Abstract
Much remains unknown regarding the relationship between anxiety, worry, sustained attention, and frontal function. Here, we addressed this using a sustained attention task adapted for functional magnetic resonance imaging. Participants responded to presentation of simple stimuli, withholding responses to an infrequent “No Go” stimulus. Dorsolateral prefrontal cortex (DLPFC) activity to “Go” trials, and dorsal anterior cingulate (dACC) activity to “No Go” trials were associated with faster error-free performance; consistent with DLPFC and dACC facilitating proactive and reactive control, respectively. Trait anxiety was linked to reduced recruitment of these regions, slower error-free performance, and decreased frontal-thalamo-striatal connectivity. This indicates an association between trait anxiety and impoverished frontal control of attention, even when external distractors are absent. In task blocks where commission errors were made, greater DLPFC-precuneus and DLPFC-posterior cingulate connectivity were associated with both trait anxiety and worry, indicative of increased off-task thought. Notably, unlike trait anxiety, worry was not linked to reduced frontal-striatal-thalamo connectivity, impoverished frontal recruitment, or slowed responding during blocks without commission errors, contrary to accounts proposing a direct causal link between worry and impoverished attentional control. This leads us to propose a new model of the relationship between anxiety, worry and frontal engagement in attentional control versus off-task thought.
Keywords: anxiety, frontal function, off-task thought, sustained attention, worry
Introduction
Worry and difficulty concentrating are symptomatic of Generalized Anxiety Disorder and also characteristic of the subclinical anxiety experienced by many individuals. The extent to which these symptoms reflect disruption to common versus unique underlying mechanisms remains unclear. An important question is whether heightened worrying is secondary to deficits in the frontal cortical control of attention or an independent feature of anxiety, understudied in terms of its brain mechanisms. Here, we addressed this by investigating the relationship between anxiety, worry, the frontal control of sustained attention, and off-task thought.
Concentration problems reported by anxious individuals suggest deficits in sustained attention. These problems can lead to impaired occupational function. Despite this, the link between anxiety and impoverished sustained attention has not been extensively investigated. Instead, many models of anxiety have focused on selective attention; in particular, the capture of visual attention by threat-related stimuli. In an experimental setting, this entails slowing in the processing of task-relevant neutral stimuli or stimulus dimensions when threat-related information is present. This slowing is increased in high trait anxious and clinically anxious individuals (Macleod and Mathews 1988; Richards and Millwood 1989). These attentional capture effects were initially proposed to result from amplification of a pre-attentive threat signal (Mathews and Mackintosh 1998; Mogg and Bradley 1998). However, recent evidence suggests that anxiety may involve a deficit in augmenting attentional control to support processing of task-relevant stimuli when competition from salient distractors is present (Bishop et al. 2004; Bishop 2009). Specifically, anxious individuals show impoverished recruitment of frontal cortical regions implicated in attentional control. This is observed regardless of whether attentional competition is created by threat-related stimuli (Bishop et al. 2004, 2007) or response conflict in the absence of emotional stimuli (Bishop 2009). Frontal regions implicated in the disrupted control of selective attention in anxiety have included the dorsolateral prefrontal cortex (DLPFC) and anterior cingulate cortex (ACC), (Bishop et al. 2004, 2007; Bishop 2009).
A number of theoretical models have attempted to specify the roles of DLPFC and ACC in attentional control (e.g., Botvinick et al. 1999). Recently, several models have focused on a distinction between “proactive” and “reactive” control (Braver et al. 2007; Aron 2011). The latter is held to involve on the spot recruitment of attentional mechanisms to inhibit prepotent responses and overcome processing interference caused by task-irrelevant distractors. It has been proposed that DLPFC and ACC are jointly involved in reactive control (Braver et al. 2007), with different accounts varying as to the precise role attributed to each region (Carter et al. 2000; Nachev 2006; Botvinick 2007; Morishima et al. 2010). DLPFC has also been implicated in proactive control, in particular the active maintenance and updating of current goals, and use of this to adjust behavior (Braver and Barch 2006; Braver et al. 2007).
Proactive and reactive control are both held to facilitate performance of sustained attention tasks (Aron 2011). These tasks are typically simple and fairly monotonous, requiring a key press response to certain stimuli and inhibition of response to others. External distractors are absent (i.e., there is no concurrent competition between target and distractor stimuli). For example, in the Sustained Attention to Response Task (SART; Robertson et al. 1997), participants are asked to respond quickly but accurately to a series of “Go” stimuli while withholding responses to occasionally presented “No Go” stimuli. The infrequent nature of the No Go stimuli necessitates the proactive control of sustained attention across Go trials in order to maintain task goals (i.e., to avoid responding so fast that it is difficult to brake when a No Go stimulus is encountered). Computational theories suggest that such proactive, ongoing control of sustained attention may be achieved by recurrent activation within lateral prefrontal regions or frontal-thalamo loops. This recurrent activation is held to enable active maintenance of current goal states. Updating of these states is achieved by input from striatal regions (Beiser and Houk 1998; Frank et al. 2001). It has been suggested that such proactive control of attention is complimented by phasic recruitment of DLPFC and ACC to facilitate response inhibition (i.e., “reactive control”) upon the actual occurrence of No Go trials (Fassbender et al. 2004). The concentration problems reported by anxious individuals suggest a deficit in proactive control processes supporting the maintenance of sustained attention. This might either occur alone or be accompanied by disruption to reactive attentional control processes.
Worry is a common feature of many anxiety disorders (ADs) and included in the diagnostic criteria of Generalized Anxiety Disorder (Olatunji et al. 2011). It has been defined as “a chain of thoughts and images” that are “negatively affect laden and relatively uncontrollable” (Borkovec et al. 1983). Standardized self-report measures have been developed to measure the extent of worry shown by different individuals (Meyer et al. 1990). The relationship between worry and impoverished attentional control has been subject to discussion within the cognitive literature. One common proposal is that there are limited executive resources. It is suggested that the extent to which these resources are allocated to maintaining attention control versus engaged in worry-related cognition is altered in anxious individuals. Such a shift in balance might either reflect worry occupying processing resources that would otherwise be allocated to attentional control (Eysenck 1979) or impoverished attentional control resulting in more processing resources being allocated to worry.
An alternative theoretical stance is suggested by the mind-wandering literature. Mind-wandering has been defined as involving a shift of attention away from external stimuli (Barron et al. 2011) and representations associated with ongoing activities and goals (McVay and Kane 2010) to occupation with spontaneously generated task-unrelated thoughts (TUTs). This literature has been fairly isolated from that on worry despite obvious theoretical connections. According to McVay and Kane (2010), mind-wandering is the product of both impoverished control of attention and increased interference from automatically elicited personal concern-related thoughts. Worry-related cognition is arguably a subclass of personal concern-related thought processes. Hence, a logical extension to McVay and Kane's position is that worry might entail the spontaneous generation and occupation with negative self-referent thoughts, but that this might be orthogonal to individual differences in attentional control. Where individuals fall on this latter control dimension might impact the ease with which worry-related thoughts can be dismissed at will, when concentration needs to be maintained on task performance. This could in turn explain the observation that worry is perceived as more uncontrollable and disruptive to everyday life when it occurs in the context of anxiety (Olatunji et al. 2011), due to its increased co-occurrence with impoverished attentional control.
The SART task has previously been used to investigate the role of DLPFC in the maintenance of task-focused attention (Fassbender et al. 2004). However, it has also been used to investigate individual differences in mind-wandering (Christoff et al. 2009). In this latter work, TUTs were reported to be associated with co-recruitment of “Default Mode” (precuneus, posterior cingulate) regions and “Executive” regions including DLPFC. If anxiety is not only associated with impoverished proactive control of sustained attention, but also with increased spontaneous off-task thought (i.e., worry), a key challenge will be to dissociate engagement of DLPFC in each of these processes. In the current study, we aimed to accomplish this by investigating changes in DLPFC activation and connectivity as a function of both within-subject performance on an fMRI-optimized version of the SART task, and between-subject measures of individual differences.
Our hypotheses were as follows. First, that trait anxiety would be associated with impoverished proactive maintenance of sustained attention, with this being reflected by reduced DLPFC activation and reduced connectivity between DLPFC and thamalo-striatal regions across SART “Go” trials. A related but more open question was whether trait anxiety-related deficits in attentional control would extend to include impoverished reactive control and reduced recruitment of DLPFC and ACC on No Go trials. Our second hypothesis was that trait anxiety would independently be linked to increased DLPFC engagement in off-task thought, with this being accompanied by increased DLPFC-Default Mode network connectivity. Given previous TUT findings (Christoff et al. 2009), it was anticipated that this would be observed to a greatest extent in blocks containing commission errors. Our third hypothesis was that individual differences in worry, as assessed by the Penn State Worry Questionnaire (PSWQ, Meyer et al. 1990) would be positively correlated with extent of DLPFC engagement in off-task thought, and DLPFC–default mode connectivity, but would be orthogonal to individual differences in frontal engagement in attentional control. Together, these hypotheses reflected our underlying proposal that there are 2 separate dimensions of function that vary across participants that predispose individuals to 1) spontaneous negative cognitions (worry) and 2) impoverished attentional control, with the joint function of the position an individual has on each of these dimensions being linked to trait vulnerability to anxiety.
Materials and Methods
Participants
Participants were recruited from volunteer databases. Individuals with a history of psychiatric care, neurological disease, or head injury were excluded, as were individuals on psychotropic medication or contra-indicated for MRI participation. The study was approved by the UC Berkeley Committee for the Protection of Human Subjects and carried out in compliance with their guidelines. All participants gave written informed consent. Twenty-three right-handed adults aged 18–26 years with normal or corrected vision took part. Five were excluded due to problems staying awake and performing the task (omission errors > 10%). The final sample comprised 15 females and 3 males, mean age = 21.5 years, SD = 2.3 years.
Procedure
During the main session, participants performed an fMRI optimized version of the SART (Robertson et al. 1997; Fassbender et al. 2004) within a Siemens 3T Tim Trio MRI scanner. Prior to entering the scanner, they completed the Spielberger State Trait Anxiety Inventory (STAI; Spielberger et al. 1983) and the PSWQ followed by a practice run of the SART task. The STAI trait subscale is a widely used measure of trait vulnerability to anxiety. Scores on this subscale are elevated in individuals meeting criteria for ADs across subtypes (Bieling et al. 1998; Chambers et al. 2004) and predict future AD diagnosis (Plehn and Peterson 2002). Some of the STAI trait subscale items assess general propensity to negative affect, others are related to cognitive style, and others to physiological arousal. The PSWQ was developed to more specifically assess the construct of worry. It shows high internal consistency and good test re-test reliability (Meyer et al. 1990). The PSWQ is the predominant measure of worry used in both healthy and clinical populations.
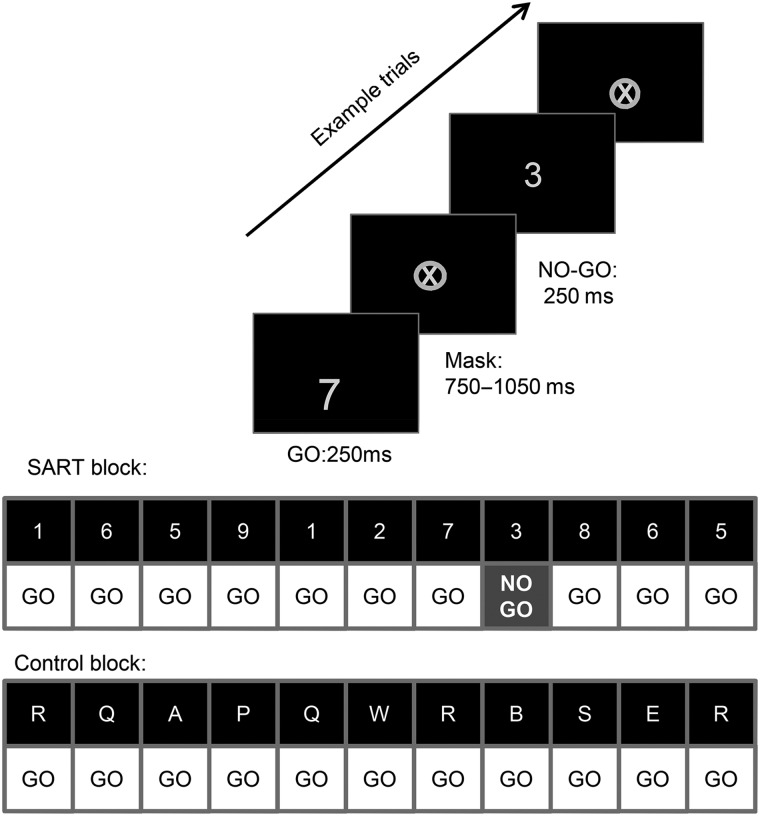
Within the scanner, localizer and structural scans were obtained and participants performed 6 6-min “runs” of the SART task while fMRI data were acquired. Visual stimuli were back-projected onto a screen behind the bore of the magnet and viewed via an angled mirror above the participant’s head. In SART task blocks, participants were presented with single digits in a randomized order. Participants were instructed to press a button with their right index finger for every digit that appeared (“Go” trials) with the exception of the digit “3” (No Go trials). Each trial comprised digit presentation (250 ms) followed by mask presentation (750–1050 ms, mean = 900 ms), Figure 1. Digit size varied from trial to trial, subtending 1.4° × 1° to 3° × 2.1° visual angle. Masks were light grey ovals containing a cross, subtending 3° × 2.9°. No Go stimuli occurred infrequently, 2 or 3 times per block of 28 digits. There were 30 SART task blocks. These were alternated with control task blocks in which stimuli were letters and there were only “Go” trials (i.e., participants pressed for every letter). These provided a control for the low level perceptual and motor demands of the SART task while placing less demand on sustained attention and response inhibition. A 2400-ms cue indicating block type was presented at the beginning of each block.
Figure 1.
The sustained attention to response task (SART), adapted for functional magnetic resonance imaging (fMRI). In SART blocks, participants responded by key press to all digits except the digit “3”, these “No Go” trials were infrequent (2 or 3 per block of 28). In Control blocks, participants responded by key press to all letters; in these blocks there were only “Go” trials.
Participants were invited to return for a follow-up session in which they performed a vigilance task with thought probes to directly assess individual differences in mind-wandering. This was completed on a computer in a behavioral testing room. Participants were shown a series of crosses at fixation, with a presentation time per cross of 100 ms and interstimulus interval of 1400 ms. Each cross subtended 9° × 9° visual angle, this decreased to 5° × 5° on 10% of trials. Participants responded to the smaller crosses by speeded button press. Every 48–80 trials, participants were presented with a “thought probe” asking “What were you thinking just now?” and instructed to press “A” if their thoughts had been task-related, “Z” if task-unrelated. Prior to task performance, participants were given definitions and examples of task-related and task-unrelated thoughts, and completed a practice set of trials with one thought probe. A score was calculated for each participant indicating the percentage of probes for which they reported task-unrelated thoughts. This score was used as an index of mind-wandering, that is, attention being “switched,” or at least partially reallocated, to off-task thoughts (McVay and Kane 2010). Fourteen participants (78%) completed this additional session, on average 3 months after the SART session.
fMRI Data Acquisition
Blood oxygenation level–dependent (BOLD) contrast functional images were acquired with echo-planar T2*-weighted (EPI) imaging using a 12-channel head coil. Each image volume comprised 36 3 mm thick slices (interslice gap: 0.75 mm; inplane resolution: 3.5 × 3.5 mm; flip angle: 78°; echo time: 30 ms; bandwidth: 2232 Hz; repetition time: 2210 ms). Slice acquisition was descending and axial oblique, angled to avoid the eyeballs and to maximize whole brain coverage. Data were acquired in 6 6-min scanning runs. The first 5 volumes of each run were discarded to allow for T1 equilibration effects. T1-weighted structural images were acquired at a resolution of 1 × 1 × 1 mm.
fMRI Imaging Analysis
This was conducted using SPM5 (Wellcome Trust Centre for Neuroimaging, London, UK). Images were converted from DICOM to NIfTI format. Following this, diagnostics were run on the time-series for each imaging run. Volumes characterized by unusually high changes in volume to volume signal intensity (assessed using the mean squared signal change across the brain) were marked as bad volumes and replaced by interpolation of the volumes on either side. (This approach is closely related to those adopted by Power et al. 2012 and Carp 2011). Regressors were created to model out the (replaced) volumes in the final analysis. These bad volumes tend to correspond to those with notable movement spikes (in line with findings by Power et al. 2012). Subsequent to this initial data cleaning step, image realignment (correcting for head movement) was conducted, followed by slice time correction, and normalization of each participant's EPI data to the MNI template (Tzourio-Mazoyer et al. 2002). Images were resampled into this space with 2 mm isotropic voxels and smoothed with a Gaussian kernel of 8 mm FWHM.
General linear modeling of the BOLD data was conducted within SPM 5. Regions of interest (ROIs) were created for right DLPFC and dorsal ACC (dACC) using 10 mm radius spheres centered on peak activations from a meta-analysis of cognitive control tasks (Duncan and Owen 2000) as described previously (Bishop et al. 2008). The MNI coordinates for the center of these spherical ROIs were as follows: right DLPFC 42, 24, 24; dACC 0, 30, 21.
Two linear models were created. In both models, in addition to regressors of interest, realignment (movement) regressors, regressors indicating volumes where “bad scans” had been replaced by interpolation of neighboring volumes, and mean time-series extracted from white matter and outside of brain masks were included to reduce task-unrelated variance (noise). In the first model, SART Go, SART No Go, and Control Go trials were modeled by delta functions convolved with the canonical hemodynamic response function to form regressors. Building on prior work (e.g., Fassbender et al. 2004), we worked on the premise that engagement of frontal regions in response inhibition (reactive control) would best be indexed by activity on No Go trials, while engagement of frontal regions in the maintenance of sustained attention (proactive control) would best be indexed by activity across Go trials in SART blocks relative to activity across Go trials in Control blocks.
A limitation of this first model was the possibility that DLPFC activity across Go trials might also reflect engagement in off-task self-referential thought processes. This might obscure any link between trait anxiety and reduced DLPFC engagement in the proactive maintenance of sustained attention. Hence, in our second model, SART blocks were broken down, on a subject-by-subject basis, according to whether or not errors of commission were made on No Go trials within each block. (One participant did not achieve error-free performance in any block and hence was excluded from analyses using this model. The remaining participants made errors during a minimum of 4 of the 30 SART blocks, M = 18, SD = 7.37. The number of blocks containing commission errors did not vary significantly as a function of trait anxiety, P > 0.1). This gave 5 regressors of interest: Control Gos, SART Error-Free (EF) block Gos, SART EF block No Gos, SART Error-Made (EM) block Gos, SART EM block No Gos. Given the performance decrement often associated with off-task thought (Christoff et al. 2009), we reasoned that DLPFC activity to Go trials in blocks where commission errors were made on No Go trials was likely to have a greater component attributable to off-task thought than DLPFC activity to Go trials in blocks where no commission errors were made (note the blocks are split by errors on No Go trials). In contrast, we anticipated that DLPFC recruitment to Go trials within EF blocks would provide a less contaminated measure of DLPFC engagement in the proactive control of sustained attention.
These models were used to conduct both ROI-based activation analyses and also ROI-seeded functional connectivity analyses. The latter allowed us to examine the regions with which DLPFC was co-activated, across Go trials, as a function of SART block type (i.e., EF vs EM). This enabled us to investigate whether the patterns of DLPFC functional connectivity observed were consistent with differential engagement in proactive control of sustained attention versus off-task thought.
The ROI activation analyses were conducted using the MARSBAR toolbox (Brett et al. 2002). We extracted the mean activity associated with each task regressor from right DLPFC and dACC ROIs, on a subject-by-subject basis. These activation indices were then submitted to analyses of covariance with STAI trait anxiety scores or PSWQ worry scores entered as the covariate of interest. Greenhouse–Geisser estimates were used to correct for violations of sphericity.
Psychophysiological interaction (PPI) analyses (Friston 1997; Gitelman et al. 2003) were used to examine changes in functional connectivity between regions of interest as a function of SART block type (EF or EM) and participant anxiety or worry levels. Using right DLPFC as a “seed” region, we investigated changes in the regions with which right DLPFC was co-activated across Go trials by block type (SART EM or SART EF vs. Control) and STAI trait or PSWQ scores. We restricted these analyses to consideration of a number of a priori ROIs. Specifically, the MNI Automated Anatomical Labeling (AAL) template was used to define ROIs for “Default Mode” regions implicated in self-referential processing (bilateral precuneus and posterior cingulate) as well as regions held, together with DLPFC, to support the proactive control of sustained attention and maintenance of task goals (bilateral thalamus, caudate). Subject-wise estimates of mean functional connectivity between right DLPFC and these 8 target ROIs were calculated for 1) Go trials in SART EF blocks versus Control blocks and 2) Go trials in SART EM blocks versus Control blocks. This enabled us to test specific hypotheses about DLPFC co-activation with these target regions as a function of SART performance while avoiding problems of multiple comparisons and effect size inflation associated with selection of peak voxels from voxelwise connectivity maps (Vul et al. 2009).
No Go trials were modeled with a single regressor. As an additional check, the analyses described above were repeated with No Go trials broken down into “correct” and “error” trials. Effectively, in a block labeled as containing errors, this distinguished which No Go trials were performed correctly and which were not. These additional analyses did not result in any notable differences in the results obtained.
Results
DLPFC and dACC Activity During Sustained Attention Task Performance
Dorsal ACC activity was greater for SART No Go trials than for SART Go or Control Go trials, t(17) = 5.02, P < 0.001, t(17) = 5.48, P < 0.001, respectively, and did not differ between SART Go and Control Go trials (P > 0.1). This is consistent with the proposed role of dACC in reactive control—specifically in response inhibition (Braver et al. 2007). Right DLPFC activity was also greatest for SART No Go trials, but this did not differ significantly from activity for SART Go trials, t(17) = 1.70, P > 0.1. Further, both SART No Go and Go trials showed greater right DLPFC activity than Control Go trials, t(17) = 2.15, P < 0.05, t(17) = 2.41, P < 0.05, respectively. This is in line with right DLPFC playing a role in the proactive control of sustained attention across SART Go trials, as well as in reactive control as observed in response to SART No Go stimuli. Additional evidence for the respective importance of right DLPFC to proactive control and dACC to reactive control comes from the finding that right DLPFC activity across SART Go trials (vs. Control Go trials) and dACC activity to SART No Go trials both significantly predicted faster speed of error-free performance, r(18) = −0.48, P = 0.05, r(18) = −0.84, P < 0.001, respectively, but this was not the case for the reverse contrasts (DLPFC to No Gos, dACC to SART Go vs. Control Go trials, Ps > 0.1).
Trait Anxiety and Frontal Recruitment to No Go Trials
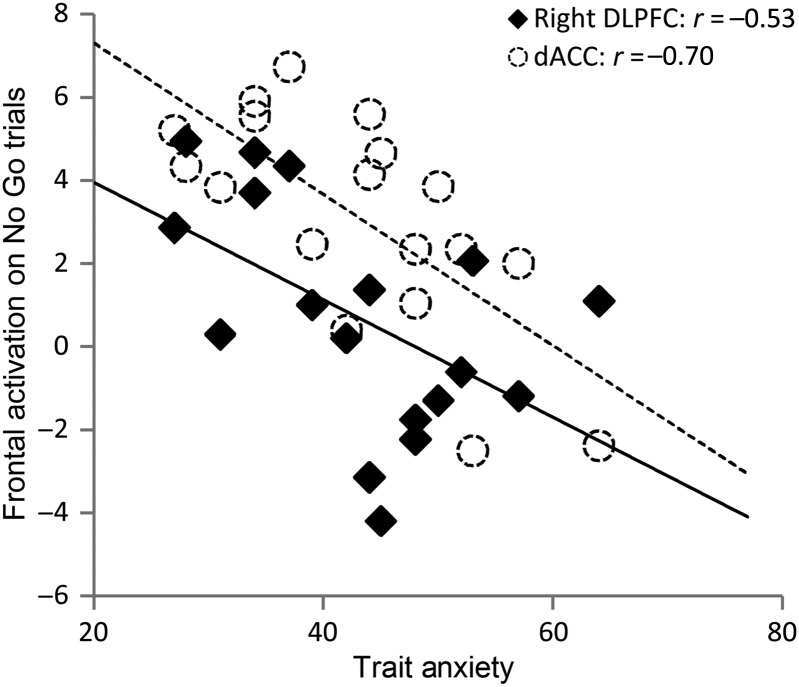
Trait anxiety was associated with both lower dACC and lower DLPFC activity to SART No Go trials (dACC: r(18) = −0.70, P < 0.05; DLPFC: r(18) = −0.53, P < 0.05), Figure 2. When this analysis was repeated for only correct No Go trials, both associations remained significant (dACC: r(18) = −0.80, P < 0.05; DLPFC: r(18) = −0.58, P < 0.05). This is in line with trait anxiety being associated with reduced reactive control upon the occurrence of infrequent No Go trials.
Figure 2.
Trait anxiety was associated with reduced activity in both dorsal ACC and right DLPFC ROIs for SART No Go trials versus baseline (the BOLD signal was averaged across each ROI for each participant). dACC, dorsal anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; SART, Sustained Attention to Response Task.
Trait Anxiety and DLPFC Recruitment to Go Trials
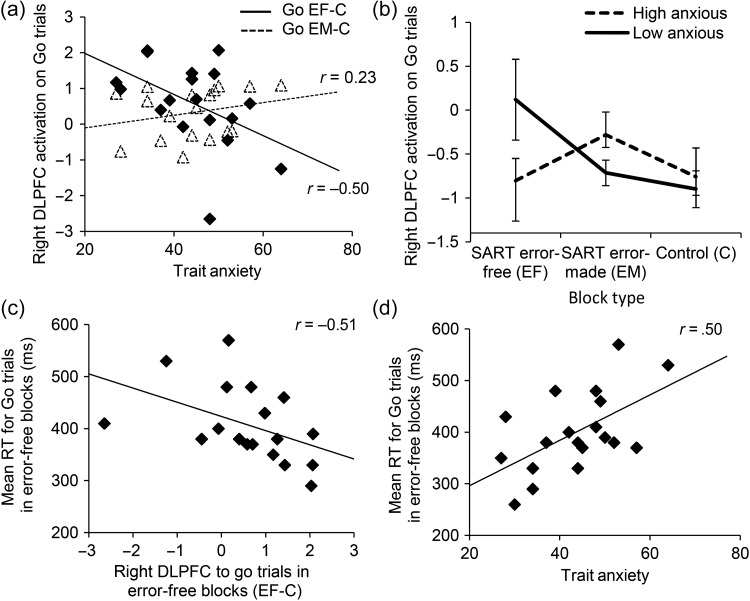
Examination of DLPFC activity to Go trials as a function of block type (SART EF, SART EM, Control (C)) revealed a striking differential pattern of recruitment as a function of trait anxiety, F(2, 30) = 7.44, P < 0.005. High trait anxiety was associated with reduced DLPFC activity to Go trials in SART EF blocks and increased DLPFC activity to Go trials in SART EM blocks, Figure 3a,b. DLPFC activity to Go trials in EF (vs. Control) blocks was associated with faster error-free performance, r(17) = −0.51, P < 0.05, Figure 3c, which in turn was negatively associated with trait anxiety, r(17) = 0.50, P < 0.05 (i.e., trait anxiety was linked to longer reactions times (RTs)—hence the positive correlation coefficient), Figure 3d. In contrast, DLPFC activity to Go trials in EM (vs. Control) blocks was unrelated to RT measures, Ps > 0.1. This provides initial tentative support for the contention that DLPFC activity across Go trials in SART blocks where no commission errors are made primarily reflects engagement of this region in proactive attentional control, facilitating fast error-free task performance, but that DLPFC activity in blocks containing commission errors may, at least in part, reflect an alternate process, such as engagement in off-task thought. There was no significant relationship between trait anxiety and dACC activity to Go trials in either EF (vs. Control) or EM (vs. Control) blocks (Ps > 0.4).
Figure 3.
Anxiety, DLPFC activation and SART performance. (a) Right DLPFC activity on SART Go trials - Control Go trials is plotted against STAI trait anxiety as a function of block type (EF, commission Error-Free; EM, commission Error-Made; C, Control). (b) To illustrate this result further, we used a median split on STAI trait anxiety scores to show DLPFC activation on Go trials by block type (Sart Error-Free, Sart Error-Made, Control) and anxiety level (low, high). Trial-specific activity is calculated against the implicit baseline. (c) DLPFC activation on Go trials in Error-Free (vs. Control) blocks is linked to faster error-free performance. (d) Trait anxiety, in turn, is linked to slower error-free performance. DLPFC, dorsolateral prefrontal cortex; SART, Sustained Attention to Response Task; STAI, Spielberger State Trait Anxiety Inventory.
Further support for 2 distinct dimensions of function being captured by DLPFC activity to Go trials in EF versus EM blocks was obtained from a hierarchical regression analysis; this showed that these 2 indices had not only opposite but additive effects in predicting trait vulnerability to anxiety, Table 1. This is consistent with anxiety being linked to both impoverished engagement of DLPFC in the proactive control of sustained attention and increased activation of this region by off-task thought. It also suggests that there is not a direct trade-off between engagement of DLPFC resources in one of these processes versus the other.
Table 1.
Hierarchical regression models predicting participants' level of trait anxiety
| Predictors | β | P | R2 for model | Adj. R2 | P for model | |
|---|---|---|---|---|---|---|
| a | Right DLPFC to Go trials EF-C Right DLPFC to Go trials EM-C |
−0.67 0.47 |
0.007 0.045 |
0.44 | 0.36 | 0.017 |
| b | Right DLPFC to Go trial EF-C Worry (PSWQ scores) Right DLPFC to go trial EM-C (*excluded) |
−0.52 0.63 *0.15 |
0.006 0.001 *0.47 |
0.65 | 0.59 | 0.001 |
Notes: DLPFC, dorsolateral prefrontal cortex; EF, Commission Error-Free SART block; EM, Commission Error-Made SART block; C, Control block; PSWQ, Penn State Worry Questionnaire.
The extent of differential DLPFC activity during SART EM versus SART EF blocks was not only very strongly linked to trait anxiety, r(18) = 0.65, P < 0.005, but also strongly linked to the mind-wandering index—of intrusion into conscious awareness of TUTs—measured during the vigilance task conducted in the follow-up session, r(13) = 0.69, P < 0.01. As in the case for trait anxiety, hierarchical regression analysis indicated that increased DLPFC activity to Go trials in SART blocks containing commission errors (relative to Control block Go trials) and decreased DLPFC activity to Go trials in SART EF blocks (relative to Control block Go trials) had additive effects in prediction of TUT scores, β DLPFC EF-C = −0.69, P < 0.01, β DLPFC EM-C = 0.62, P < 0.05, overall model R2 = 0.56, P < 0.01, l-tailed. Further, trait anxiety—as measured at the start of the SART session—was directly associated with this TUT measure of intrusive off-task cognition, indexed 2–3 months later, r(16) = 0.42, P = 0.05, 1-tailed.
Trait Anxiety and DLPFC Engagement in Brain Networks Supporting the Maintenance of Sustained Attention Versus off-Task Thought
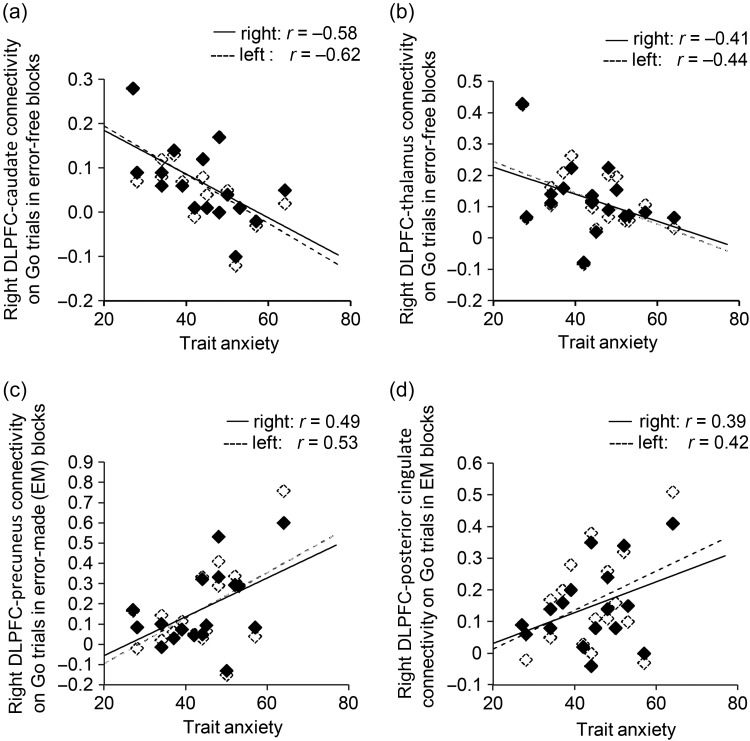
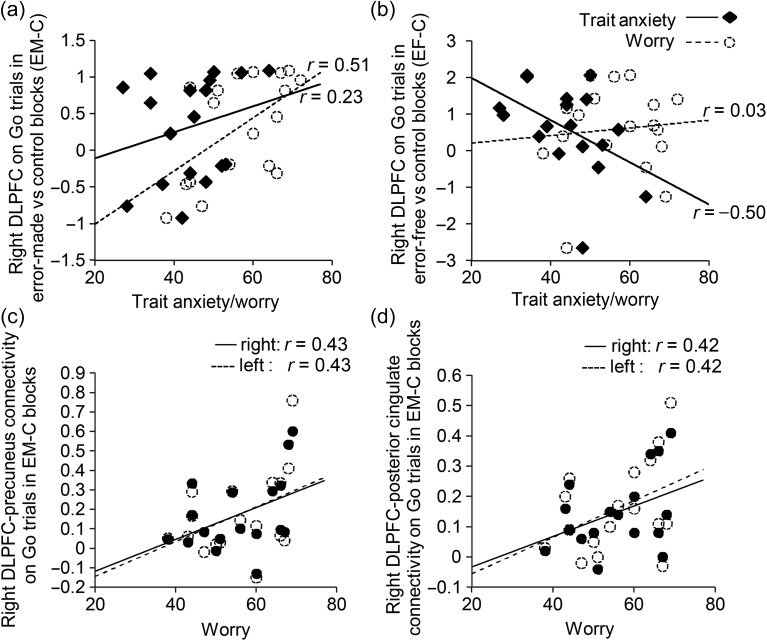
Trait anxiety was linked to reduced functional connectivity between right DLPFC and bilateral caudate and thalamus ROIs across Go trials during EF (vs. Control) blocks, (Ps < 0.05 except for right thalamus, where P = 0.053), Figure 4a,b. This is consistent with reduced engagement of frontal-thalamo-striatal “loops” in the proactive maintenance of sustained attention. In addition, high trait anxiety was associated with increased connectivity between right DLPFC and bilateral precuneus and posterior cingulate ROIs across Go trials during SART EM (vs. Control) blocks (Ps < 0.05, except for right posterior cingulate connectivity, where P = 0.06), Figure 4c,d. This is consistent with previous findings of DLPFC and Default Mode co-activation during spontaneous task unrelated thought. These findings provide additional compelling evidence that the opposing patterns of DLPFC activation linked to trait anxiety as a function of SART performance reflect engagement of this region in different cognitive processes, specifically the proactive control of sustained attention versus off-task thought.
Figure 4.
Results from psychophysiological interaction (PPI) based functional connectivity analyses of DLPFC seeded connectivity as a function of block type and STAI trait anxiety. Trait anxiety was significantly associated with decreased connectivity between right DLPFC and bilateral caudate (a) and thalamus (b) ROIs across Go trials in Error-Free (EF) versus Control (C) blocks. No significant association between trait anxiety and connectivity between these regions was observed in Error-Made versus Control blocks. In addition, trait anxiety was significantly associated with increased connectivity between right DLPFC and bilateral precuneus (c) and posterior cingulate (d) ROIs across Go trials in Error-Made (EM) versus Control (C) blocks. There was no significant association between trait anxiety and connectivity between these regions in Error-Free versus Control blocks. DLPFC, dorsolateral prefrontal cortex; STAI, Spielberger State Trait Anxiety Inventory.
Worry and Frontal Activity During SART Performance
If trait anxious participants’ increased DLPFC activation and elevated DLPFC-Default Mode connectivity for Go trials during SART blocks where commission errors are made reflects increased engagement in off-task thought, in particular occupation with intrusive self-relevant concerns, we might expect these patterns of regional brain activation and connectivity to show an even closer relationship with a specific measure of propensity to worry.
Scores on the PSWQ both significantly predicted DLPFC activity to Go trials during SART EM versus Control blocks, r(17) = 0.51, P < 0.05, Figure 5a, and mediated the relationship between trait anxiety and this index of DLPFC activity, Sobel Test, z = 1.70, P < 0.05, one-tailed. In contrast, worry was not significantly associated with DLPFC recruitment to Go trials in SART EF (vs. Control) blocks (Fig. 5b), dACC or DLPFC activity to Sart No Go trials, or RT measures of error-free performance (all Ps > 0.1 except for DLPFC to No Go trials where P > 0.05). This supports the contention that impoverished attentional control and worry are independent facets of anxiety. Furthermore, when added to the regression model described in Table 1a, PSWQ scores replaced DLPFC activity to Go trials in SART EM blocks as a predictor of anxiety, and together with DLPFC recruitment to Go trials during SART EF blocks (held to primarily reflect proactive attentional control) jointly predicted individual variability in trait anxiety (Table 1b).
Figure 5.
Relationship between worry and frontal function. (a) PSWQ worry scores correlated positively with DLPFC activity on Go trials in the Error-Made (EM) versus Control (C) blocks, showing a similar but stronger relation to trait anxiety, and statistically mediating the relationship between STAI trait scores and this index of DLPFC activity. (b) Unlike trait anxiety, worry (PSWQ scores) did not show a significant relationship with DLPFC activation on Go trials during SART Error-Free (EF) versus Control (C) blocks. (c,d) Worry (PSWQ scores) was associated with increased connectivity between DLPFC and Default Mode regions—precuneus (c) and posterior cingulate (d) across Go trials in SART Error-Made (EM) versus Control (C) blocks. There was no significant association between worry and DLPFC connectivity with thalamus and caudate for Go trials during Error-Free (EF) versus Control (C) blocks. DLPFC, dorsolateral prefrontal cortex; SART, Sustained Attention to Response Task; STAI, Spielberger State Trait Anxiety Inventory; PSWQ, Penn State Worry Questionnaire.
Turning to the functional connectivity analyses, high PSWQ scorers showed increased connectivity between right DLPFC and bilateral precuenus and posterior cingulate ROIs during SART EM (vs. Control) blocks (P ’s < 0.05), Figure 5c,d. However, there was no significant relationship between worry and DLPFC connectivity with regions implicated in the proactive maintenance of sustained attention (namely bilateral caudate and thalamus ROIs) during EF (vs. Control) blocks (Ps > 0.1). This suggests that while trait anxiety may be linked to altered engagement of DLPFC in both the maintenance of sustained attention (decreased) and task unrelated thought (increased), worry may only be linked to altered DLPFC engagement in the latter.
Discussion
Anxious individuals often report concentration problems and high levels of worrying. To date, there has been little research into the neurocognitive mechanisms underlying these symptoms of anxiety, or attempts to determine whether they reflect disruption to one or more underlying processes. Concentration problems may reflect deficits in sustained attention. This aspect of attentional function has not been extensively investigated within the neuroimaging literature on anxiety. Hence, we explored this here, using the well-established SART task (Robertson et al. 1997). Our findings revealed that trait anxiety, but not worry, was associated with impoverished recruitment of frontal regions to support the proactive control of sustained attention. Specifically, elevated STAI trait anxiety was linked to diminished DLPFC activity, reduced connectivity within a DLPFC-thalamo-striatal network previously implicated in the ongoing maintenance and prioritization of task goals (Beiser and Houk 1998; Frank et al. 2001), and slower responding on Go trials in SART blocks without commission (No Go) errors. This pattern is consistent with impoverished proactive control of attention resulting in RT slowing on Go trials being required to maintain No Go accuracy levels. Our findings here are consistent with prior suggestions that, in order to maintain performance levels, anxious participants compensate for impoverished attentional control by reducing speed (Eysenck and Calvo 1992). However, contrary to brain-based translations of efficiency theory (Eysenck et al. 2007), this reduced speed of accurate performance was linked to reduced, not increased, activation in frontal regions.
Trait anxiety was also linked to reduced DLPFC and dACC activity on trials requiring reactive control and response inhibition (i.e., No Go trials). It is of note that we did not observe an inverse relationship between trait anxiety and engagement of frontal regions in proactive versus reactive control. In at least the sustained attention task used here, impoverished recruitment of both proactive and reactive control processes seem to go hand in hand. Indeed, across individuals, ACC recruitment on No Go trials was positively correlated with DLPFC activity to Go trials in EF minus Control blocks, r(17) = 0.58, P < 0.05. As in the case of proactive control, there was no significant relationship between worry (PSWQ scores) and dACC or DLPFC activity to no go trials. These findings run contrary to the suggestion that worry is tightly coupled with, and possibly even secondary to, the disruption of frontal control of attention observed in anxiety.
The occurrence of commission errors—failing to withhold responses on No Go trials—may indicate that individuals are attempting to maintain a Go trial response speed that is too fast for inhibition of response on No Go trials to be effective. In some individuals, however, it also reflects pre-occupation with off-task thought (Christoff et al. 2009). Our findings revealed that in task blocks containing commission errors, the relationship between trait anxiety and DLPFC activity across Go trials reversed. In these blocks, trait anxiety was positively linked with DLPFC activity to Go trials. Evidence that this DLPFC activation may reflect a different process from DLPFC activity to Go trials in blocks without commission errors was provided by a hierarchical regression analysis which showed that these indices of DLPFC activity had not only opposite but additive effects in predicting trait anxiety.
Importantly, in task blocks containing commission errors, worry (PSWQ scores) mediated the positive relationship between trait anxiety and DLPFC activity. In addition, in these blocks, both trait anxiety and worry were linked to increased DLPFC functional connectivity with Default Mode regions implicated in self-referent processing (precuneus, posterior cingulate). These connectivity findings provide support for our proposal that increased anxiety and worry-related DLPFC activity during blocks containing commission errors may reflect spontaneous off-task self-referent thought. They also highlight the value of connectivity analyses for providing insight into within session and within condition changes in the functional role of a given region due to altered engagement in on- versus off-task processes, which could otherwise easily be overlooked.
Overall, the findings reported here increase our understanding of the relationship between anxiety and worry at both the neural and cognitive level. They are consistent with information processing accounts that link worry to increased rates of stimulus-independent, internally generated task unrelated thoughts; and suggest that this may involve an interplay between regions of the brain that support self-referential processing and others (namely, DLPFC) that have been implicated in reasoning (Goel and Dolan 2003). Critically, our results suggest that while this worry-related processing is elevated in trait anxious individuals, it is independent of anxiety-related impoverished recruitment of frontal attentional control mechanisms. This suggests that worry is not merely a secondary symptom to impoverished frontal control of attention in anxiety. It is similarly inconsistent with the reverse proposal that elevated levels of worry account, through competition for common limited processing resources, for anxiety-related deficits in attentional control (Eysenck and Calvo 1992).
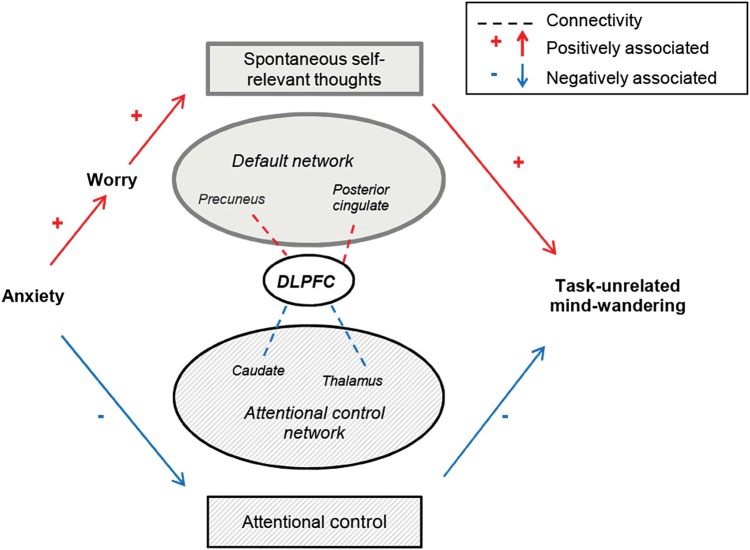
By highlighting and disentangling the dual roles of DLPFC in attentional control and spontaneous task unrelated thought, we provide initial support for a new model of the relationship between anxiety, worry, and frontal dysfunction (Fig. 6). According to this model, worry is independent of trait anxiety-related deficits in DLPFC engagement in the proactive control of sustained attention (as well as deficits in ACC engagement in reactive control). One intriguing possibility is that this model may provide a potential non-circular account for why worry is perceived as more “pathological”—i.e., more disruptive and distressing—in patients with ADs (Olatunji et al. 2011). Specifically, this might reflect the position an individual falls on 2 distinct dimensions of function, one pertaining to elevated spontaneous generation of self-referent negative thoughts and the other to impoverished attentional control, both involving alteration in DLPFC function but being differently characterized by DLPFC interaction with regions belonging to Default Mode versus attentional brain networks. Very preliminary evidence for this proposal comes from the finding that the DLPFC indices held to tap these 2 dimensions jointly predicted the level of intrusive TUTs reported by participants in a vigilance task with thought probes conducted during the follow-up session. Additional exploration of this is needed, but we hope that the model described here provides an empirically testable framework of value to future investigation of altered brain networks in anxiety and worry, and associated profiles of disruption to cognitive processing. It is hoped that this will in turn facilitate the development of theoretically grounded cognitive interventions aimed at tackling the symptoms of poor concentration and worry which contribute to the distress and dysfunction experienced by anxious individuals.
Figure 6.
Illustration of the proposed relationship between trait anxiety, worry, attentional control, and generation of spontaneous self-referent thoughts. In this model, trait anxiety is associated with both impoverished attentional control and increased worry, the latter being in turn linked to elevated levels of spontaneous thoughts about personal concerns. The attentional control dimension is held to entail reduced engagement of DLPFC in a frontal-thalamo-striatal network that supports the proactive control of attention, as well as reduced ACC and DLPFC engagement in reactive control (not shown here). The worry/spontaneous thought dimension is held to entail increased interaction between DLPFC and Default Mode regions implicated in self-referent processing, in particular the precuneus and posterior cingulate. Of note, worry is not directly associated with impoverished attentional control. A possibility that requires additional investigation is that individual variation in both these dimensions might contribute to the extent to which disruptive task-unrelated mind-wandering, focused on self-related concerns, occurs during attempts to perform everyday tasks. DLPFC, dorsolateral prefrontal cortex; ACC, anterior cingulate cortex.
Funding
This work was supported by NIH (grant number R01MH091848 to S.J.B.) and the U.K. Economic and Social Research Council (grant number ES/J006564/1 to S.F.). Funding to pay the Open Access publication charges for this article was provided by NIH and University of California, Berkeley.
Notes
The authors thank Matthew Brett and J.B. Poline for advice on imaging analysis. Conflict of Interest: None declared.
References
- Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry. 2011;69:55–68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron E, Riby LM, Greer J, Smallwood J. Absorbed in thought: the effect of mind wandering on the processing of relevant and irrelevant events. Psychol Sci. 2011;22(5):596–601. doi: 10.1177/0956797611404083. [DOI] [PubMed] [Google Scholar]
- Beiser DG, Houk JC. Model of cortical-basal ganglionic processing: encoding the serial order of sensory events. J Neurophy. 1998;796:3168–3188. doi: 10.1152/jn.1998.79.6.3168. [DOI] [PubMed] [Google Scholar]
- Bieling PJ, Antony MM, Swinson RP. The state-trait anxiety inventory, trait version: structure and content re-examined. Behav Res Ther. 1998;36:777–788. doi: 10.1016/S0005-7967(98)00023-0. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nat Neurosci. 2009;12:92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Duncan J, Brett M, Lawrence A. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Fossella J, Croucher C, Duncan J. COMT Val 158 met genotype affects recruitment of neural mechanisms supporting fluid intelligence. Cereb Cortex. 2008;18:2132–2140. doi: 10.1093/cercor/bhm240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ, Jenkins R, Lawrence A. The neural processing of task-irrelevant fearful faces: effects of perceptual load and individual differences in trait and state anxiety. Cereb Cortex. 2007;17:1595–1603. doi: 10.1093/cercor/bhl070. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Robinson E, Pruzinsky T, DePree JA. Preliminary exploration of worry: some characteristics and processes. Behav ResTher. 1983;21:9–16. doi: 10.1016/0005-7967(83)90121-3. [DOI] [PubMed] [Google Scholar]
- Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. J Cogn Affect Behav Neurosci. 2007;7:356–366. doi: 10.3758/CABN.7.4.356. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Nystrom L, Fissell K, Carter CS, Cohen JD. Conflict monitoring vs. selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM. Extracting core components of cognitive control. Trends Cogn Sci. 2006;10(12):529–532. doi: 10.1016/j.tics.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: dual mechanisms of cognitive control Chapter 4. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variation in Working Memory. Oxford, UK: Oxford University Press; 2007. pp. 76–106. [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16(Suppl. 1):1141. [Google Scholar]
- Carp J. Optimizing the order of operations for movement scrubbing: comment on Power et al. Neuroimage. 2011;76:436–438. doi: 10.1016/j.neuroimage.2011.12.061. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JA, Power KG, Durham RC. The relationship between trait vulnerability and anxiety and depressive diagnoses at longterm follow-up of generalized anxiety disorder. J Anxiety Disord. 2004;18:587–607. doi: 10.1016/j.janxdis.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci. 2009;106(21):8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;10:475–483. doi: 10.1016/S0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Eysenck MW. Anxiety, learning and memory: a reconceptualization. J Res Pers. 1979;13(4):363–385. doi: 10.1016/0092-6566(79)90001-1. [DOI] [Google Scholar]
- Eysenck MW, Calvo MG. Anxiety and performance: the processing efficiency theory. Cogn Emo. 1992;6:409–434. doi: 10.1080/02699939208409696. [DOI] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Fassbender C, Murphy K, Foxe J, Wylie G, Javitt D, Robertson IH, Garavan H. A topography of executive functions and their interactions revealed by functional magnetic resonance imaging. Brain Res Cogn Brain Res. 2004;20:132–143. doi: 10.1016/j.cogbrainres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Loughry B, O'Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn Affect Behav Neurosci. 2001;1:137–160. doi: 10.3758/CABN.1.2.137. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Analyzing brain images: principles and overview. In: Frackowiak RSJ, Friston KJ, Frith C, Dolan R, Mazziotta JC, editors. Human Brain Function. Salt Lake City, UT: Academic Press USA; 1997. pp. 25–41. [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. NeuroImage. 2003;19:200–207. doi: 10.1016/S1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. Reciprocal neural response within lateral and ventral medial prefrontal cortex during hot and cold reasoning. NeuroImage. 2003;20(4):2314–2321. doi: 10.1016/j.neuroimage.2003.07.027. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A. Anxiety and the allocation of attention to threat. Q J Exp Psychol. 1988;40:653–670. doi: 10.1080/14640748808402292. [DOI] [PubMed] [Google Scholar]
- Mathews A, Mackintosh B. A cognitive model of selective processing in anxiety. Cogn Ther Res. 1998;22:539–560. doi: 10.1023/A:1018738019346. [DOI] [Google Scholar]
- McVay JC, Kane MC. Does mind wandering reflect executive function or executive failure? Comment on Smallwood and Schooler (2006) and Watkins (2008) Psychol Bull. 2010;136:188–197. doi: 10.1037/a0018298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the penn state worry questionnaire. Behav Res Ther. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behav Res Ther. 1998;36:809–848. doi: 10.1016/S0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Okuda J, Sakai K. Reactive mechanism of cognitive control system. Cereb Cortex. 2010;20(11):2675–2683. doi: 10.1093/cercor/bhq013. [DOI] [PubMed] [Google Scholar]
- Nachev P. Cognition and medial frontal cortex in health and disease. Curr Opin Neurol. 2006;19:587–592. doi: 10.1097/01.wco.0000247609.36482.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olatunji BO, Wolitzky-Taylor KB, Sawchuk CN, Ciesielski BG. Worry and the anxiety disorders: a metaanalytic synthesis of specificity to GAD. App Prev Psychol. 2011;14:1–24. [Google Scholar]
- Plehn K, Peterson RA. Anxiety sensitivity as a predictor of the development of panic symptoms, panic attacks, and panic disorder: a prospective study. J Anxiety Disord. 2002;16:455–474. doi: 10.1016/S0887-6185(02)00129-9. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards A, Millwood B. Colour-identification of differentially valenced words in anxiety. Cognition Emotion. 1989;3:171–176. doi: 10.1080/02699938908408078. [DOI] [Google Scholar]
- Robertson TH, Manly T, Andrade J, Baddeley BT, Yiend J. “Oops”: performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologica. 1997;356:747–758. doi: 10.1016/S0028-3932(97)00015-8. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, Jacobs AG. Manual for the State-Trait Anxiety Inventory Form Y. Palo Alto: Consulting Psychologists Press, Inc; 1983. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazover B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Persp Psych Sci. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]