Abstract
Witnessing a traumatic event but not directly experiencing it can be psychologically quite damaging. In North America alone, ~30% of individuals who witness a traumatic event develop post-traumatic stress disorder (PTSD). While effects of direct trauma are evident, consequences of indirect or secondary trauma are often ignored. Also unclear is the role of social support in the consequences of these experiences. The social defeat paradigm, which involves aggressive encounters by a large Long–Evans male rat (resident) towards a smaller Sprague–Dawley male rat (intruder), is considered a rodent model of PTSD. We have modified this model to create a trauma witness model (TWM) and have used our TWM model to also evaluate social support effects. Basically, when an intruder rat is placed into the home cage of a resident rat, it encounters an agonistic behavior resulting in intruder subordination. The socially defeated intruder is designated the SD rat. A second rat, the cage mate of the SD, is positioned to witness the event and is the trauma witnessing (TW) rat. Experiments were performed in two different experimental conditions. In one, the SD and TW rats were cagemates and acclimatized together. Then, one SD rat was subjected to three sessions of social defeat for 7 d. TW rat witnessed these events. After each social defeat exposure, the TW and SD rats were housed together. In the second, the TW and SD rats were housed separately starting after the first defeat. At the end of each protocol, depression-anxiety-like behavior and memory tests were conducted on the SD and TW rats, blood withdrawn and specific organs collected. Witnessing traumatic events led to depression- and anxiety-like behavior and produced memory deficits in TW rats associated with elevated corticosterone levels.
Keywords: depression and anxiety, PTSD, stress, trauma, witness
Introduction
Post-traumatic stress disorder (PTSD) is a condition that occurs following exposure to a traumatic experience (Yehuda, 2002; Owens et al., 2005; Reeves et al., 2005). PTSD patients exhibit a broad range of symptoms including hyperarousal, avoidance, intrusive memories, anxiety, depression and poor cognition which can impair social and occupational function. Most people associate PTSD with military combat but the fact is that much of the PTSD in the United States originates not from combat exposures but from far more common events such as criminal victimization, domestic violence, accidents, and physical, sexual, or emotional abuse (Kessler et al., 1995; Kessler, 2000; Stein, 2002). More importantly, PTSD can be triggered not only in people who personally experience these traumatic events, but also in those who witness it. For example a child who repeatedly witnesses physical and emotional abuse of his/her mother or sibling, can develop PTSD. In fact, the Diagnostic and Statistical Manual of Mental Disorders (DSM-IVTR; American Psychiatric Association [APA], 2013) has recognized that learning of or witnessing traumatic events experienced by friends or family can contribute to PTSD.
Considering the increased vulnerability of witnessing a traumatic event, from natural (hurricane, Tsunami) or man-made (war, violence, crime, abuse) causes, this issue warrants extensive investigation. Much credit goes to clinical psychologists for identifying negative effects of secondary trauma, often referred to as vicarious trauma or compassion fatigue, in helping professionals (therapists, counselors and aid workers who are exposed to narratives of traumatic events). Basic scientists have addressed this issue but have not extensively studied the neurobiology underlying secondary trauma. For example, some studies have reported occurrence of emotional distress in animals that witness another animals suffering either from cage fighting (Hadfield,1983), electric shock (Church, 1959; Kaneyuki et al.,1991; Langford et al., 2006; Jeon et al., 2010; Kim et al.,2010; Atsak et al., 2011) or aversive odor (Zalaquett and Thiessen, 1991). Others have reported increased sensitivity in nociceptive mechanisms in mice produced solely by exposure to their cagemates, but not to strangers, in pain (Langford et al., 2006). Furthermore, Jeon et al. (2010) reported that fear can be acquired vicariously through social observation of others suffering from aversive stimuli and found that mice (observers) developed freezing behavior by observing other mice (demonstrators) receive repetitive foot shocks (Jeon et al., 2010). Similarly, Atsak et al. (2011) have demonstrated that rats display vicarious freezing behavior upon witnessing a cage-mate experiencing footshocks (Atsak et al., 2011). Finally, Kim et al. (2010) have suggested that conditioned fear-induced ultrasonic vocalization calls emitted by one rat evoke fear behavior in a partner rat, provided that the receiver rat had previously experienced fear (Kim et al., 2010). Although all of these studies demonstrated presence of distress in rodents from witnessing traumatic events, depression-like or anxiety-like behaviors, common occurrences of witnessing traumatic events, were not evaluated. A recent study by Warren et al., is the only one to report enhanced anxiety and depression-like behaviors in mice after witnessing other mice undergo traumatic events of social defeat (Warren et al., 2013).
The social defeat model mimics some aspects of societal stress and is postulated to model effects of human aggression, bullying, chronic subordination and humiliation (Bjorkqvist, 2001; Rohde, 2001). Moreover, societal stress is known to contribute to the severity and course of PTSD (Maercker and Horn, 2013). Relevant to this, Warren et al., suggested that the stress of witnessing social defeat induced PTSD-like symptomatology including anxiety and depression-like behavior in mice (Warren et al., 2013). Therefore, animal models to examine the consequences of witnessing traumatic events are critical for a more sophisticated understanding of the response to witnessing trauma.
A related and equally complex matter is the issue of coping with the aftermath of acquiring PTSD. The inverse relationship between PTSD and social support is one of the most consistent associations observed in stress/trauma research (Brewin et al., 2000; Ozer et al., 2003). It is well known that social support in humans is an important component of PTSD coping strategy (Andrews et al., 2003; Clapp and Gayle Beck, 2009). Interestingly, rodents have been postulated to be capable of sharing emotional experiences, and their behaviors have been equated to empathy, which previously was considered a high-level affective process expressed exclusively by humans (Panksepp and Lahvis, 2011). The importance of social support and the effect of enriched environment are well documented. It is reported that social housing reduces detrimental effects of social defeat on heart rate, temperature and prevents social defeat-induced weight loss. (Ruis et al., 1999; de Jong et al., 2005; Lehmann and Herkenham, 2011). Social buffering also has been evaluated and the emotional response of the witness and the demonstrator (animal directly exposed to the trauma) examined (Kiyokawa et al., 2004; Atsak et al., 2011). Kiyokawa and colleagues observed that the presence of the partner rat attenuated stress-induced hyperthermia, as well as behavioral responses and c-Fos expression in response to fearful stimuli in rats (Kiyokawa et al.,2004). Furthermore, in a study where a witness observed a demonstrator experience a series of footshocks, it was reported that witnesses who previously experienced footshocks displayed vicarious freezing behavior upon witnessing a cage-mate experience footshocks. Interestingly, the demonstrator’s behavior was modulated by the behavior of the witness: demonstrators froze more following footshocks if their witness froze (Atsak et al., 2011).
Therefore, assessing whether social buffering might protect against negative effects of secondary trauma in our model, as has been previously reported in animals directly exposed to trauma, is quite significant. Finally, using a modified version of the social defeat witness model (Warren et al., 2013), our study not only replicates the study of Warren et al., but also extends the initial assessment conducted in mice by this group, by examining the emotional response of primary and secondary social trauma and the important relationship between social support and PTSD in rats.
Methods and materials
Animals
Male Sprague–Dawley rats (225–250 g) were used as controls or intruders, and male Long–Evans (LE) retired breeders (400–500 g) served as resident aggressors (Charles River, USA). Rats were housed with a 12-h light/dark cycle in a climate-controlled room with ad libitum food and water. Experiments were conducted in accordance with the National Institutes of Health (NIH) guidelines using protocols approved from the University of Houston Animal Care and Use Committee.
Selection of aggressors
LE rats exhibiting aggressive behaviors were identified as previously published by others and us (Golden et al., 2011; Patki et al., 2013). Appropriate selection of aggressors is critical for successful application of social defeat stress to Sprague–Dawley rats.
Trauma witness model
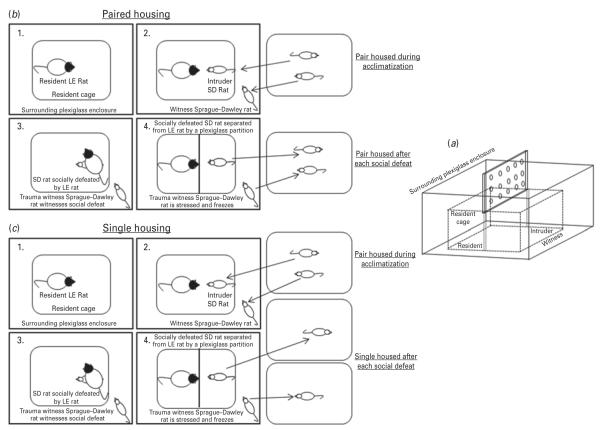
The social defeat model originally developed by Miczek (Miczek, 1979), which involves aggressive encounters between a large LE male rat (resident) and a smaller Sprague–Dawley male rat (intruder) is an accepted rodent model of PTSD (Bhatnagar and Vining, 2003; Bhatnagar et al., 2006; Wood et al., 2010, 2012; Patki et al., 2013). We have modified the resident–intruder model to create a rat trauma witness model (TWM) (Fig. 1a), resembling a recently published mice TWM (Warren et al., 2013). We have modified the protocol of Warren et al., by incorporating assessment of social support using paired or single housing conditions. This is significant as detrimental effects owing to loss of social support and the benefits of continued social interaction, are considered as the core components of PTSD coping strategy (Andrews et al., 2003; Clapp and Gayle Beck, 2009). Briefly, two Sprague–Dawley rats were housed together to allow bonding (social support) during acclimatization period. Later, one Sprague–Dawley rat considered as an intruder was introduced into the cage of the resident LE rat. This resulted in a typical social defeat behavior indicated by the intruder surrendering, when attacked by the resident LE rat. After defeat, a perforated Plexiglas partition was placed for 10 min, between the resident and the intruder to avoid injury to the intruder. The partition allowed visual, auditory, and olfactory interactions (Fig. 1a). The cage mate of the intruder designated as the trauma witnessing (TW) rat was put in an enclosure surrounding the cage and witnessed social defeat of its cage mate. This initiated a freezing response in the TW rat. Two more bouts of social defeat were performed with 5-min separation, in order to reinforce the visual stress in the TW rat. In paired housing (Fig. 1b), after social defeat sessions, intruder and TW rats were housed together until the next day of social defeat protocol.
Fig 1.
A schematic representation of the apparatus (a), paired housing (b) and single housing (c). LE, Long–Evans; SD, Socially defeated.
For single housing condition (Fig. 1c), procedures similar to Fig. 1b were followed except that TW and intruder rats were housed separately after each social defeat exposure.
Several controls were included. For paired housing condition, one Sprague–Dawley rat was placed in a novel cage (SD-CON) and another Sprague–Dawley rat, a cage mate, was put outside (TW-CON) the cage, in the enclosure for 30 min daily for 7 d. The resident LE rat was not present. After each 30 min control session/exposure, they were pair-housed. In experimental condition II, similar controls were included, except that rats were single housed after each 30 min control exposure. An additional control included, placing a Sprague–Dawley rat in the enclosure outside the cage of the resident LE rat, but no social defeat session was conducted. This was done to examine whether the changes in behavior are specific to the social defeat process or due to the mere sighting of a bigger, more aggressive rat that is different from the Sprague–Dawley rat.
Body weight was recorded on days 1, 8 and 14. Daily food and water intake also was measured (Scheme 1). Two days after the conclusion of 7 d social defeat paradigm , depression- and anxiety-like behavior tests were conducted as published by us (Salim et al., 2010; Vollert et al., 2011; Patki et al., 2014b). Cognitive functions including short-term memory (STM) and long-term memory LTM) tests using radial arm water maze (RAWM) paradigm were performed as published (Alhaider et al., 2010a, 2010b; Aleisa et al., 2011). Rats continued to remain in their respective paired or isolated housing environment until completion of the 7-d social defeat protocol. Rats were sacrificed 10 d after the social defeat protocol. Blood was collected and thymuses, adrenal glands and spleen were removed. The brains also were removed; snap frozen and stored at −80 °C for further studies (Scheme 1).
Scheme 1.
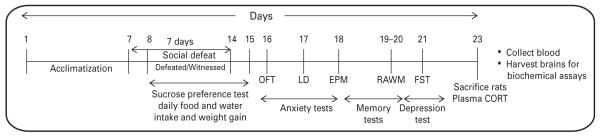
A schematic representation of the experimental regimen. Male Sprague–Dawley rats were acclimatized for one week and then subjected to the social defeat protocol as previously published by us (Patki et al., 2013) with some modifications. Behavior tests including anxiety-like behavior [Open-field (OFT), light–dark (LD), elevated plus maze tests (EPM)], radial arm water maze (RAWM) and forced swim test (FST) were conducted as previously published by us (Patki et al., 2013) and others (Cougle et al., 2009; Beck, 2011; Warren et al., 2013). Sucrose intake was measured over the 24 h period during the entire experimental period (day 8 to day 15, Scheme 1). Rats were sacrificed after the conclusion of all behavior tests and blood was collected for corticosterone (CORT) analysis and brains harvested for future analysis.
Plasma corticosterone
Corticosterone level in plasma was measured using an enzyme immunoassay (EIA)-based kit 9 d after completion of the social defeat protocol (Patki et al., 2013).
Anxiety-like behavior tests
First, open-field test was conducted followed by light–dark (LD) and elevated-plus maze (EPM) tests as previously published by us (Patki et al., 2013).
Open field (OF) test
Rats were placed in the center of the OF (60×40 cm) and left free to explore the arena for 15 min. Movement was quantified using a Opto-Varimex Micro Activity Meter v2.00 system (Optomax, Columbus Instruments, USA) as previously published by us (Salim et al., 2010; Vollert et al., 2011; Patki et al., 2014a). Ambient light intensity was adjusted at 300 lux. Total activity, ambulatory activity and distance covered were determined. The open field test was performed 2 d after completion of the 7-d social defeat protocol.
Light–dark (LD) exploration
Time spent in light is considered a measure of anxiety-like behavior. The LD box consisted of a light and a dark compartment separated by a barrier with a single opening for passage from one compartment to the other. Total time spent in the lit area was recorded (Salim et al., 2010; Vollert et al., 2011). The LD test was performed 3 d after completion of the 7-d social defeat protocol.
Elevated plus-maze (EPM)
A standard rat elevated plus-maze with 43 cm arms extending from a 10 cm central area 90 cm above the floor was used (Med Associates Inc., USA). The rat’s movements were tracked visually. The observer was blinded to treatment. Each session lasted 5 min and was started by placing the rat in the central area facing the open arms of the maze. The amount of time the rat spent in the open arms was determined (Bert et al., 2002; Patki et al., 2014b). The EPM was performed 4 d after completion of the 7-d social defeat protocol.
Depression-like behavior tests
Forced swim stress (FST)
The FST is a characteristic test used for measuring depression-like behavior in rodents. Rats were individually placed for 5 min into a water tank (24 cm in diameter and 50 cm in height) containing water at 25 °C. At some point after being placed in the water the rat assumes an immobile posture, marked by motionless floating and cessation of struggling. The total time spent immobile was recorded (Cougle et al., 2009; Beck, 2011). The FST was performed a week after completion of the social defeat protocol.
Sucrose consumption
The sucrose consumption test has been used extensively to assess stress-induced anhedonia (Barrot et al., 2002; Iniguez et al., 2010; Warren et al., 2013) in rodents. Reduced sucrose consumption is regarded as a sign of depressive behavior (Yu et al., 2011). This test consists of a two-bottle choice paradigm in which rats are given the choice between consuming water and a 1% sucrose solution. The preference for sucrose over water is considered a measure of reward-seeking behavior. Thus, anhedonia and depression-like behavior is revealed by a reduction in sucrose preference (Papp et al., 1991; Willner et al., 1992). The sucrose preference test was carried out throughout the 7-d social defeat protocol and 24 h after the last social defeat session. All groups of rats were subjected to the 2-bottle paradigm during the entire time of the social defeat (day 1 to day 8). The water or 1% sucrose consumed in the pair-housed groups was averaged per rat.
Memory function test
Short-term learning and long-term memory tests in radial arm water maze (RAWM)
The RAWM procedures were done as previously published (Alhaider et al., 2010a, 2010b; Aleisa et al., 2011). The rats were subjected to the first set of six learning trials (trials # 1–6) followed by a 5 min rest period and then a second set of six learning trials (trials # 7–12). STM was assessed by repeating the six trials a third time 30 min after the end of 12th trial. LTM was assessed by returning the rats to their home cages and then repeating the six trials a fourth time 24 h after the end of the 12th trial. The RAWM was performed days 5–6 after completion of the social defeat protocol.
Data analysis
Data are expressed as mean±s.e.m. Significance was determined by two-way analysis of variance (ANOVA) was performed and individual effects as well as interactions between housing and different animal groups were measured and further individual groups were analyzed by applying Tukey’s post-hoc test (GraphPad Software, Inc., USA). A value of p<0.05 was considered significant. The results indicating significance values between different rat groups, and housing effect as well as interaction (F value) between the two effects, are summarized in Table 1.
Table 1.
Two way analysis of variance (ANOVA) was performed and the effect between animal groups and housing was determined. The interaction between groups is provided. The F and P values are also listed
| Measurement | Animal group effect | Housing effect | Interaction |
|---|---|---|---|
| Food intake | F=0.07, p=0.97 (NS) | F=0.01, p=0.99 (NS) | NS |
| Water intake | F=8.08, p<0.001 | F=1.48, p=0.23 (NS) | NS |
| Body weight | F=16.06, p<0.001 | F=6.54, p<0.01 | F=3.03, p<0.05 |
| Thymus weight | F=2.98, p<0.05 | F=1.48, p=0.23 (NS) | NS |
| Adrenals weight | F=6.02, p<0.001 | F=8.93, p<0.01 | NS |
| Spleen weight | F=1.67, p=0.18 (NS) | F=1.36, p=0.24 (NS) | NS |
| Corticosterone | F=42.7, p<0.001 | F=17.7, p<0.001 | F=8.27, p<0.001 |
| Light–dark | F=9.08, p<0.001 | F=2.79, p<0.05 | F=2.88, p<0.05 |
| Elevated plus maze | F=4.07, p<0.01 | F=2.97, p<0.05 | F=2.72, p<0.05 |
| Swim stress | F=20.9, p<0.001 | F=0.03, p=0.85 (NS) | NS |
| Sucrose preference | F=15.1, p<0.001 | F=14.52, p<0.001 | F=3.02, p<0.05 |
| Total activity | F=6.44, p<0.001 | F=11.43, p<0.001 | F=3.36, p<0.05 |
| Ambulatory activity | F=4.88, p<0.05 | F=3.03, p<0.05 | F=2.98, p<0.05 |
| Distance traveled | F=6.36, p<0.01 | F=3.26, p<0.05 | F=2.78, p<0.05 |
| Short-term memory | F=0.33, p=0.80 (NS) | F=0.84, p=0.36 (NS) | NS |
| Long-term memory | F=7.79, p<0.001 | F=0.15, P0.70 (NS) | NS |
NS: Not significant.
Results
General body parameters and tissue weights
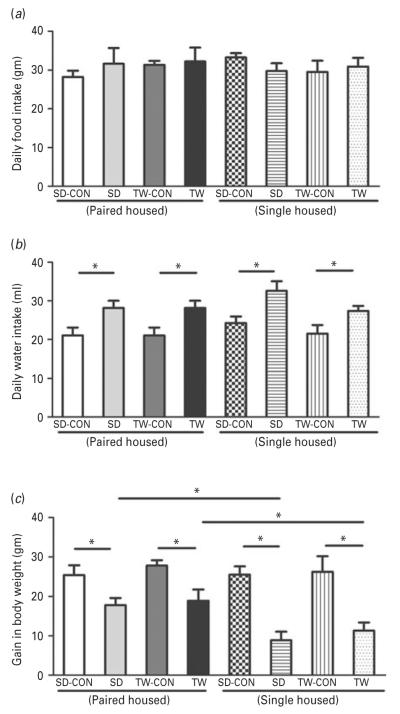
Daily food intake during the 7-d social defeat/witness protocol was not different between control, socially defeated (SD) and TW rats. Additionally, housing SD and TW rats paired or singly after social defeat had no effect on food consumption (Fig. 2a). However, daily water intake increased in SD and TW rats compared to their matched controls (Fig. 2b). Housing condition (singly or pair housing) did not affect daily water intake. SD and TW rats gained less weight during the 7-d social defeat/witness protocol compared to their matched controls (Fig. 2c). Interestingly, rats that were singly housed showed reduced body weight gain compared to rats that were pair housed regardless of whether they were in the defeated or witness group (Fig. 2c).
Fig 2.
Examination of general body parameters: (a) daily food, (b) water intake, and (c) weight gain in rats. Paired housing: Pair housed rats include; CON: Sprague–Dawley rat placed inside a novel cage and not subjected to social defeat, SD: Sprague–Dawley rat subjected to social defeat, TW-CON: Sprague–Dawley rat placed within the enclosure surrounding the novel cage and not subjected to witnessing social defeat, TW: Sprague–Dawley rat who witnessed social defeat. Single housing: Single housed rats otherwise same as pair housed condition. (*) significantly different p<0.05. Bars represent means±s.e.m, n=10 rats/group.
The overall cage behavior of the rats was closely observed by an experimenter blinded to treatment. In experimental condition I, SD rats after undergoing defeat sessions were greeted by a very curious and stressed ‘buddy’ when returned to the home cage and pair housed with the cage mate TW rat (witness of the defeat sessions). Initially, the TW rat was aloof and restless as evident from its repetitive movements in the cage, but then tried to huddle with the SD rat and spent time licking and surrounding its mate for the next hour. These qualitative assessments are representative of comforting and supportive behavior. Facilitation of this behavior by one animal after detecting distress in another animal might be an attempt to comfort the afflicted animal, as has been previously suggested in rats (Knapska et al.,2010) and primates (Preston and de Waal, 2002). In experimental condition II, SD and TW rats were housed separately after being subjected to social defeat or witnessing social defeat, respectively. Isolation after either experience led to a near freezing behavior by SD and TW rats in their home cages. In addition, neither rat made any effort to drink or eat for a long time. Earlier, Atsak et al., reported freezing behavior in rats upon witnessing other rats receive foot shocks (Atsak et al., 2011).
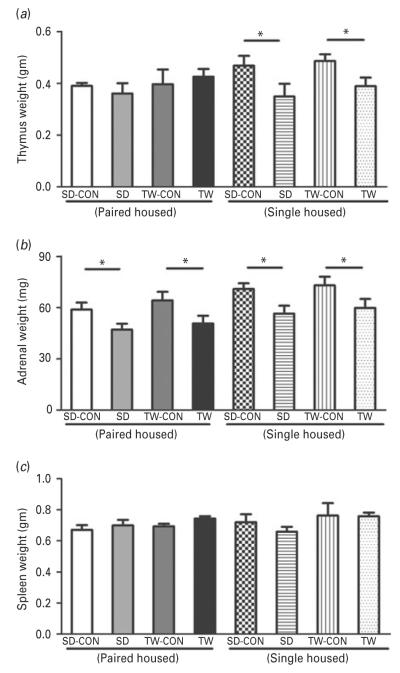
Significant changes in thymus and adrenal gland but not spleen weights were observed in the SD and TW rats compared to their matched controls. The absolute weight of the thymuses of the single housed SD and TW rats were significantly decreased compared to those from their matched controls However, this difference between the SD or TW groups and their controls was eliminated by paired housing (Fig. 3a). The absolute weights of the adrenal glands were significantly reduced in SD or TW rats when compared to their matched controls and the differences were not affected by housing environment (Fig. 3b). No differences were observed in the spleen weight between the SD or TW and the control groups, regardless of housing environment (Fig. 3c).
Fig 3.
Effect of social defeat or witnessing social defeat on absolute tissue weights: (a) thymus, (b) adrenals and (c) spleen (c). Same as in Fig. 2. (*) significantly different at p<0.05. Bars represent means±s.e.m, n=10 rats/group. CON: Sprague–Dawley rat placed inside a novel cage and not subjected to social defeat, SD: Sprague–Dawley rat subjected to social defeat, TW-CON: Sprague–Dawley rat placed within the enclosure surrounding the novel cage and not subjected to witnessing social defeat, TW: Sprague–Dawley rat who witnessed social defeat.
Plasma corticosterone
Plasma corticosterone levels were significantly increased in SD and TW rats in response to the 7-d social defeat/witness protocol when compared to their matched control groups (Fig. 4). Interestingly, single housed rats had significantly exaggerated increase in plasma corticosterone levels in both SD and TW groups as compared to pair housed rats (Fig. 4). We also measured corticosterone levels in the Sprague–Dawley rat which was placed in the enclosure outside the cage of the resident LE rat, but no social defeat session was conducted. The corticosterone levels were similar to that of the control (CON) group and hence further behavioral tests were not performed.
Fig 4.
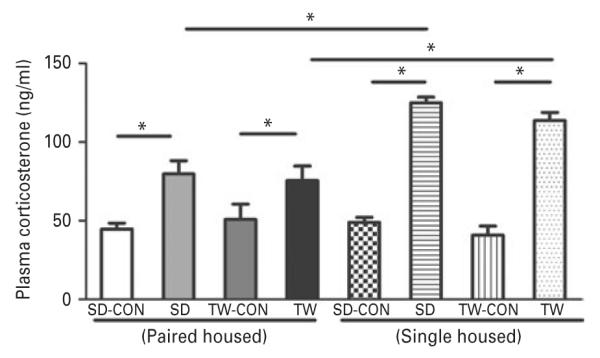
Effect of social defeat and witnessing social defeat on corticosterone levels: Social defeat or witnessing social defeat significantly elevated the plasma corticosterone levels measured using a commercially available ELISA kit. Same as in Fig. 2. (*) significantly different at p<0.05. Bars represent means±s.e.m, n=10 rats/group. CON: Sprague–Dawley rat placed inside a novel cage and not subjected to social defeat, SD: Sprague–Dawley rat subjected to social defeat, TW-CON: Sprague–Dawley rat placed within the enclosure surrounding the novel cage and not subjected to witnessing social defeat, TW: Sprague–Dawley rat who witnessed social defeat.
Analysis of anxiety-like behavior of rats
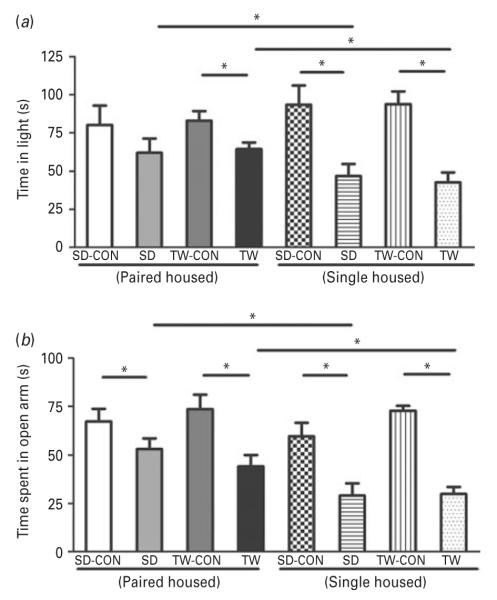
In the LD test, both social defeat and witness groups showed evidence of increased anxiety. Control rats spent more time in the light compartment, when compared to the matched socially defeated/witness groups which were singly housed. Notably, however, although the pair-housed witness group was significantly different from its matching control, the pair-housed SD group although spent reduced time in the lit area but was not significantly different from its control. Additionally, the singly-housed SD and TW rats spent significantly less time in light than pair-housed SD and TW rats (Fig. 5a).
Fig 5.
Examination of anxiety-like behavior using light–dark and elevated plus maze tests: (a) light dark and (b) elevated plus maze tests were conducted in all rats. Same as in Fig. 2. (*) significantly different at p<0.05. Bars represent means±s.e.m, n=10 rats/group. CON: Sprague–Dawley rat placed inside a novel cage and not subjected to social defeat, SD: Sprague–Dawley rat subjected to social defeat, TW-CON: Sprague–Dawley rat placed within the enclosure surrounding the novel cage and not subjected to witnessing social defeat, TW: Sprague–Dawley rat who witnessed social defeat.
In the elevated-plus maze test both the SD and TW groups also exhibited behavior suggesting increased anxiety. The control rats spent significantly more time in the open arms compared to their matching SD/TW groups. Similar to the LD test, both SD and TW singly-housed rats spent significantly less time in the open arms than the pair-housed SD and TW rats (Fig. 5b). Increased time spent in the closed arms during a 5-min session is indicative of high anxiety-like behavior.
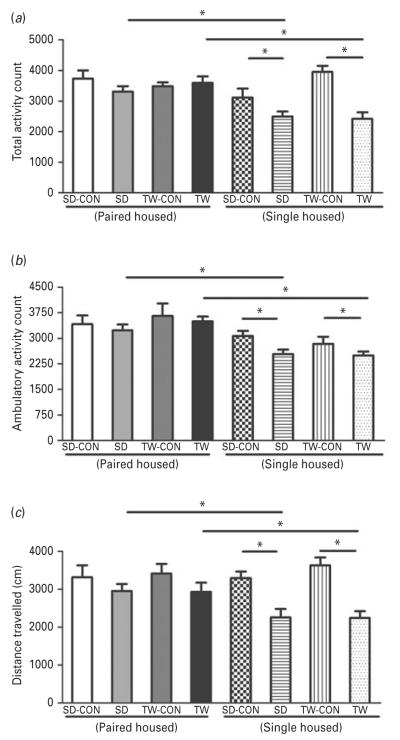
Finally, SD and TW rats had lower total (Fig. 6a) and ambulatory (Fig. 6b) activity counts and covered less distance (Fig. 6c) than their matched controls. Interestingly, the total and ambulatory activity and distance traveled in the singly-housed SD and TW rats were significantly lower than the SD and TW rats that were pair-housed (Fig. 6a, b, c). In summary, in three distinct tests assessing anxiety-like behavior, SD and TW rats exhibited greater anxiety than matched controls and pair-housed exhibited reduced anxiety compared to singly-housed SD and TW rats.
Fig 6.
Examination of anxiety-like behaviors using open-field test: The open-field test determined total (a), ambulatory (b) activities and distance traveled (c) in rats. Same as in Fig. 2. (*) significantly different at p<0.05. Bars represent means±s.e.m, n=10 rats/group. CON: Sprague–Dawley rat placed inside a novel cage and not subjected to social defeat, SD: Sprague–Dawley rat subjected to social defeat, TW-CON: Sprague–Dawley rat placed within the enclosure surrounding the novel cage and not subjected to witnessing social defeat, TW: Sprague–Dawley rat who witnessed social defeat.
Analysis of depression-like behavior of rats
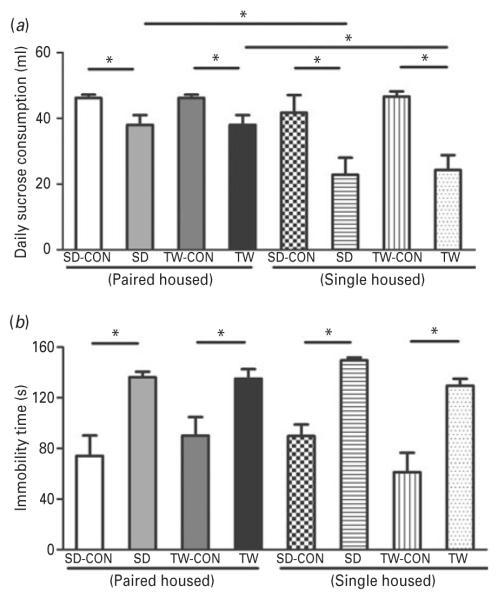
Two assays, the sucrose consumption assay and the forced swim stress test, also indicated increased depression-like behavior in SD and TW rats compared to their matched control groups. The SD and TW rats exhibited a decrease preference for sucrose when compared to their matched control groups (Fig. 7a) 24 h after the last stress, a sign of anhedonia. Additionally, the pair- housed SD and TW rats consumed significantly more sucrose than the SD and TW rats that were singly housed (Fig. 7a), indicating less anhedonia in the pair-housed groups.
Fig 7.
Examination of depression-like behavior using sucrose consumption and forced swim test: (a) sucrose consumption and (b) forced swim tests were conducted in all rats. Same as in Fig. 2. (*) significantly different at p<0.05. Bars represent means±s.e.m, n=10 rats/group. CON: Sprague–Dawley rat placed inside a novel cage and not subjected to social defeat, SD: Sprague–Dawley rat subjected to social defeat, TW-CON: Sprague–Dawley rat placed within the enclosure surrounding the novel cage and not subjected to witnessing social defeat, TW: Sprague–Dawley rat who witnessed social defeat.
The FST was performed 7 d after the last stress session. The SD and TW rats spent more time being immobile compared to their matched control groups (Fig. 7b), a sign of increased depression like behavior. The housing environments did not affect the immobility time (Fig. 7b).
Analysis of memory deficits in rat
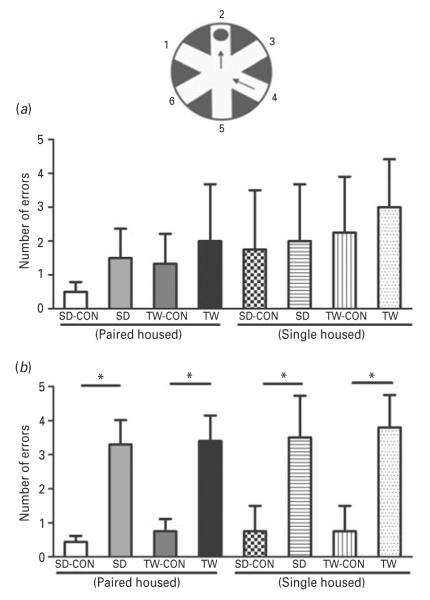
LTM but not STM was significantly impaired in SD and TW rats compared to their matched controls in the RAWM apparatus. Both SD and TW rats made comparable errors when compared to their matched controls in the STM test (Fig. 8a). On the other hand, in the LTM test 24 h after the STM test, SD and TW rats committed significantly higher number of errors when compared to their matched controls (Fig. 8b). The housing environment (single or paired) did not affect the STM or LTM performance (Fig. 8a, b).
Fig 8.
Effect on memory function using radial arm water maze (RAWM) test: (a) short-term memory and (b) long-term memory tests were conducted in all rats. Same as in Fig. 2. (*) significantly different at p<0.05. Bars represent means±s.e.m, n=10 rats/group. CON: Sprague–Dawley rat placed inside a novel cage and not subjected to social defeat, SD: Sprague–Dawley rat subjected to social defeat, TW-CON: Sprague–Dawley rat placed within the enclosure surrounding the novel cage and not subjected to witnessing social defeat, TW: Sprague–Dawley rat who witnessed social defeat.
Discussion
We have previously shown that 7 d of social defeat stress produces significant behavioral and memory impairments as well as biochemical alterations in the brain (Patki et al., 2013). In this study, we demonstrate that witnessing social defeat also causes severe behavioral deficits in rats. It is quite interesting to note that not only SD but also TW rats exhibited increased anxiety-like behavior, displayed increased anhedonia and depression-like behavior, and showed impaired LTM function. Earlier, some studies have reported that witnessing another animals distress from cage fighting (Hadfield, 1983), electric shock (Church, 1959; Kaneyuki et al., 1991; Langford et al., 2006; Jeon et al., 2010; Kim et al., 2010; Atsak et al., 2011) or aversive odor (Zalaquett and Thiessen, 1991), leads to fearful and freezing behaviors. Anxiety-like or depression-like behaviors or learning and memory function tests were not conducted in those studies. Nevertheless, these studies provided evidence that rodents experience stress from witnessing distress in conspecifics. But, physical stress of footshock, pain, exercise, and cold stress is likely to elicit behavioral and physiological responses different from those resulting from social or psychological stressors (Sawchenko et al., 1996; Herman and Cullinan, 1997; Koolhaas et al., 1997; Martinez et al., 1998), such as social defeat, which bears more resemblance to societal stress (Bjorkqvist, 2001; Rohde, 2001).
A recent study using a 10-d social defeat paradigm is the only one to examine some aspects of witnessing trauma in C57/BL6 mice (Warren et al., 2013). In this study, adult male C57BL/6J mice were exposed to either emotional (ES) or physical stress (PS) for 10 min per day for 10 d. Like PS mice, ES mice exhibited a range of depression- and anxiety-like behaviors both 24 h and 1 month after the stress. Increased levels of serum corticosterone, part of the stress response, accompanied these behavioral deficits (Warren et al., 2013). Several features distinguish our study from the previous one. First, in addition to assessing anxiety and depression-like behavior, we also have examined cognitive function in our 7-d social defeat model. Second, we also have examined the effect of social housing after experiencing vs. witnessing social defeat in rats. In agreement with Warren et al., we observed that both SD and TW rats displayed heightened anxiety-like behavior and elevated corticosterone levels, but we observed increased anhedonia in both witness and defeated rats. This is in agreement with previous work where stress induced anhedonia has been reported in rats witnessing electric shocks (Church, 1959), but in contrast to Warren et al., who observed reduced sucrose preference in defeated and witness groups after one month but not 24 h after conclusion of social defeat protocol (Warren et al., 2013). In our study daily sucrose intake was measured and averaged for the entire 7-d social defeat protocol (day 8 to day 15, scheme 1), whereas Warren et al., conducted a one-time measurement, either 24 h or 1 month after 10 d of social defeat. Perhaps, adaptive responses in brain’s reward circuitry that regulate anhedonia (Papp et al., 1991; Berlin et al., 1998; Bolanos et al., 2003; Nocjar et al., 2012) are time based depending on stress duration and intensity. Our data also show that SD and TW rats both do not attain normal rodent weight gain profile (Wood et al., 2010; Patki et al., 2013) even though they consume comparable amount of diet. Interestingly, SD and TW rats consumed more water suggesting stress associated dehydration (Greenleaf, 1992; Bhatnagar et al., 2006). Although, dehydration may be one potential cause of increased water drinking, changes in vasopressin or angiotensin could also contribute to increased drinking behavior (Fitzsimons, 1978; Bhatnagar et al., 2006).
Interestingly, we found that both the defeated and witness rats exhibited impaired LTM but no deficits in STM were noted. Previous evidence suggests that emotion displayed by a conspecific influences learning and memory function of other animals (Bredy and Barad, 2009; Knapska et al., 2010). It is reported that social exposure of witness mice to fear-conditioned familiar mouse impairs acquisition of conditioned fear and facilitates fear extinction (Bredy and Barad, 2009; Knapska et al.,2010). The lack of effect on STM in our study could be the result of increased alertness caused by previous defeat or witnessing of defeat, rendering them less prone to errors. However, as LTM is assessed 24 h later when alertness has lessened, the effects on cognition are revealed. Previous studies have reported that stress induces enhancement in memory consolidation (Roozendaal et al.,1999, 2009; Molteni et al., 2001), by supporting hippocampal long-term potentiation (LTP) and stimulating adult hippocampal neurogenesis (Korz and Frey, 2005).
A unique aspect of our study is the assessment of social housing on the consequences of experiencing vs. witnessing defeat. It is reported that rodents are capable of emotional contagion, social buffering and shared effect or empathy (Church, 1959; Zentall and Levine, 1972; Heyes, 1994; Galef and Giraldeau, 2001; Valsecchi et al., 2002; Kiyokawa et al., 2004; Carlier and Jamon, 2006; Guzman et al., 2009; Knapska et al., 2010; Atsak et al., 2011), suggesting that a rodent can be attuned by the affective state of a social partner (Church, 1959; Rice and Gainer, 1962; Langford et al., 2006). Relevant to this, effect of social support and enriched environment has been examined in the social defeat paradigm (Ruis et al., 1999; de Jong et al., 2005; Lehmann and Herkenham, 2011). However, the effect of social support on witnessing social defeat was not known prior to the current work. As witnessing traumatic events also causes PTSD-like behaviors in rodents and because social support is considered as an integral part of PTSD coping strategy (Andrews et al., 2003; Clapp and Gayle Beck, 2009), we compared the consequences of paired vs. isolated housing in social defeat experiencing and witnessing rats. Our results suggest that anxiety, anhedonia and depression-like behavior are all significantly greater when the rats are isolated in a single cage after undergoing the traumatic experience in both social defeat experiencing and witnessing rats.
Interestingly, the housing environment after the traumatic stress did not impact the deficits in LTM observed in either the defeated or defeat witnessing rat. This is intriguing as impairment in learning with isolation stress is reported (Schrijver et al., 2004) and an interaction between cognitive behaviors and social buffering also is known (Bredy and Barad, 2009; Knapska et al., 2010). However, it must be noted that social buffering studies were conducted using foot shock method, or fear conditioning findings could additionally be affected by pain sensitivity (Imbe et al., 2004). Moreover, it is important to consider that stress can produce diverse effects on cognitive functions depending on stress paradigms employed, intensity and duration applied as well as learning tasks and intervals between stress exposures and learning/memory tests used (Sandi and Pinelo-Nava, 2007). Finally, it is likely that LTM deficits observed in our SD and TW rats get too pronounced, which cannot be restored with social housing perhaps owing to severely compromised neurobiological mechanisms.
Furthermore, stress-induced changes in the weight of adrenal glands, thymus and spleen have been reported (Blanchard et al., 1995; Tamashiro et al., 2004). Stressful conditions reportedly cause enlargement of adrenals and reduction in thymus and spleen (Selye, 1936; Barnett, 1958; Blanchard et al., 1995; McKittrick et al., 1995). Moreover, increased adrenal and decreased thymus weight in SD animals has been previously linked to activation of hypothalamic–pituitary–adrenal (HPA) axis (Berton et al., 1998). In our study we observed, significant decrease in thymus and adrenal gland but not spleen weight in SD and TW rats compared to their matched controls. Previous studies have reported differential effects on these organs depending on the stressor applied (Berton et al., 1998; Kioukia-Fougia et al., 2002; Calvo et al., 2011). Many stressors have been associated with involution of the thymus (Selye, 1936). Interestingly, adrenal glands respond in three distinct stages, at the initial stage, the adrenal glands enlarge in size but as the stress continues, the glands start to shrink; we think our model depicts this sub-chronic phase of stress (Selye, 1936).
Historically, vicarious trauma or secondary traumatic stress identified as compassion fatigue and physical or emotional exhaustion has been studied primarily in trauma counselors, trauma nurses, aid workers and other helping professionals (Figley, 1995). This type of trauma has much broader and complex implications requiring thoughtful analysis. Psychological trauma resulting from witnessing traumatic events often leads to PTSD (McCann and Pearlman, 1990), a severe form of anxiety disorder frequently accompanied by comorbid psychiatric illnesses associated with high rates of disability with a lifetime prevalence rate of 8–10% (Stein et al., 2000; Liebschutz et al., 2007). The prevalence, however, is expected to increase to almost 20% following chronic societal stress (Blake et al., 1992). Therefore, neurobiology of vicarious trauma or secondary traumatic stress must be fully understood. Present study and that of Warren et al., both support occurrence of secondary trauma after witnessing social defeat, a trauma considered to resemble societal stress of bullying and humiliation. In conclusion, we report that social support, similar to antidepressant treatment (Warren et al., 2013), reversed the effects of direct or indirect emotional distress. Whether such support induces the same neuroprotective effects as antidepressant drug treatment, in response to emotional or physical stress would be an interesting area for future PTSD research.
Acknowledgments
Funding for this research was provided by R15MH093918 grant awarded to S.S. Special thanks to Prof Karim Alkadhi and Dr Douglas Eikenburg for many helpful suggestions and comments during the course of this study. Special thanks to Dr Eikenburg for critical reading and editing several versions of this manuscript.
Footnotes
Statement of Interest None.
References
- Aleisa AM, Helal G, Alhaider IA, Alzoubi KH, Srivareerat M, Tran TT, Al-Rejaie SS, Alkadhi KA. Acute nicotine treatment prevents REM sleep deprivation-induced learning and memory impairment in rat. Hippocampus. 2011;21:899–909. doi: 10.1002/hipo.20806. [DOI] [PubMed] [Google Scholar]
- Alhaider IA, Aleisa AM, Tran TT, Alkadhi KA. Caffeine prevents sleep loss-induced deficits in long-term potentiation and related signaling molecules in the dentate gyrus. Eur J Neurosci. 2010a;31:1368–1376. doi: 10.1111/j.1460-9568.2010.07175.x. [DOI] [PubMed] [Google Scholar]
- Alhaider IA, Aleisa AM, Tran TT, Alzoubi KH, Alkadhi KA. Chronic caffeine treatment prevents sleep deprivation-induced impairment of cognitive function and synaptic plasticity. Sleep. 2010b;33:437–444. doi: 10.1093/sleep/33.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed American Psychiatric Association; Washington, DC: 2013. [Google Scholar]
- Andrews B, Brewin CR, Rose S. Gender, social support, and PTSD in victims of violent crime. J Trauma Stress. 2003;16:421–427. doi: 10.1023/A:1024478305142. [DOI] [PubMed] [Google Scholar]
- Atsak P, Orre M, Bakker P, Cerliani L, Roozendaal B, Gazzola V, Moita M, Keysers C. Experience modulates vicarious freezing in rats: a model for empathy. PLoS ONE. 2011;6:e21855. doi: 10.1371/journal.pone.0021855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett SA. Physiological effects of social stress in wild rats. I. The adrenal cortex. J Psychosom Res. 1958;3:1–11. doi: 10.1016/0022-3999(58)90012-6. [DOI] [PubMed] [Google Scholar]
- Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC, Zachariou V, Nestler EJ. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck CT. Secondary traumatic stress in nurses: a systematic review. Arch Psychiatr Nurs. 2011;25:1–10. doi: 10.1016/j.apnu.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Berlin I, Givry-Steiner L, Lecrubier Y, Puech AJ. Measures of anhedonia and hedonic responses to sucrose in depressive and schizophrenic patients in comparison with healthy subjects. Eur Psychiatry. 1998;13:303–309. doi: 10.1016/S0924-9338(98)80048-5. [DOI] [PubMed] [Google Scholar]
- Bert B, Fink H, Huston JP, Voits M. Fischer 344 and wistar rats differ in anxiety and habituation but not in water maze performance. Neurobiol Learn Mem. 2002;78:11–22. doi: 10.1006/nlme.2001.4040. [DOI] [PubMed] [Google Scholar]
- Berton O, Aguerre S, Sarrieau A, Mormede P, Chaouloff F. Differential effects of social stress on central serotonergic activity and emotional reactivity in Lewis and spontaneously hypertensive rats. Neuroscience. 1998;82:147–159. doi: 10.1016/s0306-4522(97)00282-0. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C. Facilitation of hypothalamic-pituitary-adrenal responses to novel stress following repeated social stress using the resident/intruder paradigm. Horm Behav. 2003;43:158–165. doi: 10.1016/s0018-506x(02)00011-9. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C, Iyer V, Kinni V. Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. J Neuroendocrinol. 2006;18:13–24. doi: 10.1111/j.1365-2826.2005.01375.x. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist K. Social defeat as a stressor in humans. Physiol Behav. 2001;73:435–442. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- Blake DD, Cook JD, Keane TM. Post-traumatic stress disorder and coping in veterans who are seeking medical treatment. J Clin Psychol. 1992;48:695–704. doi: 10.1002/1097-4679(199211)48:6<695::aid-jclp2270480602>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20:117–134. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Barrot M, Berton O, Wallace-Black D, Nestler EJ. Methylphenidate treatment during pre- and periadolescence alters behavioral responses to emotional stimuli at adulthood. Biol Psychiatry. 2003;54:1317–1329. doi: 10.1016/s0006-3223(03)00570-5. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Barad M. Social modulation of associative fear learning by pheromone communication. Learn Mem. 2009;16:12–18. doi: 10.1101/lm.1226009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:748–766. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- Calvo N, Cecchi M, Kabbaj M, Watson SJ, Akil H. Differential effects of social defeat in rats with high and low locomotor response to novelty. Neuroscience. 2011;183:81–89. doi: 10.1016/j.neuroscience.2011.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier P, Jamon M. Observational learning in C57BL/6j mice. Behav Brain Res. 2006;174:125–131. doi: 10.1016/j.bbr.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Church RM. Emotional reactions of rats to the pain of others. J Comp Physiol Psychol. 1959;52:132–134. doi: 10.1037/h0043531. [DOI] [PubMed] [Google Scholar]
- Clapp JD, Gayle Beck J. Understanding the relationship between PTSD and social support: the role of negative network orientation. Behav Res Ther. 2009;47:237–244. doi: 10.1016/j.brat.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougle JR, Resnick H, Kilpatrick DG. Does prior exposure to interpersonal violence increase risk of PTSD following subsequent exposure? Behav Res Ther. 2009;47:1012–1017. doi: 10.1016/j.brat.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong JG, van der Vegt BJ, Buwalda B, Koolhaas JM. Social environment determines the long-term effects of social defeat. Physiol Behav. 2005;84:87–95. doi: 10.1016/j.physbeh.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Figley CR. Compassion Fatigue: Coping with secondary traumatic stress disorder in those who treat the traumatized. 1995.
- Fitzsimons JT. Angiotensin, thirst, and sodium appetite: retrospect and prospect. Fed Proc. 1978;37:2669–2675. [PubMed] [Google Scholar]
- Galef BG, Jr., Giraldeau LA. Social influences on foraging in vertebrates: causal mechanisms and adaptive functions. Anim Behav. 2001;61:3–15. doi: 10.1006/anbe.2000.1557. [DOI] [PubMed] [Google Scholar]
- Golden SA, Covington HE, III, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenleaf JE. Problem: thirst, drinking behavior, and involuntary dehydration. Med Sci Sports Exerc. 1992;24:645–656. [PubMed] [Google Scholar]
- Guzman YF, Tronson NC, Guedea A, Huh KH, Gao C, Radulovic J. Social modeling of conditioned fear in mice by non-fearful conspecifics. Behav Brain Res. 2009;201:173–178. doi: 10.1016/j.bbr.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield MG. Dopamine: mesocortical vs. nigrostriatal uptake in isolated fighting mice and controls. Behav Brain Res. 1983;7:269–281. doi: 10.1016/0166-4328(83)90019-0. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Heyes CM. Social learning in animals: categories and mechanisms. Biol Rev Camb Philos Soc. 1994;69:207–231. doi: 10.1111/j.1469-185x.1994.tb01506.x. [DOI] [PubMed] [Google Scholar]
- Imbe H, Murakami S, Okamoto K, Iwai-Liao Y, Senba E. The effects of acute and chronic restraint stress on activation of ERK in the rostral ventromedial medulla and locus coeruleus. Pain. 2004;112:361–371. doi: 10.1016/j.pain.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Iniguez SD, Warren BL, Bolanos-Guzman CA. Short- and long-term functional consequences of fluoxetine exposure during adolescence in male rats. Biol Psychiatry. 2010;67:1057–1066. doi: 10.1016/j.biopsych.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, Rabah D, Kinet JP, Shin HS. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci. 2010;13:482–488. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneyuki H, Yokoo H, Tsuda A, Yoshida M, Mizuki Y, Yamada M, Tanaka M. Psychological stress increases dopamine turnover selectively in mesoprefrontal dopamine neurons of rats: reversal by diazepam. Brain Res. 1991;557:154–161. doi: 10.1016/0006-8993(91)90129-j. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry. 2000;61(Suppl. 5):4–12. discussion 13–14. [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Kim ES, Covey E, Kim JJ. Social transmission of fear in rats: the role of 22-kHz ultrasonic distress vocalization. PLoS ONE. 2010;5:e15077. doi: 10.1371/journal.pone.0015077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioukia-Fougia N, Antoniou K, Bekris S, Liapi C, Christofidis I, Papadopoulou-Daifoti Z. The effects of stress exposure on the hypothalamic-pituitary-adrenal axis, thymus, thyroid hormones and glucose levels. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:823–830. doi: 10.1016/s0278-5846(01)00297-4. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Partner’s stress status influences social buffering effects in rats. Behav Neurosci. 2004;118:798–804. doi: 10.1037/0735-7044.118.4.798. [DOI] [PubMed] [Google Scholar]
- Knapska E, Mikosz M, Werka T, Maren S. Social modulation of learning in rats. Learn Mem. 2010;17:35–42. doi: 10.1101/lm.1670910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A. Social stress in rats and mice. Acta Physiol Scand Suppl. 1997;640:69–72. [PubMed] [Google Scholar]
- Korz V, Frey JU. Bidirectional modulation of hippocampal long-term potentiation under stress and no-stress conditions in basolateral amygdala-lesioned and intact rats. J Neurosci. 2005;25:7393–7400. doi: 10.1523/JNEUROSCI.0910-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford DJ, Crager SE, Shehzad Z, Smith SB, Sotocinal SG, Levenstadt JS, Chanda ML, Levitin DJ, Mogil JS. Social modulation of pain as evidence for empathy in mice. Science. 2006;312:1967–1970. doi: 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- Lehmann ML, Herkenham M. Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. J Neurosci. 2011;31:6159–6173. doi: 10.1523/JNEUROSCI.0577-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebschutz J, Saitz R, Brower V, Keane TM, Lloyd-Travaglini C, Averbuch T, Samet JH. PTSD in urban primary care: high prevalence and low physician recognition. J Gen Intern Med. 2007;22:719–726. doi: 10.1007/s11606-007-0161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maercker A, Horn AB. A socio-interpersonal perspective on PTSD: the case for environments and interpersonal processes. Clin Psychol Psychother. 2013;20:465–481. doi: 10.1002/cpp.1805. [DOI] [PubMed] [Google Scholar]
- Martinez M, Phillips PJ, Herbert J. Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. Eur J Neurosci. 1998;10:20–33. doi: 10.1046/j.1460-9568.1998.00011.x. [DOI] [PubMed] [Google Scholar]
- McCann IL, Pearlman LA. Vicarious traumatization: a framework for understanding the psychological effects of working with victims. J Trauma Stress. 1990;3:131–149. [Google Scholar]
- McKittrick CR, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Serotonin receptor binding in a colony model of chronic social stress. Biol Psychiatry. 1995;37:383–393. doi: 10.1016/0006-3223(94)00152-s. [DOI] [PubMed] [Google Scholar]
- Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl) 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- Molteni R, Lipska BK, Weinberger DR, Racagni G, Riva MA. Developmental and stress-related changes of neurotrophic factor gene expression in an animal model of schizophrenia. Mol Psychiatry. 2001;6:285–292. doi: 10.1038/sj.mp.4000865. [DOI] [PubMed] [Google Scholar]
- Nocjar C, Zhang J, Feng P, Panksepp J. The social defeat animal model of depression shows diminished levels of orexin in mesocortical regions of the dopamine system, and of dynorphin and orexin in the hypothalamus. Neuroscience. 2012;218:138–153. doi: 10.1016/j.neuroscience.2012.05.033. [DOI] [PubMed] [Google Scholar]
- Owens GP, Baker DG, Kasckow J, Ciesla JA, Mohamed S. Review of assessment and treatment of PTSD among elderly American armed forces veterans. Int J Geriatr Psychiatry. 2005;20:1118–1130. doi: 10.1002/gps.1408. [DOI] [PubMed] [Google Scholar]
- Ozer EJ, Best SR, Lipsey TL, Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol Bull. 2003;129:52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Lahvis GP. Rodent empathy and affective neuroscience. Neurosci Biobehav Rev. 2011;35:1864–1875. doi: 10.1016/j.neubiorev.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp M, Willner P, Muscat R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl) 1991;104:255–259. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- Patki G, Solanki N, Allam F, Salim S. Depression and anxiety-like behavior as well as memory impairment is associated with increased oxidative stress in a rat model of social defeat. Brain Res. 2013;1539:73–86. doi: 10.1016/j.brainres.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patki G, Li L, Allam F, Solanki N, Dao AT, Alkadhi K, Salim S. Moderate treadmill exercise rescues anxiety and depression-like behavior as well as memory impairment in a rat model of posttraumatic stress disorder. Physiol Behav. 2014a;130C:47–53. doi: 10.1016/j.physbeh.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patki G, Solanki N, Ansari A, Allam F, Atrooz F, Jannise B, Maturi J, Salim S. Novel mechanistic insights into treadmill exercise based rescue of social defeat-induced anxiety -like behavior and learning-memory impairment in rats. Physiol Behav. 2014b;130:135–144. doi: 10.1016/j.physbeh.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SD, de Waal FB. Empathy: its ultimate and proximate bases. Behav Brain Sci. 2002;25:1–20. doi: 10.1017/s0140525x02000018. discussion 20–71. [DOI] [PubMed] [Google Scholar]
- Reeves RR, Parker JD, Konkle-Parker DJ. War-related mental health problems of today’s veterans: new clinical awareness. J Psychosoc Nurs Ment Health Serv. 2005;43:18–28. doi: 10.3928/02793695-20050701-06. [DOI] [PubMed] [Google Scholar]
- Rice GE, Gainer P. ‘Altruism’ in the albino rat. J Comp Physiol Psychol. 1962;55:123–125. doi: 10.1037/h0042276. [DOI] [PubMed] [Google Scholar]
- Rohde P. The relevance of hierarchies, territories, defeat for depression in humans: hypotheses and clinical predictions. J Affect Disord. 2001;65:221–230. doi: 10.1016/s0165-0327(00)00219-6. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Williams CL, McGaugh JL. Glucocorticoid receptor activation in the rat nucleus of the solitary tract facilitates memory consolidation: involvement of the basolateral amygdala. Eur J Neurosci. 1999;11:1317–1323. doi: 10.1046/j.1460-9568.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McReynolds JR, Van der Zee EA, Lee S, McGaugh JL, McIntyre CK. Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala. J Neurosci. 2009;29:14299–14308. doi: 10.1523/JNEUROSCI.3626-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruis MA, te Brake JH, Buwalda B, De Boer SF, Meerlo P, Korte SM, Blokhuis HJ, Koolhaas JM. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology. 1999;24:285–300. doi: 10.1016/s0306-4530(98)00050-x. [DOI] [PubMed] [Google Scholar]
- Salim S, Sarraj N, Taneja M, Saha K, Tejada-Simon MV, Chugh G. Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behav Brain Res. 2010;208:545–552. doi: 10.1016/j.bbr.2009.12.039. [DOI] [PubMed] [Google Scholar]
- Sandi C, Pinelo-Nava MT. Stress and memory: behavioral effects and neurobiological mechanisms. Neural Plast. 2007;2007:78970. doi: 10.1155/2007/78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko PE, Brown ER, Chan RK, Ericsson A, Li HY, Roland BL, Kovacs KJ. The paraventricular nucleus of the hypothalamus and the functional neuroanatomy of visceromotor responses to stress. Prog Brain Res. 1996;107:201–222. doi: 10.1016/s0079-6123(08)61866-x. [DOI] [PubMed] [Google Scholar]
- Schrijver NC, Pallier PN, Brown VJ, Wurbel H. Double dissociation of social and environmental stimulation on spatial learning and reversal learning in rats. Behav Brain Res. 2004;152:307–314. doi: 10.1016/j.bbr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Selye H. Thymus and Adrenals in the response of the Organism to injuries and Intoxication. Br J Exp Pathol. 1936;17:234–248. [Google Scholar]
- Stein MB. Taking aim at posttraumatic stress disorder: understanding its nature and shooting down myths. Can J Psychiatry. 2002;47:921–922. doi: 10.1177/070674370204701002. [DOI] [PubMed] [Google Scholar]
- Stein MB, Walker JR, Forde DR. Gender differences in susceptibility to posttraumatic stress disorder. Behav Res Ther. 2000;38:619–628. doi: 10.1016/s0005-7967(99)00098-4. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Nguyen MM, Fujikawa T, Xu T, Yun Ma L, Woods SC, Sakai RR. Metabolic and endocrine consequences of social stress in a visible burrow system. Physiol Behav. 2004;80:683–693. doi: 10.1016/j.physbeh.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Valsecchi P, Bosellini I, Sabatini F, Mainardi M, Fiorito G. Behavioral analysis of social effects on the problem-solving ability in the house mouse. Ethology. 2002;108:1115–1134. [Google Scholar]
- Vollert C, Zagaar M, Hovatta I, Taneja M, Vu A, Dao A, Levine A, Alkadhi K, Salim S. Exercise prevents sleep deprivation-associated anxiety-like behavior in rats: potential role of oxidative stress mechanisms. Behav Brain Res. 2011;224:233–240. doi: 10.1016/j.bbr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Warren BL, Vialou VF, Iniguez SD, Alcantara LF, Wright KN, Feng J, Kennedy PJ, Laplant Q, Shen L, Nestler EJ, Bolanos-Guzman CA. Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry. 2013;73:7–14. doi: 10.1016/j.biopsych.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795–1805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, McFadden KV, Grigoriadis D, Bhatnagar S, Valentino RJ. Depressive and cardiovascular disease comorbidity in a rat model of social stress: a putative role for corticotropin-releasing factor. Psychopharmacology (Berl) 2012;222:325–336. doi: 10.1007/s00213-012-2648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Post-traumatic stress disorder. N Engl J Med. 2002;346:108–114. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]
- Yu T, Guo M, Garza J, Rendon S, Sun XL, Zhang W, Lu XY. Cognitive and neural correlates of depression-like behaviour in socially defeated mice: an animal model of depression with cognitive dysfunction. Int J Neuropsychopharmacol. 2011;14:303–317. doi: 10.1017/S1461145710000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalaquett C, Thiessen D. The effects of odors from stressed mice on conspecific behavior. Physiol Behav. 1991;50:221–227. doi: 10.1016/0031-9384(91)90524-r. [DOI] [PubMed] [Google Scholar]
- Zentall TR, Levine JM. Observational learning and social facilitation in the rat. Science. 1972;178:1220–1221. doi: 10.1126/science.178.4066.1220. [DOI] [PubMed] [Google Scholar]