Abstract
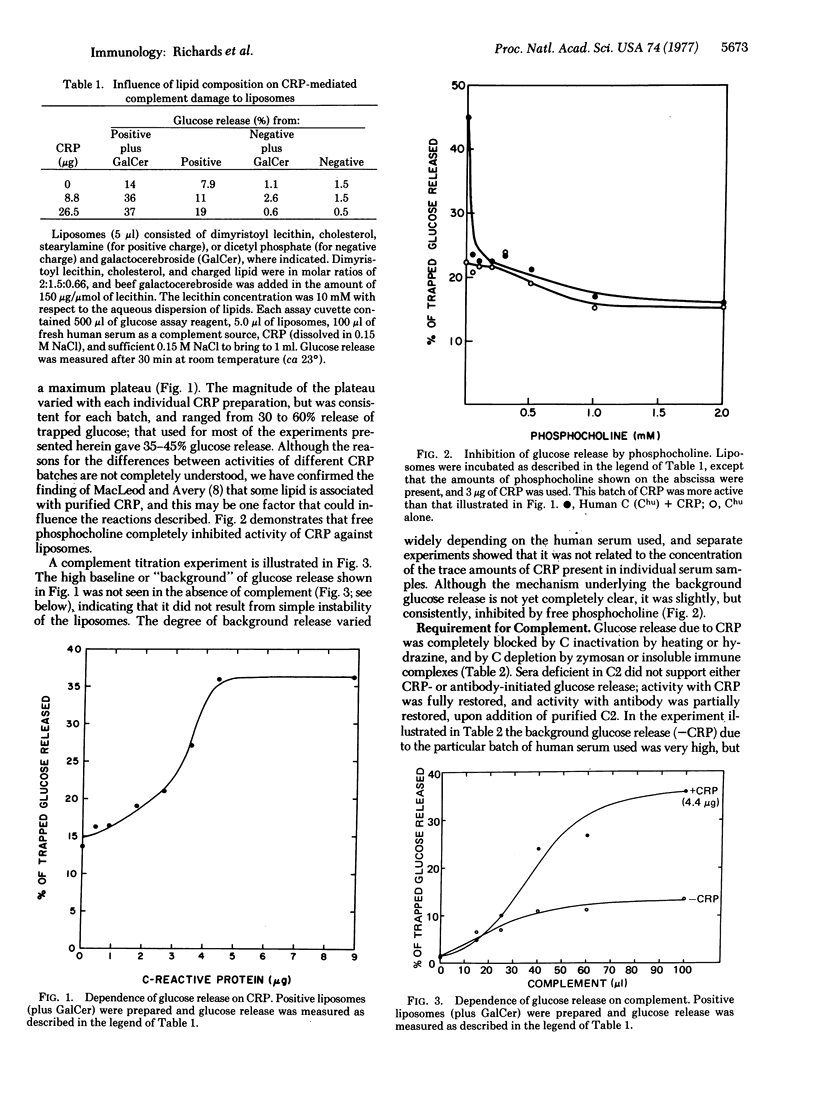
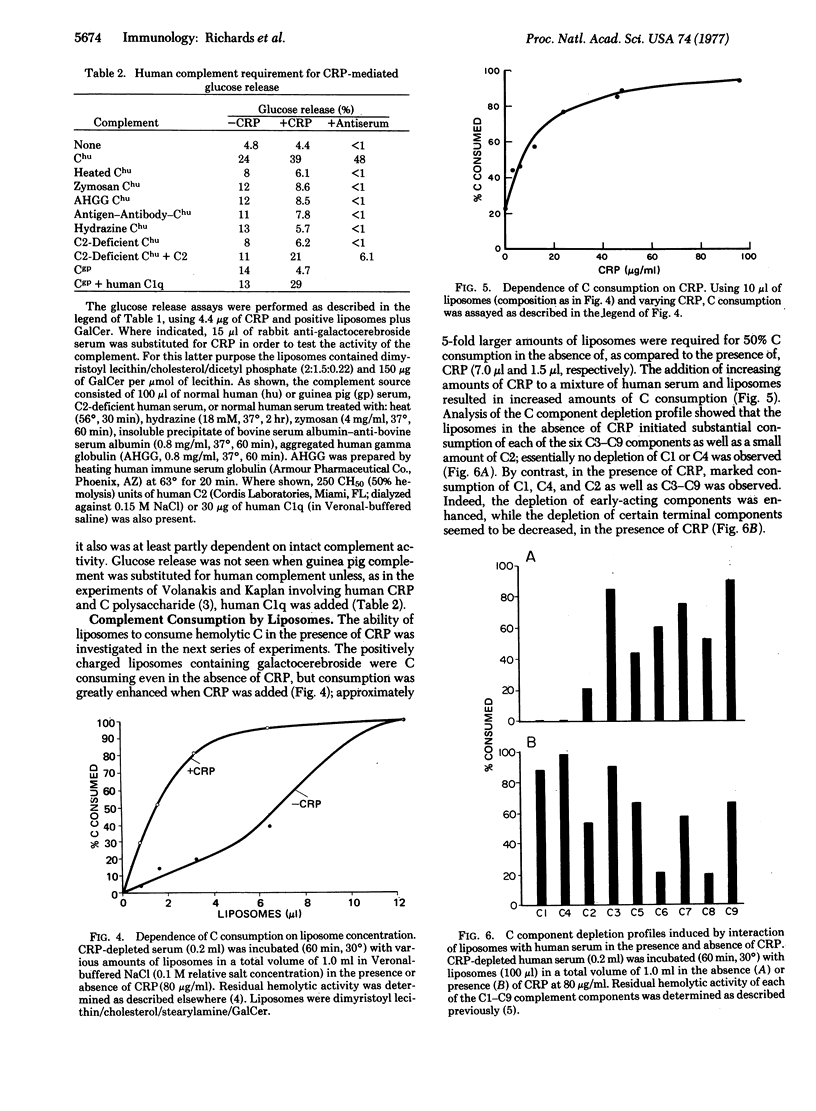
Interactions between C-reactive protein (CRP) and liposomal model membranes containing phosphatidylcholine were investigated. These interactions, in the presence of human serum, resulted in consumption of each of the components of the classical complement pathway (C1-C9) and also resulted in complement-dependent damage and release of trapped glucose from certain types of liposomes. CRP-initiated lysis of liposomes was strongly dependent upon membrane lipid composition. Optimal activity occurred with positively charged liposomes containing galactosylceramide (galactocerebroside); positively charged liposomes lacking galactocerebroside released much less glucose, while negatively charged liposomes, either with or without galactocerebroside, did not release glucose at all. Glucose release was inhibited by free phosphocholine. Lesser, but significant, “background” glucose release independent of the presence of CRP also was observed with positively charged liposomes containing galactocerebroside, and this was associated with marked preferential consumption of the later-acting complement components (C3-C9). C2-deficient human serum failed to support CRP-dependent glucose release, but glucose release was observed upon reconstitution of the serum with C2. Guinea pig complement also did not support CRP-mediated glucose release, but upon addition of human C1q substantial glucose release was observed. We conclude that (i) CRP can sensitize appropriate liposomes for complement-dependent damage via the primary complement pathway starting at the level of C1q; (ii) of those studied, liposomes that are most susceptible to membrane damage contain phosphatidylcholine, have a positive charge, and contain a ceramide glycolipid; and (iii) such liposomes also are sensitive, although to a much lesser degree, to complement-dependent lysis initiated in the absence of CRP and involving consumption of terminal in excess of early acting complement components.
Keywords: phosphocholine, phospholipids, galactocerebroside, membrane permeability
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alving C. R., Richards R. L., Guirguis A. A. Cholesterol-dependent human complement activation resulting in damage to liposomal model membranes. J Immunol. 1977 Jan;118(1):342–347. [PubMed] [Google Scholar]
- Brundish D. E., Baddiley J. Pneumococcal C-substance, a ribitol teichoic acid containing choline phosphate. Biochem J. 1968 Dec;110(3):573–582. doi: 10.1042/bj1100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus D. R., Osmand A. P., Gewurz H. Radioimmunoassay of human C-reactive protein and levels in normal sera. J Lab Clin Med. 1976 Jan;87(1):120–128. [PubMed] [Google Scholar]
- Demel R. A., Geurts van Kessel W. S., Zwaal R. F., Roelofsen B., van Deenen L. L. Relation between various phospholipase actions on human red cell membranes and the interfacial phospholipid pressure in monolayers. Biochim Biophys Acta. 1975 Sep 16;406(1):97–107. doi: 10.1016/0005-2736(75)90045-0. [DOI] [PubMed] [Google Scholar]
- Gotschlich E. C., Edelman G. M. Binding properties and specificity of C-reactive protein. Proc Natl Acad Sci U S A. 1967 Mar;57(3):706–712. doi: 10.1073/pnas.57.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEDLUND P. Clinical and experimental studies on C-reactive protein (acute phase protein). Acta Med Scand Suppl. 1961;361:1–71. [PubMed] [Google Scholar]
- Haxby J. A., Götze O., Müller-Eberhard H. J., Kinsky S. C. Release of trapped marker from liposomes by the action of purified complement components. Proc Natl Acad Sci U S A. 1969 Sep;64(1):290–295. doi: 10.1073/pnas.64.1.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. H., Volanakis J. E. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-polysaccharide and with the choline phosphatides, lecithin and sphingomyelin. J Immunol. 1974 Jun;112(6):2135–2147. [PubMed] [Google Scholar]
- Kinsky S. C. Antibody-complement interaction with lipid model membranes. Biochim Biophys Acta. 1972 Feb 14;265(1):1–23. doi: 10.1016/0304-4157(72)90017-2. [DOI] [PubMed] [Google Scholar]
- Kinsky S. C. Preparation of liposomes and a spectrophotometric assay for release of trapped glucose marker. Methods Enzymol. 1974;32:501–513. doi: 10.1016/0076-6879(74)32050-2. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J., Munn E. A., Weissmanng Complement-mediated lysis of liposomes produced by the reactive lysis procedure. Immunology. 1970 Dec;19(6):983–986. [PMC free article] [PubMed] [Google Scholar]
- Siegel J., Osmand A. P., Wilson M. F., Gewurz H. Interactions of C-reactive protein with the complement system. II. C-reactive protein-mediated consumption of complement by poly-L-lysine polymers and other polycations. J Exp Med. 1975 Sep 1;142(3):709–721. doi: 10.1084/jem.142.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J., Rent R., Gewurz H. Interactions of C-reactive protein with the complement system. I. Protamine-induced consumption of complement in acute phase sera. J Exp Med. 1974 Sep 1;140(3):631–647. doi: 10.1084/jem.140.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volanakis J. E., Kaplan M. H. Interaction of C-reactive protein complexes with the complement system. II. Consumption of guinea pig complement by CRP complexes: requirement for human C1q. J Immunol. 1974 Jul;113(1):9–17. [PubMed] [Google Scholar]
- Volanakis J. E., Kaplan M. H. Specificity of C-reactive protein for choline phosphate residues of pneumococcal C-polysaccharide. Proc Soc Exp Biol Med. 1971 Feb;136(2):612–614. doi: 10.3181/00379727-136-35323. [DOI] [PubMed] [Google Scholar]
- Yonemasu K., Stroud R. M. Clq: rapid purification method for preparation of monospecific antisera and for biochemical studies. J Immunol. 1971 Feb;106(2):304–313. [PubMed] [Google Scholar]


