Selected MS ion chromatograms of synthetic 1,2-diacyl-sn-3-glycerol molecular species. DAG species were resolved and identified by LC/MS/MS using specific retention time, acyl chains, and a unique MS/MS signature ion for each molecular species as indicated. B, Quantitation of total DAG molecular species identified by LC/MS/MS from patients with LAP and asymptomatic individuals (n = 6, p < 0.05). C, Evaluation of 1,2-dipalmitoyl-sn-3-glycerol (n = 6, p < 0.05).
Discussion
PMN from patients with LAP gave a ligand-specific decrement in transendothelial migration with LTB4 adding a new feature to the phenotype of LAP. Transendothelial PMN migration was decreased by 82% while superoxide anion generation from these patients was dramatically increased by 487% with LTB4. Dysregulation of these critical responses of PMN would impact both microbial clearance and PMN-mediated tissue injury, which are both hallmarks of LAP. Hence, the present findings (Fig. 2) provide evidence for the key role of PMN signal transduction in the pathogenesis of LAP as extravasation from the vasculature along a chemotactic gradient and subsequent superoxide anion generation as essential components of both innate immunity and host tissue injury (1–4).
Ligand-selective responses depend on the orchestrated spatial and temporal formation of second messengers. It is of interest that a combined approach of microarray analysis and real-time PCR with PMN uncovered reduced RNA expression for a key enzyme that terminates the second messenger DAG, namely, DAGKα (21, 22, 27). DAG binds to cytosolic protein kinase C isoforms, promoting rapid translocation of DAGKα and protein kinase C to membranes where signaling cascades are then terminated via rapid DAG conversion (28–32). In this study, we provide the first evidence that DAGKα expression as well as its activity are markedly decreased in PMN from patients with LAP. Following activation of G protein-coupled receptors, specific phospholipases are generally held to produce specific temporal and ligand DAG species that modulate protein kinase C (33, 34). These highly regulated intracellular molecular signaling mechanisms are involved in both PMN chemotaxis and superoxide anion generation. Indeed, pharmacological inhibition of DAGK led to amplification of the respiratory burst in normal PMN findings that are consistent with Refs. 25 and 26 and are reviewed in Ref. 27. Moreover, DAGK inhibition or treatment with synthetic DAG species leads to alterations in normal PMN motility (35–37). Therefore, defective DAGK expression provides a potential molecular basis that may explain disparate functional responses in PMN from patients with LAP, namely, agonist-specific reduced motility and enhanced production of superoxide anions and related reactive oxygen species. The importance of DAGK to leukocyte function is emphasized by DAGKα’s integral role in attenuating receptor signaling in lymphocytes and linking plasma receptors to nuclear responses (23).
The notion that reduced DAGKα RNA expression manifests itself in DAG signal transduction is substantiated by the dramatically reduced activity of DAGK in PMN from patients with LAP (Fig. 4). The nine mammalian isoforms of DAGK exhibit apparently little substrate specificity, namely, individual molecular species of 1,2-DAG (i.e., carrying different acyl chains), except for the ε isoform present in testis (27). Numerous structurally distinct molecular species of 1,2-DAG and phosphatidic acid can be generated via phospholipase C and phospholipase D activation, respectively, and their temporal formation is characteristically biphasic upon cell activation (28–31, 33, 34). Moreover, DAG phosphorylation also yields phosphatidic acid, which itself carries bioactivity (1, 38, 39). Thus, DAGK is a pivotal component in the intracellular signaling cascades in PMN.
In this study, we provide the first molecular analysis of intra-cellular 1,2-DAG species in human PMN where specific DAG molecules are elevated in patients with LAP. Notably, 1,2-dipalmi-toyl-sn-3-glycerol species of DAG was elevated. This is an efficient substrate for PMN DAGK (Fig. 4) as well as a product of the phospholipase D signaling pathway (27, 31). The accumulation of specific DAG species appears to arise from the diminished DAGK in LAP PMN. This molecular event is associated with elevated signaling responses to agonists such as LTB4 that rely heavily on the PMN DAG-protein kinase C pathway to activate NADPH oxidase. In addition, DAG accumulation is also likely responsible for reduced transmigration in LAP PMN (Fig. 1), a process that requires regulated protein kinase C (34). These findings implicate a shift in the set point for DAG signals in patients with LAP and are in agreement with the observed reduced DAGK activity. Taken together, these results provide previously unknown links between elevated DAG levels and impaired DAGK function that manifest as altered PMN phenotype. Moreover, in LAP-defective RNA expression of the leukocyte type DAGKα appears as a key molecular lesion underlying abnormalities in DAG-associated signal transduction in PMN.
Acknowledgments
We thank Drs. G. Stahl and M. Montalto for discussions on real-time PCR. We also thank the Asthma Research Center at Brigham and Women’s Hospital for kindly providing samples.
Footnotes
This work was supported in part by Grants P01-DE13499 (to C.N.S) and General Clinical Research Center M01-RR00533 (to T.E.V.D.) from the National Institutes of Health.
Abbreviations used in this paper: PMN, polymorphonuclear neutrophil; LAP, localized aggressive periodontitis; LTB4, leukotriene B4; CP, chronic periodontitis; DAG, diacylglycerol; DAGK, DAG kinase; TCP, threshold crossing point; LC, liquid chromatography; MS, mass spectrometry.
References
- 1.Weissmann G, Smolen JE, Korchak HM. Release of inflammatory mediators from stimulated neutrophils. N Engl J Med. 1980;303:27. doi: 10.1056/NEJM198007033030109. [DOI] [PubMed] [Google Scholar]
- 2.Lekstrom-Himes JA, Gallin JI. Immunodeficiency diseases caused by defects in phagocytes. N Engl J Med. 2000;343:1703. doi: 10.1056/NEJM200012073432307. [DOI] [PubMed] [Google Scholar]
- 3.Dennison DK, Van Dyke TE. The acute inflammatory response and the role of phagocytic cells in periodontal health and disease. Periodontology. 1997;14:54. doi: 10.1111/j.1600-0757.1997.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 4.Gallin JI, Snyderman R, Fearon DT, Haynes BF, Nathan C. Inflammation: Basic Principles and Clinical Correlates. Lippincott Williams & Wilkins; Philadelphia: 1999. [Google Scholar]
- 5.Cainciola LJ, Genco RJ, Patters MR, McKenna J, van Oss CJ. Defective polymorphonuclear leukocyte function in a human periodontal disease. Nature. 1977;265:445. doi: 10.1038/265445a0. [DOI] [PubMed] [Google Scholar]
- 6.Perez HD, Kelly E, Elfman F, Armitage G, Winkler J. Defective polymorphonuclear leukocyte formyl peptide receptor(s) in juvenile periodontitis. J Clin Invest. 1991;87:971. doi: 10.1172/JCI115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pouliot M, Clish CB, Petasis NA, Van Dyke TE, Serhan CN. Lipoxin A4 analogues inhibit leukocyte recruitment to Porphyromonas gingivalis: a role for cyclooxygenase-2 and lipoxins in periodontal disease. Biochemistry. 2000;39:4761. doi: 10.1021/bi992551b. [DOI] [PubMed] [Google Scholar]
- 8.Tyagi SR, Uhlinger DJ, Lambeth JD, Champagne C, Van Dyke TE. Altered diacylglycerol level and metabolism in neutrophils from patients with localized juvenile periodontitis. Infect Immun. 1992;60:2481. doi: 10.1128/iai.60.6.2481-2487.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy BD, Petasis NA, Serhan CN. Polyisoprenyl phosphates in intracellular signalling. Nature. 1997;389:985. doi: 10.1038/40180. [DOI] [PubMed] [Google Scholar]
- 10.Colgan SP, Serhan CN, Parkos CA, Delp-Archer C, Madara JL. Lipoxin A4 modulates transmigration of human neutrophils across intestinal epithelial monolayers. J Clin Invest. 1993;92:75. doi: 10.1172/JCI116601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takano T, Fiore S, Maddox JF, Brady HR, Petasis NA, Serhan CN. Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: evidence for anti-inflammatory receptors. J Exp Med. 1997;185:1693. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preiss JE, Loomis CR, Bell RM, Niedel JE. Quantitative measurement of sn-1,2-diacylglycerols. Methods Enzymol. 1987;141:294. doi: 10.1016/0076-6879(87)41077-x. [DOI] [PubMed] [Google Scholar]
- 13.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:E45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunstone FD, Harwood JL, Padley FB. The Lipid Handbook. Chapman & Hall; London: 1994. [Google Scholar]
- 15.Smith MA, Braswell LD, Collins JG, Boyd DL, Jeffcoat MK, Reddy M, Li KL, Wilensky S, Vogel R, Alfano M, et al. Changes in inflammatory mediators in experimental periodontitis in the rhesus monkey. Infect Immun. 1993;61:1453. doi: 10.1128/iai.61.4.1453-1459.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heasman PA, Collins JG, Offenbacher S. Changes in crevicular fluid levels of interleukin-1β, leukotriene B4, prostaglandin E2, thromboxane B2 and tumour necrosis factor α in experimental gingivitis in humans. J Periodontal Res. 1993;28:241. doi: 10.1111/j.1600-0765.1993.tb02090.x. [DOI] [PubMed] [Google Scholar]
- 17.Samuelsson B, Dahlén SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 18.Chee M, Yang R, Hubbell E, Berno A, Huang XC, Stern D, Winkler J, Lockhart DJ, Morris MS, Fodor SPA. Accessing genetic information with high-density DNA arrays. Science. 1996;274:610. doi: 10.1126/science.274.5287.610. [DOI] [PubMed] [Google Scholar]
- 19.Mills JC, Roth KA, Cagan RL, Gordon JI. DNA microarrays and beyond: completing the journey from tissue to cell. Nat Cell Biol. 2001;3:E175. doi: 10.1038/35087108. [DOI] [PubMed] [Google Scholar]
- 20.Modlin RL, Bloom BR. Immunology: chip shots–will functional genomics get functional? Science. 2001;294:799. doi: 10.1126/science.1066314. [DOI] [PubMed] [Google Scholar]
- 21.Fujikawa K, Imai S, Sakane F, Kanoh H. Isolation and characterization of the human diacylglycerol kinase gene. Biochem J. 1993;294:443. doi: 10.1042/bj2940443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart TC, Champagne C, Zhou J, Van Dyke TE. Assignment of the gene for diacylglycerol kinase (DAGK) to human chromosome 12. Mamm Genome. 1994;5:123. doi: 10.1007/BF00292343. [DOI] [PubMed] [Google Scholar]
- 23.Sanjuan MA, Jones DR, Izquierdo M, Merida I. Role of diacylglycerol kinase α in the attenuation of receptor signaling. J Cell Biol. 2001;153:207. doi: 10.1083/jcb.153.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 25.Ohtsuka T, Hiura M, Yoshida K, Okamura N, Ishibashi S. A diacylglycerol kinase inhibitor, R59022, potentiates superoxide anion production and 46-kDa protein phosphorylation in guinea pig polymorphonuclear leukocytes. J Biol Chem. 1990;265:15418. [PubMed] [Google Scholar]
- 26.Mege JL, Tao W, Molski TF, Gomez-Cambronero J, Huang CK, Becker EL, Sha’afi RI. Diacylglycerol kinase inhibitor R59022 and stimulated neutrophil responses. Am J Physiol. 1988;255:C589. doi: 10.1152/ajpcell.1988.255.5.C589. [DOI] [PubMed] [Google Scholar]
- 27.Topham MK, Prescott SM. Mammalian diacylglycerol kinases, a family of lipid kinases with signaling functions. J Biol Chem. 1999;274:11447. doi: 10.1074/jbc.274.17.11447. [DOI] [PubMed] [Google Scholar]
- 28.Feng X, Becker KP, Stribling SD, Peters KG, Hannun YA. Regulation of receptor-mediated protein kinase C membrane trafficking by auto-phosphorylation. J Biol Chem. 2000;275:17024. doi: 10.1074/jbc.275.22.17024. [DOI] [PubMed] [Google Scholar]
- 29.Ganong BR, Loomis CR, Hannun YA, Bell RM. Specificity and mechanism of protein kinase C activation by sn-1,2-diacylglycerols. Proc Natl Acad Sci USA. 1986;83:1184. doi: 10.1073/pnas.83.5.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodgkin MN, Pettitt TR, Martin A, Michell RH, Pemberton AJ, Wakelam MJ. Diacylglycerols and phosphatidates: which molecular species are intracellular messengers? Trends Biochem Sci. 1998;23:200. doi: 10.1016/s0968-0004(98)01200-6. [DOI] [PubMed] [Google Scholar]
- 31.Asaoka Y, Nakamura S, Yoshida K, Nishizuka Y. Protein kinase C, calcium and phospholipid degradation. Trends Biochem Sci. 1992;17:414. doi: 10.1016/0968-0004(92)90011-w. [DOI] [PubMed] [Google Scholar]
- 32.Rando RR. Regulation of protein kinase C activity by lipids. FASEB J. 1988;2:2348. doi: 10.1096/fasebj.2.8.3282960. [DOI] [PubMed] [Google Scholar]
- 33.Werner MH, Bielawska AE, Hannun YA. Multiphasic generation of diacylglycerol in thrombin-activated human platelets. Biochem J. 1992;282:815. doi: 10.1042/bj2820815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reibman J, Korchak HM, Vosshall LB, Haines KA, Rich AM, Weissmann G. Changes in diacylglycerol labeling, cell shape, and protein phosphorylation distinguish “triggering” from “activation” of human neutrophils. J Biol Chem. 1988;263:6322. [PubMed] [Google Scholar]
- 35.Nourshargh S, Hoult JR. Divergent effects of co-carcinogenic phorbol esters and a synthetic diacylglycerol on human neutrophil chemokinesis and granular enzyme secretion. Br J Pharmacol. 1987;91:557. doi: 10.1111/j.1476-5381.1987.tb11249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurttia HM, Pelto LM, Leino L. Evidence of an association between functional abnormalities and defective diacylglycerol kinase activity in peripheral blood neutrophils from patients with localized juvenile periodontitis. J Periodontal Res. 1997;32:401. doi: 10.1111/j.1600-0765.1997.tb00550.x. [DOI] [PubMed] [Google Scholar]
- 37.Boonen GJ, de Koster BM, VanSteveninck J, Elferink JG. Neutrophil chemotaxis induced by the diacylglycerol kinase inhibitor R59022. Biochim Biophys Acta. 1993;1178:97. doi: 10.1016/0167-4889(93)90114-5. [DOI] [PubMed] [Google Scholar]
- 38.Serhan CN, Broekman MJ, Korchak HM, Smolen JE, Marcus AJ, Weissmann G. Changes in phosphatidylinositol and phosphatidic acid in stimulated human neutrophils: relationship to calcium mobilization, aggregation and superoxide radical generation. Biochim Biophys Acta. 1983;762:420. doi: 10.1016/0167-4889(83)90007-1. [DOI] [PubMed] [Google Scholar]
- 39.Siddiqui RA, Akard LP, Garcia JG, Cui Y, English D. Chemotactic migration triggers IL-8 generation in neutrophilic leukocytes. J Immunol. 1999;162:1077. [PubMed] [Google Scholar]