Abstract
Background
Loss of control is a prominent feature of cannabis use disorders (CUD) and involves orchestrated activity from several brain inhibitory control networks.
Objectives
In this study, we determined the associations between inhibitory control network activation and connectivity and CUD severity.
Methods
To that end, we compared cannabis-dependent (N = 44) vs. nondependent (N = 30) users during a Stop Signal Task. First, we compared differences in neural response during response inhibition via general linear model analysis within a priori regions of interest. Second, we examined functional connectivity via psychophysiological interaction (PPI) analysis between the right frontal control network (seed region) and inhibitory control networks.
Results
There was no significant difference in network activation between cannabis-dependent and nondependent users in any of the inhibitory control networks. However, preliminary findings using the PPI analysis showed that during successful response inhibition, cannabis-dependent users had greater connectivity between right frontal control network and substantia nigra/subthalamic nucleus (STN) network compared to nondependent users (small volume correction, FWE-corrected p<0.05). Further, multiple regression analyses on the PPI maps showed modulatory effects of age of onset and quantity of cannabis use in the nondependent users.
Conclusions
Taken together, these findings suggest that functional connectivity between frontal control and substantia nigra/STN networks during response inhibition is sensitive to the effects of CUD severity unlike behavioral task performance and neural activation in inhibitory control networks. Further, modulators of this connectivity, such as onset and quantity of cannabis use, show attenuated effects with progression of CUD.
Keywords: Impulsivity, prefrontal cortex, response inhibition, stop signal task, substantia nigra
Introduction
Inhibitory control or the ability to inhibit a prepotent response is one of the factors within the construct of impulsivity that has been widely reported in substance use disorders (SUD) and is suggested to underlie the loss of behavioral control over drug use (1). Deficits in inhibitory control are apparent in all stages of substance abuse—initiation, transition toward dependence, maintenance, abstinence and relapse (2,3). Indeed, cannabis users often self-report greater impulsivity (4,5). Cognitive testing confirms these self-reports (6-9), although this has not been consistent (10). However, despite unimpaired behavioral task performance, abnormalities in neural activation are often reported (11).
Response inhibition paradigms such as the Stop Signal Task (SST) in functional MRI (fMRI) have allowed the characterization of the neural mechanisms that underlie inhibitory control in SUD (3,12). For instance, Boehler and colleagues suggested a network of brain regions that underlie successful response inhibition, which includes the lateral inferior frontal and medial frontal cortical areas as well as basal ganglia regions, such as the caudate nucleus (13,14). They propose that this network underlies the cancellation of a planned action (i.e. motor response), which occurs via communication between the frontal cortex and the basal ganglia. A recent study (N=1896) extended this model by suggesting a system involving neural activation from 7 multiple control networks during response inhibition (15): bilateral basal ganglia network, right frontal network, bilateral substantia nigra/subthalamic nucleus (STN) network, bilateral orbital network, bilateral pre-supplementary motor area (pre-SMA) and precentral gyrus network, bilateral parietal network and bilateral medial orbital network. Of these, orbital, frontal and pre-SMA networks distinguished substance-abusing individuals, with the right frontal network being associated with severity of substance use.
Studies that examine time-coupled response in brain regions corroborate the existence of an inhibitory control system by demonstrating correlated activity or functional connectivity within these areas during response inhibition. For example, effective connectivity analyses during the SST in healthy controls demonstrated the importance of communication between right inferior frontal gyrus (IFG)–right caudate during motor inhibition and that successful (16) and reactive (vs. proactive) (17,18) inhibition are associated with stronger connectivity in this network. Similarly correlated activation between IFG and STN supports a pathway between these regions during response inhibition, particularly in those with faster stop signal response times (SSRT) (19). These studies suggest that PFC’s role in inhibition is in exerting control over sensorimotor regions reflecting a top-down modulatory mechanism. Taken together, effective connectivity studies using SST suggest that stronger connectivity reflects increased effort in order to inhibit a planned or ongoing action.
With regard to the effects of cannabis on this system, functional connectivity within inhibitory control networks has only been examined using a selective attention task (vs. response inhibition) (20). Specifically, Harding et al. reported that while no difference was found in regional task activation between chronic (>10 years) cannabis users and nonusing controls, the magnitude of functional connectivity between fronto-occipitoparietal regions differed between the groups such that cannabis users exhibited greater connectivity compared to controls (20). Further, they also noted that age of onset of regular cannabis use modulated the strength of this connectivity, which suggests a role of severity in the degree to which functional connectivity is altered as a result of cannabis use. In sum, there is concordant evidence to suggest that (1) the inhibitory control system relies on top-down modulatory control from PFC regions to sensorimotor regions, and (2) that these connections may be encumbered in chronic cannabis use. However, to date, no direct examination of functional connectivity during response inhibition (as opposed to selective attention as a construct of impulsivity (20)) and how CUD severity affects it has been reported in the literature.
In the current study, we examined how severity of CUD affects functional connectivity in inhibitory control networks in terms of response inhibition. To that end, we examined differences in the inhibitory control networks of dependent and nondependent cannabis-using adults. Given the literature suggesting that greater functional connectivity reflects greater effort to inhibit a response (despite unimpaired behavioral task performance) and that chronic use leads to alterations, we expected greater functional connectivity between inhibitory control networks in cannabis-dependent users vs. nondependent cannabis users.
Methods
This study was approved by the University of New Mexico and University of Texas at Dallas institutional review boards.
Participants
A total of 103 cannabis users from the general population in the Albuquerque metro area provided informed consent to participate in this study (some of whom were previously described in an earlier paper (21)). Eligibility for the study required self-reported regular cannabis use of at least 4 uses per week for at least 6 months prior (confirmed by a positive urinalysis for THC metabolites), right-handedness, English as the primary language, absence of current or history of psychosis, traumatic brain injury and MRI contraindications (e.g. pregnancy, nonremovable metallic implants, claustrophobia). The participants were excluded if drugs (other than cannabis) were detected in the urinalysis.
SCID-I (for DSM-IV) Research Version (22) was used to categorize the cannabis users into cannabis-dependent users and cannabis-nondependent users. Detailed demographics for the populations are provided in Table 1.
Table 1.
Demographics for the population (mean±SD).
| Cannabis dependent (N = 44) |
Nondependent (N = 30) |
Two-tailed p value |
|
|---|---|---|---|
| Gender | 34 M, 10 F | 21 M, 9 F | 0.50 |
| Age (years) | 23.7 ± 6.5 | 24.8 ± 8.2 | 0.55 |
| Education (years) | 13.6 ± .3 | 13.4 ± 2.2 | 0.82 |
| Mean age of onset (years)a | 17.3 ± 2.5 | 17.4 ± 2.6 | 0.88 |
| Mean duration of cannabis use (years) |
5.5 ± 5.5 | 7.7 ± 7.5 | 0.17 |
| Mean # cannabis use occasions/dayb |
3.4 ± 2 | 4 ± 4 | 0.53 |
| Total score on MPSc | 4.8 ± 5.2 | 2.3 ± 2.6 | 0.02 |
| Mean # alcohol drinks/month | 7.8 ± 6.6 | 7.1 ± 7.2 | 0.71 |
| Mean # cigarettes/day | 3 ± 5.2 | 5.6 ± 7.5 | 0.14 |
| IMPSSd | 9.4 ± 3.8 | 8.9 ± 4.1 | 0.63 |
| BIS-Briefe | 14.8 ± 3.6 | 13.8 ± 4.1 | 0.35 |
MPS, Marijuana Problem Scale; IMPSS, Impulsivity and Sensation Seeking Scale; BIS, Barratt Impulsiveness Scale.
Dependent (n = 34), nondependent (n = 24).
Dependent (n = 31), nondependent (n = 21).
Dependent (n = 33), nondependent (n = 22).
Dependent (n = 26), nondependent (n = 21).
Dependent (n = 35), nondependent (n = 24).
Prior to the experiment, the participants were asked to abstain from cannabis use for 72 h in order to control for the acute effects of cannabis. To verify abstinence, we used a bogus pipeline similar to previous studies, including ours (23,24), during which a urinalysis (rather than gas chromatography/mass spectrometry [GC/MS]) of THC metabolites was conducted at baseline and also during the experimental session (i.e. following 72-h abstinence). Although insensitive to 72-h abstinence, this method has been shown to increase accuracy of self-report (25). Only those who reported 72-h abstinence were included in the study.
MRI acquisition
MRI images were collected using a 3T Siemens whole-body scanner with a 12-channel head phased array coil combined with body coil transmission. High-resolution structural MRI scans were collected with a multiecho MPRAGE (MEMPR) sequence with the following parameters: TR/TE/TI=2300/ 2.74/900 ms, flip angle=8°, FOV = 256 × 256 mm2, slab thickness = 176 mm, voxel size = 1 × 1 × 1 mm3, number of echos = 4, pixel bandwidth = 650 Hz. fMRI scans were collected using a gradient echo, echoplanar sequence with ramp sampling correction using the intercomissural line (AC-PC) as a reference (TR = 2.0 s, TE = 27 ms, α = 70°, matrix size = 64 × 64, 32 slices, slice thickness = 3.5 mm, voxel size = 3 × 3 × 4 mm3). Additionally, in order to improve the signal dropout and warping in the orbitofrontal cortex (OFC), a tilting acquisition was applied (26). Images were collected in the oblique axial plane and whole-brain coverage was achieved for all participants.
SST task
We utilized a version of the Stop Signal Task previously described by Aron and Poldrack to assess inhibitory control mechanisms in the cannabis users (27). The SST measures the ability to inhibit a prepotent response by presenting Go and Stop signal trials. The task was presented in 3 runs with each run consisting of 96 Go trials and 32 Stop trials per run (128 total). The total task duration was 17m 18 s. Each trial began with a fixation circle followed by an arrow to which participants responded by button press depending on the direction of the arrow (direction is randomized, with each direction appearing 50% of the time). A blank screen appeared after the participant responded (response time) or after 1 s (whichever came first). In the Go trials, participants were given 1 s to make a left or right button press. In the Stop trials, which were indicated by a tone, participants were withheld from button pressing. The tone (i.e. StopSignal) was presented after a random delay and the duration of the blank screen was calculated at 1 s minus the response time plus the random delay. The random delay controlled by the staircase mechanism with 50-ms step increases for events following a StopSuccess trial and 50-ms decreases for events following a StopFail trial.
Data analysis
Behavioral data
t Tests and χ2 tests were used to evaluate differences in age, gender, education and alcohol use between cannabis-dependent and nondependent groups (tested at 2-tailed p<0.05) (Table 1). With regards to SST behavioral task performance, we recorded accuracy on Go and Stop trials and response time on Go trials (GoRT). Stop signal reaction time (SSRT) was also calculated by the method proposed by Logan and Cowan (28).
fMRI Data
Preprocessing
Functional imaging time series data were preprocessed using Statistical Parametric Mapping 8 (SPM8) software (Wellcome Department of Imaging Neuroscience, London, UK) running on Matlab 7.1 (Mathworks, Natick, MA, USA). Before starting the statistical analysis, the first seven volumes of each echoplanar imaging (EPI) run were discarded to allow for T1 saturation effects. Following this, motion correction was implemented using SPM’s realignment module (29). For each subject, sessions with motion greater than 2mm (translation) or 2 degrees (rotation) were excluded from further analysis. Four subjects in our population had high motion in all three runs and were excluded from further analysis. Twenty other subjects had high motion in one or two runs of imaging data: for these subjects, available runs with motion below the selected threshold were included for further analysis. Functional images were corrected for differences in EPI slice acquisition times within each volume using the central slice (16th) as the reference slice. The data were then normalized (30) into the Montreal Neurological Institute (MNI) standard space using the template provided in SPM. The resultant time series was smoothed using a 10-mm (full-width half-maximum) Gaussian kernel.
First-level analyses
As part of the post-processing module, the fMRI signal intensity was scaled by its grand mean (averaged signal intensity over all intracerebral voxels, across all volumes acquired within a run) and the data were highpass filtered (filter frequency 1/128.0 Hz) to reduce low-frequency MR signal drifts. Serial correlations in the fMRI signal were modeled by a first-degree autoregressive AR(1) model (31). For each subject, explanatory variables were created by convolving the onsets of the successful Stop trials (StopSuccess) and StopFail trials by the canonical hemodynamic response function (HRF). These were entered as regressors into the model along with the temporal derivative of the HRF (included to improve model fit). Effect of residual head motion was accounted for by including the motion parameters from the realignment step as nuisance variables in the model. As is current practice, Go trials were not explicitly modeled (32,33) and, therefore, constituted the implicit baseline. Standard voxel-by-voxel general linear modelling (GLM) analysis (details provided in (34)) of the preprocessed functional time series was performed against the resultant model, generating beta maps corresponding to each modeled condition. Next, because we were interested in evaluating neural response during inhibitory control (vs. inhibitory failure), contrast images were generated by comparisons of StopSuccess>baseline. These contrast images were used for further group level (random effects) analysis.
Network activation
All analyses were performed using seven a priori network regions of interest (ROIs) (definitions per Whelan et al. (15)): (1) bilateral basal ganglia network, (2) right frontal network, (3) bilateral substantia nigra/subthalamic nucleus (STN) network, (4) bilateral orbital network, (5) bilateral presupplementary motor area (pre-SMA) and precentral gyrus network, (6) bilateral parietal network and (7) bilateral medial orbital network. These StopSuccess network ROIs were generated from ROIs in MNI space (available in Pickatlas software; (35)). The resulting statistical maps were thresholded at p<0.05, familywise error (FWE) corrected for multiple comparisons using a small volume correction (SVC, (36)). Further, to account for the multiple ROIs (n = 7) being tested, we applied a Bonferroni-corrected threshold of FWE p<0.007. The anatomical localization for all regions of activation was found using the AAL software (37). For visualization and display of significant activation, the z-maps were overlaid on the T1 canonical MNI template provided within SPM. Group level statistical tests were performed using one-sample t tests for within-group analyses and two-sample t tests to check for task performance group differences (cannabis-dependent vs. nondependent).
Correlations between network activation and behavior
For further voxel-by-voxel analysis, we conducted a correlation analysis of the above mentioned contrast images and (i) age of onset of cannabis use, (ii) number of cannabis use occasions per day (i.e. incidents of cannabis use), (iii) total score of marijuana problem scale (MPS) (38), (iv) total Impulsive Sensation Seeking Scale (ImpSS) and (v) Barratt Impulsiveness Scale (BIS-Brief) total score (39). To control for residual variance, demographic variables (namely, age, education, gender, frequency of cigarette smoking and alcohol consumption) were added to the model as covariates of no interest. Correlation analyses were performed for cannabis-dependent and nondependent groups separately.
Network connectivity
We examined functional connectivity between networks via psychophysiological interaction (PPI), which describes how functional connectivity between brain regions is altered as a result of experimental or psychological context (40). To that end, we conducted a PPI analysis to estimate the functional connectivity between the right frontal control network (seed region, supplementary materials Figure S1a, available online) and other StopSuccess network ROIs for the StopSuccess>baseline contrast using the gPPI toolbox (41). We selected this region as our seed because of the primary role of the right inferior frontal gyrus (IFG) in all aspects of inhibitory control (42,43).
Prior to PPI analysis, we performed a one-sample t test of the StopSuccess>baseline activation maps for all subjects. The presence of robust activation within this network ROI (supplemental materials Figure S1b, available online) for this contrast supported our selection of this network ROI as a seed region for further PPI analysis. Following this, for each subject, the first eigenvariate of fMRI signal was extracted from within this ROI, temporally filtered and corrected for nonneuronal components of the design (such as session-specific mean and estimated motion parameters). This time series was deconvolved by the canonical HRF to estimate the time series for the neural activity, which served as the physiological vector for further analysis. The psychological vector was obtained by encoding the onset of the StopSuccess trials by delta functions. The physiological and psychological vectors were multiplied to obtain the corresponding PPI vector. Similar physiological, psychological and PPI vectors were also obtained for the StopFail condition, which was included in the model to improve the model fit. This PPI regressor for StopFail is also required to obtain the correct estimate for the StopSuccess connectivity, since we are estimating connectivity changes relative to the (implicit) baseline (41). The single subject PPI GLM now comprised the PPI vectors (for StopSuccess and StopFail), the corresponding psychological vectors and physiological vector, each of which was convolved by the canonical hemodynamic response function (HRF) prior to GLM analysis. Similar to the standard single subject level GLM analysis, motion parameters were also included in the model as nuisance variables. Following the analysis, contrast images (PPI maps) were generated for each subject.
These PPI maps entered into group level analysis wherein one-sample and two-sample t tests (dependent vs. nondependent groups) were carried out in a manner similar to the standard activation analysis. As with the activation analysis, an ROI approach localized to the StopSuccess network ROIs was used to examine group differences. All statistical maps were thresholded at Bonferroni-corrected FWE p<0.007 (with SVC). In addition, to inform future investigations, we also report preliminary findings that passed a multiple comparison corrected level of FWE p<0.05 (with SVC) but was at trend level of the Bonferroni-corrected threshold.
Correlations between network connectivity and behavior
Similar to the correlation between network activation and behavior, we conducted a correlation analysis between the PPI parameter estimates and (i) age of onset of cannabis use, (ii) number of cannabis use occasions per day, and total scores from (iii) MPS, (iv) ImpSS and (v) BIS-Brief with similar covariates of no interest. Correlation analyses on the PPI connectivity maps were performed for the dependent and nondependent groups separately. Additionally, we also performed between-group tests to determine differences in correlation between dependent and nondependent users. For this, we first performed a Fisher r-to-z transformation. In cases where a group did not show voxels with significant correlation, we approximated the correlation coefficient for the group using the average signal intensity (connectivity value in the PPI map) within the entire structural ROI. Two-sample t tests were then performed to determine significant differences in correlation coefficients between groups.
Results
The mean self-reported length of abstinence for all of the participants was 3.38 days (range 2–7 days). The two groups did not differ significantly on length of reported abstinence (p=0.98) (dependent=3.34 ±0.68; nondependent=3.44±1.0). Of the 103 cannabis users, 17 were excluded from analyses due to missing behavioral data and 4 due to high motion (>2 mm) in imaging data. Of the remaining 82, 8 did not have complete SCID information and were excluded from the two-sample t-tests so that the population size for the final analysis was 44 dependent vs. 30 nondependent cannabis users.
SST behavioral performance
The percentage of StopSuccess trials was not significantly different between groups (2-tailed, p=0.33) (dependent=68.5±11.5, nondependent = 65.7±12.5). The groups also did not differ in their GoRT (dependent: 516.5±79.4 ms, nondependent: 506.1±72.5 ms) or SSRT (dependent: 185.1±30 ms, nondependent: 198.4±38.2 ms).
Effects of response inhibition on network activation
We found no significant between-group (dependent vs. nondependent cannabis group) difference within any network for StopSuccess>baseline.
Correlations between network activation and behavior
In the dependent group, there were no significant correlations between StopSuccess>baseline network activation and any of the behavioral scores. In the nondependent group, there were significant and trend-level correlations in the negative direction between network activation and age of onset, ImpSS, and BIS-Brief (peaks listed in Table 2). Specifically, the earlier the age of onset of regular use, the greater the activation in the medial orbital network (SVC, FWE p=0.027); the lower the ImpSS score, the greater the activation in the substantia nigra/STN (SVC, FWE p=0.003), basal ganglia (SVC, FWE p=0.009) and right frontal networks (SVC, FWE p=0.041); the lower the BIS-Brief total scores, the greater the activation in the basal ganglia (SVC, FWE p=0.006), right frontal (SVC, FWE p=0.034), precentral gyrus (SVC, FWE p=0.045), substantia nigra/STN (SVC, FWE p=0.045) and orbital (SVC, FWE p=0.052) networks.
Table 2.
Correlation between network activation and behavioral measures in nondependent cannabis users during StopSuccess > baseline.
| MNI coordinates |
|||||
|---|---|---|---|---|---|
| Behavioral measure (n) Network: localization of peak |
x | y | z | Total # voxels kE |
Peak T value Tmax |
| Negative correlation: Age of onset (n = 24) | |||||
| Medial orbital network: L medial orbital gyrus | −8 | 26 | −14 | 27 | 4.18** |
| Negative correlation: ImpSS (n = 21) | |||||
| Basal ganglia network: L putamen | −30 | −12 | 6 | 1481 | 6.29** |
| R frontal network: R anterior cingulum | 12 | 34 | 28 | 359 | 5.2** |
| Substantia nigra/STN network | −14 | −14 | 2 | 102 | 5.16* |
| 14 | −10 | 2 | 72 | 4.39** | |
| Negative correlation: BIS-Brief (n = 24) | |||||
| Basal ganglia network: L putamen | −28 | −10 | 2 | 1202 | 6.02* |
| 22 | −6 | −8 | 1200 | 5.11** | |
| R frontal network: R anterior cingulum | 6 | 22 | 28 | 246 | 5.01** |
| Precentral gyrus network: R precentral gyrus | 54 | 2 | 44 | 400 | 4.75** |
| Substantia nigra/STN network | −14 | −14 | 2 | 17 | 3.37** |
| 14 | −10 | −4 | 31 | 3.33** | |
| Orbital network: L middle orbital gyrus | −34 | 50 | −6 | 156 | 4.38** |
L, left; R, right.
Significant at Bonferroni-corrected threshold of FWE p < 0.007 (with SVC).
Significant at trend level.
Effects of response inhibition on network connectivity
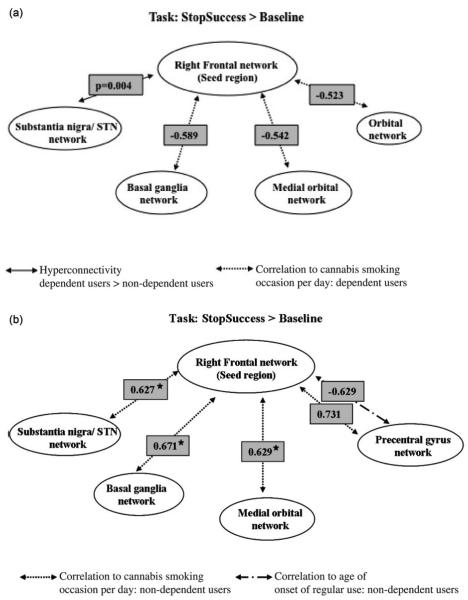
Compared to the nondependent group, the cannabis-dependent users had increased connectivity strength during StopSuccess>baseline between the right frontal seed region and the substantia nigra/STN network (SVC, FWE-corrected p=0.054, peak at [6, –16, –8], t = 2.9, k= 10 voxels) (illustrated in the path diagram in Figure 1a). The reverse comparison (nondependent>dependent groups) did not show a difference in connectivity.
Figure 1.
Connectivity in inhibitory control networks during response inhibition (StopSuccess>baseline). Path diagrams illustrating the connections between the right frontal control network and StopSuccess network ROIs for cannabis-dependent and nondependent groups. The presence of between-group differences (p) and correlations (r) with cannabis use measures are indicated for (a) dependent and (b) nondependent groups. Networks that did not show either a significant group difference or correlation with behavior are not represented in these figures. *Significant at Bonferroni corrected threshold of FWE p<0.007 (with SVC).
Correlations between network connectivity and behavior
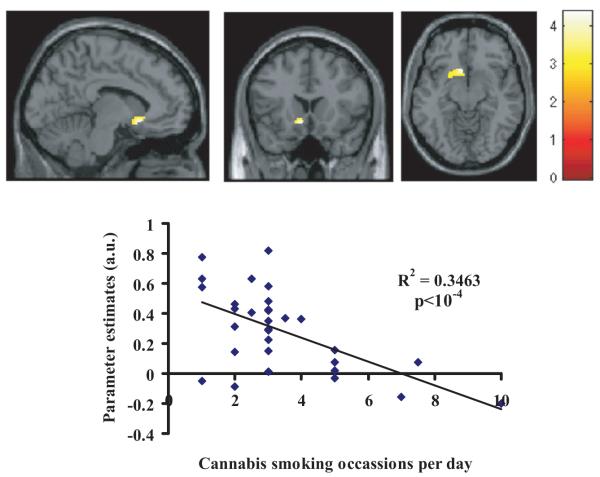
In the dependent group, the correlation analyses of the PPI maps with number of cannabis use occasions per day showed a significant negative correlation with connectivity between the seed region and medial orbital (SVC, FWE-corrected p=0.014), orbital (SVC, FWE-corrected p=0.035) and basal ganglia networks (SVC, FWE-corrected p=0.043) (Table 3, Figure 2). No significant correlations to connectivity strength for the StopSuccess>baseline were found with age of onset, MPS, ImpSS or BIS-Brief in the dependent group.
Table 3.
Correlation between network connectivity and behavioral measure per group for StopSuccess> baseline (small volume corrected FWE p<0.05).
| MNI coordinates |
|||||
|---|---|---|---|---|---|
| Behavioral measure (n) Network: localization of peak |
x | y | z | Total # voxels kE |
Peak T value Tmax |
| Cannabis dependent | |||||
| Negative correlation: number of cannabis use occasions per day (n = 31) | |||||
| Basal ganglia network: L caudate | −8 | 18 | −10 | 132 | 4.39** |
| Orbital network: L superior orbital gyrus | −18 | 46 | −12 | 88 | 4.26** |
| Medial orbital network: L medial orbital gyrus | −12 | 40 | −8 | 163 | 4.23** |
| Nondependent | |||||
| Positive correlation: age of onset (n = 24) | |||||
| Precentral gyrus network: R precentral gyrus | 56 | 2 | 50 | 179 | 5.23** |
| Negative correlation: age of onset (n = 24) | |||||
| Precentral gyrus network: R precentral gyrus | 28 | −18 | 78 | 231 | 5.24** |
| −38 | −4 | 48 | 98 | 5.05** | |
| Positive correlation: number of cannabis use occasions per day (n = 21) | |||||
| Basal ganglia network: L caudate | −6 | 10 | 0 | 414 | 8.2* |
| 12 | 8 | 10 | 588 | 7.9* | |
| Medial orbital network: R medial orbital gyrus | 12 | 36 | −14 | 79 | 6.44* |
| Substantia nigra/STN network | −8 | −16 | −10 | 67 | 5.46* |
| 12 | −16 | −10 | 94 | 5.35* | |
| Precentral gyrus network: R precentral gyrus | 34 | −24 | 72 | 310 | 5.42** |
| −30 | −24 | 76 | 301 | 5.14** | |
L, left; R, right.
Significant at Bonferroni-corrected threshold of FWE p< 0.007 (with SVC).
Significant at trend level.
Figure 2.
Negative correlations in dependent cannabis users. Number of cannabis smoking occasions (per day) negatively correlated with connectivity between right frontal control network and basal ganglia network. Activations have been overlaid onto the single subject template. The color bar indicates t values. Orientation: right=right.
In the nondependent group, the correlation analyses of the connectivity maps with age of onset showed negative trend-level correlations with the right frontal control network–precentral gyrus (SVC, FWE-corrected p=0.026) as well as right frontal control network–substantia nigra/STN network (SVC, FWE-corrected p=0.054) (Table 3, Figures 1b, 2). Additionally, number of cannabis use occasions per day showed a positive significant correlation with connectivity between the seed region and basal ganglia (SVC, FWE-corrected p=0.001), medial orbital (SVC, FWE-corrected p=0.002) and substantia nigra (SVC, FWE-corrected p=0.003). Regions of positive correlation were also observed within the precentral gyrus network, which was at trend-level significance (SVC, FWE-corrected p=0.036).
The remaining scores did not show any significant correlations to connectivity strength for the StopSuccess>baseline contrast.
Comparisons of the correlations between dependent and nondependent cannabis users
Nondependent users had significantly greater correlations of number of cannabis use occasions per day to connectivity between frontal and medial orbital (p<0.0001), basal ganglia (p<0.0001), precentral gyrus (p=0.0004) and STN (p=0.014) networks compared to cannabis-dependent users. No significant between-group difference was found between number of cannabis use occasions per day and frontal–orbital network connectivity.
Cannabis-dependent users had significantly greater correlations between age of onset and frontal–precentral gyrus networks (p=0.0003). No significant between-group difference was found between age of onset and connectivity in the rest of the networks.
Post hoc analyses
Given the behavioral similarities between the two groups (i.e. age of onset, quantity of use, Table 1), we combined dependent and nondependent users to test for effects of use measures on fMRI parameter estimates, with dependence, age, gender and education as covariates of no interest to determine how functional connectivity is related to behavioral symptoms of cannabis use. We correlated participants’ PPI data to behavioral measures (MPS, BIS-Brief, ImpSS, cannabis use occasions per day and age of onset). The results from these correlation analyses showed a significantly positive correlation between number of cannabis use occasions per day and the right frontal seed region–precentral (SVC, FWE p=0.001) connectivity. Number of cannabis use occasions per day was also positively correlated to seed region–substantia nigra connectivity at trend level of significance (SVC, FWE p=0.046). Age of onset was significantly correlated in the positive direction between the seed region and precentral gyrus network (SVC, FWE p=0.034) and in the negative direction between the right frontal control network–substantia nigra/STN network (SVC, FWE p=0.02).
Although not the primary focus of this report, we also analyzed activation during inhibitory failure (i.e. StopFail4baseline). All analyses were performed using a priori network regions of interest (ROIs) (definitions per Whelan et al. (15)): bilateral frontal network, bilateral substantia nigra/STN network, bilateral basal ganglia network, bilateral parietal network, bilateral posterior cingulate (PC)/medial orbital network and bilateral orbital network. Two-sample t-tests between nondependent and dependent cannabis users did not reveal significant group differences in connectivity during failed inhibitory response (StopFail vs. baseline).
Discussion
In this study, we examined whether severity of cannabis use influences network function within the inhibitory control system. Correlation analyses indicated that there is a relationship between network connectivity and cannabis use measures, such as frequency of use and age of onset. When determining the clinical relevance of this finding by comparing SCID-dependent vs. non-SCID-dependent cannabis users, our findings showed that despite similar behavioral performance and an absence of difference in regional network activation during response inhibition, there was greater internetwork functional connectivity between the right frontal control network and substantia nigra/STN network in dependent compared to nondependent cannabis users. Previous studies have suggested that greater neural activation during inhibitory control is due to greater effort required in order to perform cognitive processes (11). For example, Tapert et al. (12) showed that abstinent adolescent cannabis users exhibited greater neural response in PFC during response inhibition trials in the Go/NoGo task. The authors interpreted this pattern of greater neural response in terms of greater effort required in order to successfully inhibit a prepotent response. Similarly, greater connectivity between inhibitory control networks have also been postulated to reflect greater magnitude of effort in order to successfully inhibit (16,17). Although not an investigation of response inhibition, a similar pattern of enhanced connectivity between PFC-sensorimotor regions was reported in chronic cannabis users (vs. controls) during a selective attention task as a measure of impulsivity (20). In general, the existing literature supports the view that enhanced connectivity is partly a compensatory mechanism for regional deficits (e.g. PFC, sensorimotor regions). From a top-down modulation perspective, this increase in connectivity strength between networks may reflect greater required modulatory control of PFC network on sensorimotor networks (20). Taken together, our findings suggest that those with greater severity of CUD have greater alterations in the inhibitory control system such that greater coupling (increased signaling) between PFC and substantia nigra/STN is required in order to successfully inhibit a prepotent motor response.
It is established that the PFC is an important region for control processes. Within the PFC, the right inferior frontal gyrus (IFG; within our seed region) in particular is implicated in inhibitory control (15,42). Cai et al. characterized the critical role of the IFG in target detection and rule retrieval during response inhibition (43). Additionally, the STN and substantia nigra play important roles in the integration of information related to the execution of motor responses. Aron and Poldrack (28) specifically noted the key role of the STN during response inhibition in SST in the suppression of a motor response. As important, the substantia nigra is proposed to integrate emotional, cognitive and motor inputs for the execution of appropriate behavioral responses (44). Thus, in the context of response inhibition, the substantia nigra/STN network, under the modulatory control of the PFC, integrates the functions of other networks for the execution of motor response. Indeed, projections from PFC to STN to substantia nigra have been referred to as the hyperdirect pathway (vs. indirect or indirect pathway via striatum), particularly in faster SST response inhibition (19). Our findings suggest that in cannabis-dependent users, modulation of this network by the PFC is particularly hyperconnected during response inhibition.
In addition to severity of CUD, we also found neurodevelopmental effects on inhibitory system function in the nondependent users. We found that age of onset was negatively correlated with both network activation and connectivity. This suggests that the earlier the onset of regular cannabis use, the greater the alterations in inhibitory networks. This is concordant with studies highlighting the greater impact to cognitive function in those with early cannabis use onset (45,46). Indeed, brain alterations due to early cannabis use have been widely reported, such as reduced white matter integrity (via diffusion tensor imaging), which have been associated with increased impulsivity (47). It is possible that greater functional connectivity associated with early onset of cannabis use reflects this decrement in white matter integrity, such that greater signal between networks is compulsory in order to be effective. In our study, it is interesting to note that this correlation was found only in the nondependent users, but not in the dependent users. This suggests that nondependent users may be more heterogeneous in their pattern of inhibitory control network functioning than cannabis-dependent users.
Interestingly, Harding et al. (20) reported the opposite effect wherein a positive association between connectivity strength and age of onset and lifetime exposure was found. Harding et al. suggested that those with earlier ages of onset are not able to dynamically recruit greater connectivity to compensate for the regional dysfunction. In the presence of equal task performance and similar regional activation, a positive correlation suggests that early-onset users may rely on other mechanisms (besides greater connectivity) to successfully selectively attend. Because our findings did not find the same pattern, we speculate that our reported early-onset effects are specific to response inhibitory processes. This may be due to differences on the processes’ demands on regional activation. More important, the direction of connectivity differences may be network-specific (i.e. fronto-substantia nigra/STN vs. fronto-occipitoparietal).
We also found heavier cannabis use to have differential effects on connectivity in dependent and nondependent cannabis users. Whereas the expected pattern of positive correlation was found in the nondependent users, the opposite effect was found in cannabis-dependent users in fronto-basal ganglia and fronto-orbital networks. Because both basal ganglia and orbital networks also pay a role in reward salience, it is possible that an adaptation or shift in these networks occurs due to changes in reward salience. Notably, there were no significant correlations between trait impulsivity (i.e. BIS-Brief, IMPSS) and connectivity during SST (network activation correlations were only found in the nondependent group and not the dependent group).
Limitations
These findings should be taken in light of some considerations. Notably, the mean SSRT in our participants was faster (e.g. 191.3±34 ms) relative to previous reports, which was due to the higher percentage of StopSuccess rate, and interpretation of these findings must take this into account. However, because the two groups did not differ in their SSRT or rate of StopSuccess, potential implications on our findings of differences in network activation and connectivity between groups are minimal. Nevertheless, this relatively faster SSRT supports the hyperdirect pathway model of SST response inhibition (IFC-STN).
It should also be noted that we did not have an abstinence requirement for tobacco use prior to the scan or assess nicotine dependence. However, because participants were scanned approximately 1 h after their arrival for their scanning appointment (for various preparations), any tobacco user would have at least 1-h abstinence. Also, we did collect information on tobacco use and have included these measures to control for cigarette smoking in the analyses. Thus, any effects of tobacco use on the current findings are minimal.
Interpretation of these findings should also consider that we used self-reported abstinence and a bogus pipeline for the required cannabis abstinence. While quantification of THC is the most ideal means of validating compliance, bogus pipelines as noted above have been demonstrated to be effective in ensuring abstinence (25). Our required abstinence of 3 days also coincides with peak withdrawal symptoms for marijuana (typically cannabis withdrawal symptoms peak between days 2 and 6). Thus, it is possible that our findings may be explained by differences in withdrawal symptoms between dependent vs. nondependent. However, upon performing a 2-sample t test on the total score on the Marijuana Withdrawal Checklist (MWC), we found no significant between-group difference in MWC scores (p=0.84; dependent group [mean (SD)]: 6.13 (5.5), nondependent group [mean (SD)]: 6.38 (4.32)). Therefore, withdrawal symptoms do not appear to have impacted the differences seen between the two groups.
Lastly, we acknowledge that our selected networks were based on work derived from a large adolescent sample (15). While there may be concern that these inhibitory networks may be different from the mature brain, the adult literature largely concurs on the engagement of these brain regions via both general linear modeling and independent component analysis during inhibitory control with the SST task (48-50).
Conclusion
Our preliminary findings suggest that inhibitory control network connectivity is sensitive to severity of CUD. Further, modulatory effects of onset and extent of use on network connectivity are present only in nondependent users. This suggests a distinct difference in mechanisms that underlie response inhibition in CUD, which reflects a regression toward a more homogeneous pattern of network connectivity with the progression of CUD. Future studies should determine whether these changes are premorbid to or the consequence of chronic cannabis use.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- 2.Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- 3.de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Day AM, Metrik J, Spillane NS, Kahler CW. Working memory and impulsivity predict marijuana-related problems among frequent users. Drug Alcohol Depend. 2013;131:171–174. doi: 10.1016/j.drugalcdep.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simons JS, Carey KB. Risk and vulnerability for marijuana use problems: the role of affect dysregulation. Psychol Addict Behav. 2002;16:72–75. [PubMed] [Google Scholar]
- 6.Moreno M, Estevez AF, Zaldivar F, Montes JM, Gutierrez-Ferre VE, Esteban L, Sanchez-Santed F, Flores P. Impulsivity differences in recreational cannabis users and binge drinkers in a university population. Drug Alcohol Depend. 2012;124:355–362. doi: 10.1016/j.drugalcdep.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Metrik J, Kahler CW, Reynolds B, McGeary JE, Monti PM, Haney M, de Wit H, Rohsenow DJ. Balanced placebo design with marijuana: pharmacological and expectancy effects on impulsivity and risk taking. Psychopharmacology (Berl) 2012;223:489–499. doi: 10.1007/s00213-012-2740-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramaekers JG, Kauert G, van Ruitenbeek P, Theunissen EL, Schneider E, Moeller MR. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31:2296–2303. doi: 10.1038/sj.npp.1301068. [DOI] [PubMed] [Google Scholar]
- 9.Prince van Leeuwen A, Creemers HE, Verhulst FC, Ormel J, Huizink AC. Are adolescents gambling with cannabis use? A longitudinal study of impulsivity measures and adolescent substance use: the TRAILS study. J Stud Alcohol Drugs. 2011;72:70–78. doi: 10.15288/jsad.2011.72.70. [DOI] [PubMed] [Google Scholar]
- 10.Griffith-Lendering MF, Huijbregts SC, Vollebergh WA, Swaab H. Motivational and cognitive inhibitory control in recreational cannabis users. J Clin Exp Neuropsychol. 2012;34:688–697. doi: 10.1080/13803395.2012.668874. [DOI] [PubMed] [Google Scholar]
- 11.Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009;34:2450–2458. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boehler CN, Appelbaum LG, Krebs RM, Hopf JM, Woldorff MG. Pinning down response inhibition in the brain—conjunction analyses of the Stop-signal task. Neuroimage. 2010;52:1621–1632. doi: 10.1016/j.neuroimage.2010.04.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boehler CN, Appelbaum LG, Krebs RM, Chen LC, Woldorff MG. The role of stimulus salience and attentional capture across the neural hierarchy in a stop-signal task. PLoS One. 2011;6:e26386. doi: 10.1371/journal.pone.0026386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whelan R, Conrod PJ, Poline JB, Lourdusamy A, Banaschewski T, Barker GJ, Bellgrove MA, et al. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat Neurosci. 2012;15:920–925. doi: 10.1038/nn.3092. [DOI] [PubMed] [Google Scholar]
- 16.Jahfari S, Waldorp L, van den Wildenberg WP, Scholte HS, Ridderinkhof KR, Forstmann BU. Effective connectivity reveals important roles for both the hyperdirect (fronto-subthalamic) and the indirect (fronto-striatal-pallidal) fronto-basal ganglia pathways during response inhibition. J Neurosci. 2011;31:6891–6899. doi: 10.1523/JNEUROSCI.5253-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jahfari S, Verbruggen F, Frank MJ, Waldorp LJ, Colzato L, Ridderinkhof KR, Forstmann BU. How preparation changes the need for top-down control of the basal ganglia when inhibiting premature actions. J Neurosci. 2012;32:10870–10878. doi: 10.1523/JNEUROSCI.0902-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry. 2011;69:e55–e68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harding IH, Solowij N, Harrison BJ, Takagi M, Lorenzetti V, Lubman DI, Seal ML, et al. Functional connectivity in brain networks underlying cognitive control in chronic cannabis users. Neuropsychopharmacology. 2012;37:1923–1933. doi: 10.1038/npp.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Marijuana craving in the brain. Proc Natl Acad Sci U S A. 2009;106:13016–13021. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.First M, Spitzer R, Gibbon M, et al. User’s guide for the structured clinical interview for DSM-IV axis I disorders – SCID. American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- 23.Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Marijuana craving in the brain. Proc Natl Acad Sci U S A. 2009;106:13016–13021. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filbey FM, Schacht J, Myers U, Chavez R, Hutchison K. Individual and additive effects of the CNR1 and FAAH genes on brain response to marijuana cues. Neuropsychopharmacology. 2010;35:967–975. doi: 10.1038/npp.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roese NJ, Jamieson DW. Twenty years of bogus pipeline research: a critical review and meta-analysis. Psychol Bull. 1993;114:363–375. [Google Scholar]
- 26.Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage. 2003;19:430–441. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- 27.Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- 28.Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friston K, Ashburner J, Frith CD, Poline JP, Heather JD, Frackowiak RS. Spatial registration and normalization of images. Hum Brain Mapp. 1995;2:165–189. [Google Scholar]
- 30.Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Purdon PL, Weisskoff RM. Effect of temporal autocorrelation due to physiological noise and stimulus paradigm on voxel-level false-positive rates in fMRI. Hum Brain Mapp. 1998;6:239–249. doi: 10.1002/(SICI)1097-0193(1998)6:4<239::AID-HBM4>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes ME, Fulham WR, Johnston PJ, Michie PT. Stop-signal response inhibition in schizophrenia: behavioural, event-related potential and functional neuroimaging data. Biol Psychol. 2012;89:220–231. doi: 10.1016/j.biopsycho.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Padmala S, Pessoa L. Moment-to-moment fluctuations in fMRI amplitude and interregion coupling are predictive of inhibitory performance. Cogn Affect Behav Neurosci. 2010;10:279–297. doi: 10.3758/CABN.10.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friston K, Ashburner J, J KS, E NT, D PW. Statistical parametric mapping: the analysis of funtional brain images. Academic Press; London: 2007. [Google Scholar]
- 35.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 36.Worsley K, Marrett S, Neelin P, Vandal A, Friston K, Evans A. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 37.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 38.Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. J Consult Clin Psychol. 2000;68:898–908. [PubMed] [Google Scholar]
- 39.Steinberg L, Sharp C, Stanford MS, Tharp AT. New tricks for an old measure: the development of the Barratt Impulsiveness Scale-Brief (BIS-Brief) Psychol Assess. 2013;25:216–226. doi: 10.1037/a0030550. [DOI] [PubMed] [Google Scholar]
- 40.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 41.McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61:1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 43.Cai W, Leung HC. Rule-guided executive control of response inhibition: functional topography of the inferior frontal cortex. PLoS One. 2011;6:e20840. doi: 10.1371/journal.pone.0020840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haber SN, Calzavara R. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res Bull. 2009;78:69–74. doi: 10.1016/j.brainresbull.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, Pesa N, et al. Reflection impulsivity in adolescent cannabis users: a comparison with alcohol-using and non-substance-using adolescents. Psychopharmacology (Berl) 2012;219:575–586. doi: 10.1007/s00213-011-2486-y. [DOI] [PubMed] [Google Scholar]
- 46.Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, Lubman DI, Yucel M. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology (Berl) 2011;216:131–144. doi: 10.1007/s00213-011-2203-x. [DOI] [PubMed] [Google Scholar]
- 47.Gruber SA, Silveri MM, Dahlgren MK, Yurgelun-Todd D. Why so impulsive? White matter alterations are associated with impulsivity in chronic marijuana smokers. Exp Clin Psychopharmacol. 2011;19:231–242. doi: 10.1037/a0023034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Congdon E, Mumford JA, Cohen JR, Galvan A, Aron AR, Xue G, Miller E, Poldrack RA. Engagement of large-scale networks is related to individual differences in inhibitory control. Neuroimage. 2010;53:653–663. doi: 10.1016/j.neuroimage.2010.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiecki TV, Frank MJ. A computational model of inhibitory control in frontal cortex and basal ganglia. Psychol Rev. 2013;120:329–355. doi: 10.1037/a0031542. [DOI] [PubMed] [Google Scholar]
- 50.Zandbelt BB, Bloemendaal M, Neggers SF, Kahn RS, Vink M. Expectations and violations: delineating the neural network of proactive inhibitory control. Hum Brain Mapp. 2013;34:2015–2024. doi: 10.1002/hbm.22047. [DOI] [PMC free article] [PubMed] [Google Scholar]