Abstract
Animal studies suggest that structural changes occur in the maternal brain during the early postpartum period in regions such as the hypothalamus, amygdala, parietal lobe, and prefrontal cortex and such changes are related to the expression of maternal behaviors. In an attempt to explore this in humans, we conducted a prospective longitudinal study to examine gray matter changes using voxel-based morphometry on high resolution magnetic resonance images of mothers’ brains at two time points: 2–4 weeks postpartum and 3–4 months postpartum. Comparing gray matter volumes across these two time points, we found increases in gray matter volume of the prefrontal cortex, parietal lobes, and midbrain areas. Increased gray matter volume in the midbrain including the hypothalamus, substantia nigra, and amygdala was associated with maternal positive perception of her baby. These results suggest that the first months of motherhood in humans are accompanied by structural changes in brain regions implicated in maternal motivation and behaviors.
Keywords: maternal brain, maternal behavior, postpartum, brain structure, neuroimaging
Maternal care during the first several months provides the foundation for the infant’s neurobiological, socioemotional, and cognitive development (Bornstein, 2002; Champagne et al., 2004). Animal studies have identified several brain areas as important for the initiation and maintenance of maternal pup-directed behaviors. For example, lesion studies have shown that the medial preoptic areas (MPOA), located in the rostral hypothalamus, and its connections with the mesolimbic dopamine system for reward processing play a critical role in maternal motivation (Numan & Insel, 2003; Swain et al., 2004). The thalamus, parietal cortex, and brainstem also serve important functions for the processing of infant-related sometosensory information, such as smell, touch, and vocalizations (Xerri, Stern, & Merzenich, 1994). The prefrontal cortex is involved in integrating these information and monitoring parental behaviors (Afonso, Sison, Lovic, & Fleming, 2007). In human mothers, recent functional MRI (fMRI) brain studies have reported that many of these brain regions including substantia nigra, amygdala, thalamus, parietal cortex, and prefrontal cortex were activated in response to infant-related stimuli, supporting the role of these brain areas for the development and expression of parenting behaviors (Lenzi et al., 2008; Lorberbaum et al., 2002; Noriuchi, Kikuchi, & Senoo, 2008; Strathearn, Li, Fonagy, & Montague, 2008; Strathearn, Fonagy, Amico & Montague, 2009; Swain et al., 2008; see review by Swain, Lorberbaum, Kose, & Strathearn, 2007).
The increased activations of these brain regions during the early postpartum period may be further accompanied by structural changes in the brain. Animal studies have shown that increased interactions with pups over time during the early postpartum period lead to structural changes in the maternal brain. For example, the amount of experience interacting with their pups was correlated with enhanced c-fos expression and cortical representation in the hypothalamus (MPOA), basolateral amygdala, parietal cortex and prefrontal cortex of rat mothers (Featherstone, Fleming, & Ivy, 2000; Fleming & Korsmit, 1996; Kinsley et al., 1999; Lonstein, Simmons, Swann, & Stern, 1998; Xerri et al., 1994). Based on these animal studies, it is reasonable to expect that similar structural changes occur in the brain of human mothers during the early postpartum period. However, this hypothesis has not been rigorously tested in human mothers yet.
To understand the neuroplasticity related to maternal behaviors during the early postpartum period, this longitudinal MRI study examined structural changes in the brains of human mothers from 2–4 weeks to 3–4 months postpartum. We employed the optimized voxel-based morphometry (VBM) to identify region-specific changes in gray matter volumes over the first postpartum months (Mechelli, Friston, & Ashburner, 2005). We also assessed the mothers’ subjective experience of being a parent and perception of their baby at 2–4 weeks postpartum to examine whether maternal experience may facilitate longitudinal structural changes in the key maternal motivation areas of midbrain regions including hypothalamus, substantia nigra, and amygdala.
Methods
Participants
Nineteen biological mothers of full-term and healthy infants participated in this longitudinal study. Nineteen mothers (mean age = 33.27, SD = 6.07) were recruited in postpartum hospital wards at the Yale-New Haven Hospital. All mothers were right-handed, Caucasian, and either married or cohabiting and all were breastfeeding. The average educational level was 18.5 (SD = 3.40) years. Ten out of the 19 mothers had male newborns. Eight of all the mothers were multiparous. On the Beck Depression Inventory (BDI), mothers scored between 0 and 13, indicating minimal to moderate levels of depressive symptoms at both 2–4 weeks and 3–4 months postpartum (Beck, Steer, & Garbin, 1988). Informed consent was obtained from each participant according to the procedure approved by the School of Medicine Human Investigations Committee.
Mothers’ Subjective Perception on Parenting and Baby
At 2–4 weeks postpartum, during home visit, the Yale Inventory of Parental Thoughts and Actions – Revised (YIPTA-R), a semi-structured interview, was conducted by an experienced clinician. YIPTA-R was designed to elicit information concerning the specific features of new parents’ thoughts and actions. Positive Thoughts on Parenting and Baby was two of the subscales of YIPTA-R. Mothers were asked to select from a list of adjectives words that best described their perception of the baby or experience as a mother. A list of the words was based on a previous version of the YIPTA (Leckman et al., 1999). For the positive thoughts on baby, the list included 13 positive words including “Beautiful,” “Ideal,” “Perfect,” and “Special.” For the positive thoughts on parenting, this list included 32 positive words including “Blessed,” “Content,” and “Proud.” The sum of the positive words mothers chose was included as a variable in this study. Positive thoughts on baby and positive thoughts on parenting scores ranged 0–13 and 0–32, respectively. Cronbach’s alpha value for the reliability was .78 for positive thoughts on baby, and .85 for positive thoughts on parenting.
Image Acquisition
Brain imaging data were obtained twice for each mother: first, between 2 and 4 weeks postpartum (Time 1) and second, between 3 and 4 months postpartum (Time 2) at the University Magnetic Resonance Research Center. The average interval between two scans was 76.53 (SD = 9.88) days. High resolution T1-weighted structural magnetic resonance images (MRI) were obtained (3D MPRAGE; TR = 2530 ms; TE = 3.66 ms; matrix size 256 × 256; 176 slices) with a Siemens trio 3T full-body scanner (Erlangen, Germany). Head movements were restrained throughout the session with foam padding and surgical tape placed across each participant’s forehead.
Voxel-Based Morphometry Longitudinal Analysis
Voxel-based morphometry (VBM) analyses were performed with VBM2 toolbox for Statistical Parametric Mapping 2 (SPM2; Wellcome Trust Center for Neuroimaging, London, U.K.). All the structural images were processed according to the optimized VBM protocol (Ganzel, Kim, Glover, & Temple, 2008; Good et al., 2001; Kim et al., 2010). Study-specific T1 gray matter, white matter, CSF templates were first created based on the images of all participants in one group from both Time 1 and 2. Next, customized T1 gray matter templates were used for segmentation and normalization of the original images. Template creation and subsequent segmentation and normalization were performed using default options in the VBM toolbox (25 mm cut off, medium regularization, medium HMRF [Hidden Markov Random Field]) weighting for segmentation) with 16 nonlinear iterations. Additional bias correction was performed to minimize these intensity differences between the time points due to systematical differences in the bias field (Mechelli et al., 2005). To preserve the total within-voxel volume, which may have been affected by the nonlinear transformation, every voxel’s signal intensity in the segmented GM images was multiplied by the Jacobian determinants derived from the spatial normalization (Good et al., 2001; Mechelli et al., 2005). All the modulated images were smoothed with a filter of 12 mm Gaussian kernel. Finally, the modulated and smoothed images were analyzed with a paired t test (available in the VBM2 toolbox for the longitudinal analysis) to test gray matter changes between Time 1 and Time 2. Parenting experience (primiparous = −0.5; multiparous = 0.5) and scan interval were included as a control variable. Statistical significance was defined using a whole-brain false-discovery-rate (FDR) of 5% (p < .05) and an extent threshold of 100 voxels after multiple comparison correction (Genovese, Lazar, & Nichols, 2002). Multiple regression analyses was performed using SPSS (SPSS, Inc., Chicago, Ill) to test the association between behavioral measures (positive experience on parenting and baby) and gray matter volume changes in the midbrain region from Time 1 to 2, controlling for parenting experience and scan intervals. Estimates of gray matter volume changes were extracted from a cluster of the midbrain region for each participant using MarsBaR (Marseille boîte à région d’intérêt; Brett, Anton, Valabregue, & Poline, 2002).
Results
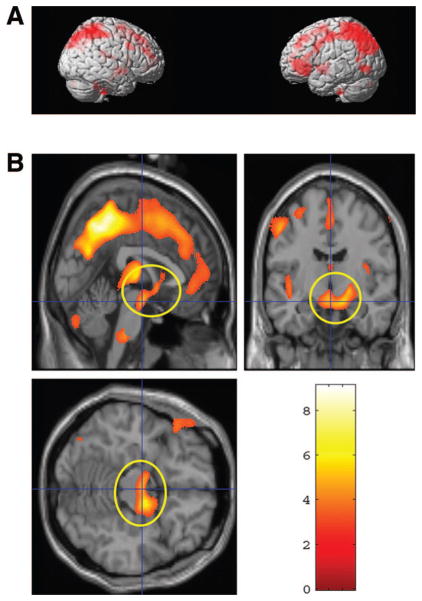
Optimized whole brain VBM analyses revealed that from Time 1 (2–4 weeks postpartum) to Time 2 (3–4 months postpartum), mothers showed an increase in gray matter volume in a number of brain regions including superior, middle and inferior prefrontal cortex, precentral and postcentral gyrus, superior and inferior parietal lobe, insula and thalamus, p < .05 (FDR corrected), > 100 voxels (see Table 1 and Figure 1A). No brain region showed a decrease in gray matter volumes from Time 1 to Time 2, p < .05 (FDR corrected), > 100 voxels.
Table 1.
Brain Regions Showed Gray Matter Increase From 2–4 Weeks to 3–4 Months Postpartum
| Regions | BA | Side | MNI coordinates (peak within a cluster)
|
Cluster size | z-value | Corrected p-level | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Inferior parietal lobe, superior parietal lobe, precuneus, medial frontal gyrus, postcentral gyrus, cingulate gyrus | 3/5/6/7/8/31/40 | L/R | −42 | −43 | 50 | 154783 | 5.50 | <.001 |
| Inferior frontal gyrus, middle frontal gyrus, precentral gyrus | 6/9/47 | L | ||||||
| Thalamus | — | R | 6 | −16 | 12 | 5778 | 4.86 | <.001 |
| Hypothalamus, substantia nigra, caudate body, caudate head, mammillary body | — | L/R | 13 | −6 | −15 | 12822 | 4.55 | <.001 |
| Amygdala, putamen, medial globus pallidus, lateral globus pallidus, anterior cingulate gyrus, parahippocampal gyrus, insula | 34 | R | ||||||
| Middle frontal gyrus, precentral gyrus | 9/46 | R | 43 | 12 | 32 | 2166 | 4.26 | <.005 |
| Middle frontal gyrus, superior frontal gyrus | 8/9 | R | 23 | 33 | 44 | 6192 | 4.10 | <.005 |
| Cerebellum (anterior & posterior lobe) | — | R | 22 | −43 | −36 | 1366 | 4.08 | <.005 |
| Superior temporal gyrus, insula | 13/21/22 | L | −42 | −3 | −8 | 2859 | 3.94 | <.005 |
| Brainstem (pons, medulla) | — | L | 5 | −28 | −44 | 1410 | 3.55 | <.05 |
| Insula | 13 | R | 34 | −26 | 18 | 1036 | 3.31 | <.05 |
| Cerebellum (posterior lobe) | — | L | −14 | −58 | −34 | 196 | 3.27 | <.05 |
| Cerebellum (posterior lobe) | — | R | 3 | −81 | −33 | 638 | 3.09 | <.05 |
| Parahippocampal gyrus | 36 | R | 28 | −20 | −27 | 178 | 2.97 | <.05 |
Note. p < .05 (FDR, corrected), >100 voxels.
Figure 1.
A. Gray matter increase from 2–4 weeks to 3–4 months postpartum, p < .05, (FDR corrected) > 100 voxels—surface areas shown in red—please see Table 1 for complete list of structures that increased in density over time; B. A cluster of midbrain regions are circled in yellow (x = 1, y = −10, z = −15; p < .05, corrected; the vertical color bar indicates t statistical values for the paired t test.), for which gray matter increase from 2–4 weeks postpartum to 3–4 months postpartum was predicted by mothers’ positive perception of own baby at 2–4 weeks postpartum, controlling for parenting experience and scan intervals, β = .44, p = .01.
Mothers’ positive thoughts on baby averaged 6.11 (SD = 2.83; Range 1–12) and the positive thoughts on parenting averaged 8.21 (SD = 7.71; Range 1–32). The mother’s positive perception of her baby at Time 1 significantly predicted gray matter volume change from Time 1 to Time 2 in a cluster of the midbrain including hypothalamus, amygdala, and substantia nigra, controlling for parenting experience and scan interval, β = .44, p = .01 (Figure 1B). The positive thoughts on parenting did not significantly predict the gray matter changes in the same region.
Discussion
Rodent studies have indicated that the expression of maternal behaviors is associated with structural changes in brain regions including the MPOA, the parietal lobe, and the prefrontal cortex (Fleming & Korsmit, 1996; Xerri et al., 1994). This study identified structural changes in similar brain regions among human mothers during the first few postpartum months. Increased gray matter volumes in large regions of the prefrontal cortex, parietal lobe, and midbrain were found. Furthermore, a mother’s positive thoughts on her baby at the first month postpartum predicted gray matter volume increase from the first month to 3–4 months post-partum. This postpartum period marks a critical time for the development of sensitive mothering and changes in these brain regions may be important to promote sensitive maternal behaviors.
Several key maternal motivation and behavior regions including bilateral hypothalamus, amygdala, substantia nigra, and globus pallidus showed increases in gray matter volume during the early postpartum period. The animal literature underlines the importance of these structures for parenting and lesions in the hypothalamus including MPOA impairs maternal motivation and in the MPOA regions increase the likelihood of infanticide (Flannelly, Kemble, Blanchard, & Blanchard, 1986; Novakova, Sterc, Kuchar, & Mozes, 1993). Structural reorganization in the MPOA was also found to be sensitive to postpartum experience; the increased amount of interactions with pups was associated with greater density in MPOA in rat mothers (Featherstone et al., 2000; Fleming & Korsmit, 1996; Lonstein et al., 1998). An increase in gray matter volumes was also found in the right substantia nigra, a key region of the mesolimbic dopaminergic system responsible for processing reward signals (Schultz, Dayan, & Montague, 1997). During the postpartum period, SN serves an important function in activating positive responses to pup stimuli through dopamine neurons. The ventral pallidum, a part of the globus pallidus, receives inputs from substantia nigra and regulates motor activities and behavioral reactivity (Nestler, 2001). Hypothalamus and globus pallidus have previously been implicated in maternal responses to infant stimuli in humans (Bartels & Zeki, 2004; Lorberbaum et al., 2002). Finally, amygdala activations has been found to be important for maternal behaviors in rodents and nonhuman primates (Kling & Steklis, 1976; Sheehan, Paul, Amaral, Numan, & Numan, 2001). Activations of the amygdala, particularly the medial amygdala, inhibit maternal responses to pup in virgin rats. However, animal studies suggest that once mothers are exposed to their offspring, such pathways involving the medial amygdala may be a key to consolidating maternal learning about the infant (Fleming, Gavarth, & Sarker, 1992; Mayes, 2006). Thus, interactions with the infant during the first postpartum months may be associated with the increased gray matter volumes in the hypothalamus, substantia nigra, globus pallidus, and amygdala may help the mothers activate their maternal motivation and respond to infant cues.
Furthermore, the structural changes in the midbrain region including the hypothalamus, substantia nigra, globus pallidus, and amygdala over time were predicted by a mother’s positive perception of her baby at the first month postpartum. Thus, the mother’s positive feelings on her baby may facilitate the increased levels of gray matter volume. fMRI studies with human mothers have similarly shown that greater substantia nigra responses to infant stimuli were correlated with the mother’s self-reported positive emotional reactions to infant stimuli (Bartels & Zeki, 2004; Noriuchi et al., 2007).
Several brain regions implicated in somatosensory information processing also showed an increase in gray matter over the first postpartum months. These findings may provide evidence that these changes in parent brain structure require exposure to infant-related stimuli. In rats, a rich amount of olfactory, auditory, somatosensorial, and visual information during physical interactions with pups and suckling stimuli during nursing were associated with the reorganization of the thalamus, parietal lobe, and someosensory cortex in lactating mothers (Kinsley et al., 2008; Lonstein et al., 1998; Xerri et al., 1994). Moreover, these changes in the parietal cortex only occurred when mothers interacted with their pups but not when they were only exposed to the pups’ smells or sounds (Fleming & Korsmit, 1996). It would be of interest to examine whether the increased gray matter volumes found here in the thalamus, precentral and postcentral gyrus, and superior parietal lobe from the first to fourth month postpartum are related to the frequency and quality of the mother’s interactions with her infant.
Another large area that showed an increase in gray matter volume was the prefrontal cortex (PFC), including the superior, middle and medial frontal cortices. Afonso and colleagues (2007) found that mother rats with medial prefrontal cortex lesions exhibited deficits in a certain maternal behaviors such as pup retrievals and licking behavior, but not in nest building or pup mouthing. Thus, it is possible that the increase in gray matter volumes in the PFC reported here is associated with the mothers’ adaptation to orchestrate a new and increased repertoire of complex interactive behaviors with infants during the early postpartum. Neuroimaging data highlights the importance of the PFC in parenting behaviors; greater activations in frontal regions including superior and middle frontal gyrus (BA 9, 10) and medial frontal guys (BA8) have been found in almost every fMRI study of human mothers’ responses to infant stimuli (reviewed in Swain et al., 2007).
In addition to parenting experience during the early postpartum period, several other factors may be associated with changes in gray matter volumes in mothers’ brain should be monitored in future studies. Animal studies demonstrate that hormones including estrogen, oxytocin, and prolactin act in several brain areas to activate maternal behaviors in response to infant-related stimuli (Pedersen, Caldwell, Peterson, Walker, & Mason, 1992; Wamboldt & Insel, 1987) and changes in these hormones during the early postpartum period affect anatomical changes (Rosenblatt, 2002). Experience during the pregnancy, for instance, increased amount of stress, may also be associated with structural changes in mothers’ brain regions susceptive to stress exposure including amygdala, hypothalamus, and PFC (McEwen, 2007). A future study may include gray matter volumes during the pregnancy as a baseline and compare them with the ones during the postpartum period. Other factors such as changes in menstrual cycles (Protopopescu et al., 2008) or in hydration, weight and nutritional status (Castro-Fornieles et al., 2009; Raji et al., 2010) may also produce alterations in the brain structure. Studies comparing the gray matter volumes between new mothers and age-matched women with no parenting experience would be helpful to control these factors to assess the apparent new learning that may be occurring (Draganski & May, 2008; Driemeyer, Boyke, Gaser, Büchel, & May, 2008).
Finally, the results should be considered in the light of the study’s limitations that suggest future studies. First, although the findings are in accord with animal literature, the study has a relatively small sample size and requires replication in larger groups. Second, although our finding suggest that experience with infants during the first few months is associated with structural development in brain areas that are important for parental motivation and behaviors, the causal directionality is not clear. Also, we measure maternal behaviors based on mothers’ subjective reports. Future research should investigate the relationship between direct measures of maternal behaviors and structural changes in maternal brain. Third, although VBM is an increasingly widely used tool to examine changes in brain structure among healthy and clinical population (Good et al., 2001; Rüsch et al., 2003), interpreting the microstructural and functional nature of the findings is limited. With this caveat in mind, we consider that the current group differences in the VBM measure indicate an increase in regional gray matter volume. This increase may be attributed to enlarged neuropil, neuronal size, or extended dendritic, or axonal arborization (Ashburner & Friston, 2001; Draganski & May, 2008). The findings promote further exploration of other gray matter properties of these populations with different measurements, such as tensor based morphometry, deformation-based morphometry or cortical thickness measurements as well as connecting brain structure changes with function. The potential change in white matter fiber tract is also worthy of future studies with diffusion tensor imaging. Finally, our findings on the plasticity of the parental brain demonstrate normative changes among human mothers since the sample included healthy mothers of healthy infants who lived in supportive environments. However, mothers with genetic and environmental risk factors such as early traumatic experiences, peripartum or postpartum mood disorders, adolescence or low socioeconomic status may show different patterns of structural changes in brain regions that are important for the expression of parenting behaviors. Such abnormal brain structures may be further associated with risk of low parental sensitivity among these parents and affect child outcome (Feldman et al., 2009; Francis, Diorio, Liu, & Meaney, 1999; Swain et al., 2004). Further research is thus required to identify distinctive changes in the maternal brain among at-risk mothers and their infants in order to devise and direct more specific and early interventions appropriately.
Acknowledgments
This work was supported by College of Human Ecology Graduate Research Grant, Cornell University (PK); the US-Israel binational science foundation (2005–273, RF, JFL), the Institute for the Study of Unlimited Love (JES, JFL); the National Alliance for Research on Schizophrenia and Depression (RF, JES), the National Institute of Mental Health (JFL: K05MH076273), the National Institute of Drug Abuse (LCM: 5K05DA020091), and the Associates of the Yale Child Study Center. We thank Dr. Gary W. Evans, Dr. Cindy Hazan, and Dr. Richard Depue for their helpful comments.
Contributor Information
Pilyoung Kim, Cornell University and Yale University School of Medicine.
James F. Leckman, Yale University School of Medicine
Linda C. Mayes, Yale University School of Medicine and The Anna Freud Centre
Ruth Feldman, Yale University School of Medicine and Bar-Ilan University.
Xin Wang, University of Michigan.
James E. Swain, Yale University School of Medicine and University of Michigan
References
- Afonso VM, Sison M, Lovic V, Fleming AS. Medial prefrontal cortex lesions in the female rat affect sexual and maternal behavior and their sequential organization. Behavioral Neuroscience. 2007;121:515–526. doi: 10.1037/0735-7044.121.3.515. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry–the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. NeuroImage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- Bornstein MH. Parenting Infants. In: Bornstein MH, editor. Handbook of parenting. Vol. 1. Mahwah, NJ: Erlbaum; 2002. pp. 3–43. [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox [abstract]. Paper presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. Jun, [Google Scholar]
- Castro-Fornieles J, Bargalló N, Lázaro L, Andrés S, Falcon C, Plana MT, Junqué C. A cross-sectional and follow-up voxel-based morphometric MRI study in adolescent anorexia nervosa. Journal of Psychiatric Research. 2009;43:331–340. doi: 10.1016/j.jpsychires.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. Journal of Neuroscience. 2004;24:4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, May A. Training-induced structural changes in the adult human brain. Behavioural Brain Research. 2008;192:137–142. doi: 10.1016/j.bbr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Driemeyer J, Boyke J, Gaser C, Büchel C, May A. Changes in gray matter induced by learning–revisited. PLoS One. 2008;3:e2669. doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone RE, Fleming AS, Ivy GO. Plasticity in the maternal circuit: Effects of experience and partum condition on brain astrocyte number in female rats. Behavioral Neuroscience. 2000;114:158–172. doi: 10.1037//0735-7044.114.1.158. [DOI] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente C, Kanety H, Kuint J, Gilboa-Schechtman E. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. Journal of American Academy of Child and Adolescent Psychiatry. 2009;48:919–927. doi: 10.1097/CHI.0b013e3181b21651. [DOI] [PubMed] [Google Scholar]
- Flannelly KJ, Kemble ED, Blanchard DC, Blanchard RJ. Effects of septal-forebrain lesions on maternal aggression and maternal care. Behavioral and Neural Biology. 1986;45:17–30. doi: 10.1016/s0163-1047(86)80002-4. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Gavarth K, Sarker J. Effects of transections to the vomeronasal nerves or to the main olfactory bulbs on the initiation and long term retention of maternal behavior in primiparous rats. Behavioral and Neural Biology. 1992;57:177–188. doi: 10.1016/0163-1047(92)90122-k. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Korsmit M. Plasticity in the maternal circuit: Effects of maternal experience on Fos-Lir in hypothalamic, limbic, and cortical structures in the postpartum rat. Behavioral Neuroscience. 1996;110:567–582. doi: 10.1037//0735-7044.110.3.567. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Ganzel BL, Kim P, Glover GH, Temple E. Resilience after 9/11: Multimodal neuroimaging evidence for stress-related change in the healthy adult brain. NeuroImage. 2008;40:788–795. doi: 10.1016/j.neuroimage.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashnurner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of aging in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Newman M, Feldman R, Swain JE. Perceived quality of maternal care in childhood and structure and function of mothers’ brain. Developmental Science. 2010;13:662–673. doi: 10.1111/j.1467-7687.2009.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsley CH, Bardi M, Karelina K, Rima B, Christon L, Friedenberg J, Griffin G. Motherhood induces and maintains behavioral and neural plasticity across the lifespan in the rat. Archives of Sexual Behavior. 2008;37:43–56. doi: 10.1007/s10508-007-9277-x. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Madonia L, Gifford GW, Tureski K, Griffin GR, Lowry C, Lambert KG. Motherhood improves learning and memory. Nature. 1999;402:137–138. doi: 10.1038/45957. [DOI] [PubMed] [Google Scholar]
- Kling A, Steklis HD. A neural basis for affiliative behavior in non-human primates. Brain, Behavior, and Evolution. 1976;13:216–238. doi: 10.1159/000123811. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Mayes LC, Feldman R, Evans DW, King RA, Cohen DJ. Early parental preoccupations and behaviors and their possible relationship to the symptoms of obsessive-compulsive disorder. Acta Psychiatrica Scandinavica, S. 1999;396:1–26. doi: 10.1111/j.1600-0447.1999.tb10951.x. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Trentini C, Pantano P, Macaluso E, Iacoboni M, Lenzi GL, Ammaniti M. Neural basis of maternal communication and emotional expression processing during infant preverbal stage. Cerebral Cortex. 2008;19:1124–1133. doi: 10.1093/cercor/bhn153. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Simmons DA, Swann JM, Stern JM. Forebrain expression of c-fos due to active maternal behaviour in lactating rats. Neuroscience. 1998;82:267–281. doi: 10.1016/s0306-4522(97)00283-2. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, George MS. A potential role for thalamocingulate circuitry in human maternal behavior. Biological Psychiatry. 2002;51:431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- Mayes LC. Arousal regulation, emotional flexibility, medial amygdala function, and the impact of early experience: Comments on the paper of Lewis et al. Annals of the New York Academy of Sciences. 2006;1094:178–192. doi: 10.1196/annals.1376.018. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel-based morphometry of the human brain: Methods and applications. Current Medical Imaging Reviews. 2005;1:1–9. [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nature Reviews Neuroscience. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Senoo A. The functional neuroanatomy of maternal love: Mother’s response to infant’s attachment behaviors. Biological Psychiatry. 2008;63:415–423. doi: 10.1016/j.biopsych.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Novakova V, Sterc J, Kuchar S, Mozes S. Maternal behaviour in septal rat females. Physiological Research. 1993;42:351–360. [PubMed] [Google Scholar]
- Numan M, Insel TR. The neurobiology of parental behavior. New York: Springer; 2003. [Google Scholar]
- Pedersen CA, Caldwell JD, Peterson G, Walker CH, Mason GA. Oxytocin activation of maternal behavior in the rat. Annals of New York Academy of Science. 1992;652:58–69. doi: 10.1111/j.1749-6632.1992.tb34346.x. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Butler T, Pan H, Root J, Altemus M, Polanecsky M, Stern E. Hippocampal structural changes across the menstrual cycle. Hippocampus. 2008;18:985–988. doi: 10.1002/hipo.20468. [DOI] [PubMed] [Google Scholar]
- Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, Thompson PM. Brain structure and obesity. Human Brain Mapping. 2010;31:353–364. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt JS. Hormonal bases of parenting in mammals. In: Bornstein MH, editor. Handbook of parenting. 2. Vol. 2. Mahwah, NJ: Erlbaum; 2002. pp. 31–60. [Google Scholar]
- Rüsch N, van Elst LT, Ludaescher P, Wilke M, Huppertz HJ, Thiel T, Ebert D. A voxel-based morphometric MRI study in female patients with borderline personality disorder. NeuroImage. 2003;20:385–392. doi: 10.1016/s1053-8119(03)00297-0. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Sheehan T, Paul M, Amaral E, Numan MJ, Numan M. Evidence that the medial amygdala projects to the anterior/ventromedial hypothalamic nuclei to inhibit maternal behavior in rats. Neuroscience. 2001;106:341–356. doi: 10.1016/s0306-4522(01)00286-x. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34:2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR. What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Leckman JF, Mayes LC, Feldman R, Constable RT, Schultz RT. Neural substrates and psychology of human parent-infant attachment in the postpartum. Biological Psychiatry. 2004;55:153S. [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: Psychology, physiology, and in vivo functional neuroimaging studies. Journal of Child Psychology and Psychiatry. 2007;48:262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Tasgin E, Mayes LC, Feldman R, Constable RT, Leckman JF. Cesarean delivery affects maternal brain responses to own baby cry. Journal of Child Psychology and Psychiatry. 2008;49:1042–1052. doi: 10.1111/j.1469-7610.2008.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamboldt MZ, Insel TR. The ability of oxytocin to induce short latency maternal behavior is dependent on peripheral anosmia. Behavioral Neuroscience. 1987;101:439–441. doi: 10.1037//0735-7044.101.3.439. [DOI] [PubMed] [Google Scholar]
- Xerri C, Stern JM, Merzenich MM. Alterations of the cortical representation of the rat ventrum by nursing behavior. Journal of Neuroscience. 1994;14:1710–1721. doi: 10.1523/JNEUROSCI.14-03-01710.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]