Abstract
Parenting behavior critically shapes human infants’ current and future behavior. The parent–infant relationship provides infants with their first social experiences, forming templates of what they can expect from others and how to best meet others’ expectations. In this review, we focus on the neurobiology of parenting behavior, including our own functional magnetic resonance imaging (fMRI) brain imaging experiments of parents. We begin with a discussion of background, perspectives and caveats for considering the neurobiology of parent–infant relationships. Then, we discuss aspects of the psychology of parenting that are significantly motivating some of the more basic neuroscience research. Following that, we discuss some of the neurohormones that are important for the regulation of social bonding, and the dysregulation of parenting with cocaine abuse. Then, we review the brain circuitry underlying parenting, proceeding from relevant rodent and nonhuman primate research to human work. Finally, we focus on a study-by-study review of functional neuroimaging studies in humans. Taken together, this research suggests that networks of highly conserved hypothalamic–midbrain–limbic–paralimbic–cortical circuits act in concert to support aspects of parent response to infants, including the emotion, attention, motivation, empathy, decision-making and other thinking that are required to navigate the complexities of parenting. Specifically, infant stimuli activate basal forebrain regions, which regulate brain circuits that handle specific nurturing and caregiving responses and activate the brain’s more general circuitry for handling emotions, motivation, attention, and empathy – all of which are crucial for effective parenting. We argue that an integrated understanding of the brain basis of parenting has profound implications for mental health.
Keywords: Attachment, brain imaging, parent–child interaction, parent–child relationships, parenting, neuropsychology, neurobiology, neurophysiology, child development
In mammals, species survival critically depends on an extensive repertoire of conserved parental behavior to sustain each infant through an extensive dependency period and contribute to long-term health (Ellison, 2006; Gerhardt, 2006; Leckman & Mayes, 1998; Schore, 2005; Sroufe, 2005). Universal parenting behaviors cross species (Clutton-Brock, 1991) as summarized in Table 1, and include pan-cultural human thoughts and activities listed in Table 2 (Hrdy, 2000). Such behaviors may be transmitted genetically or epigenetically (culturally), with the latter permitting the transmission of early life infant experiences across generations, including abusive and neglectful behavior as elaborated elsewhere in this journal. While we contend that unifying concepts across species represent a useful starting point to understand the general scaffolding underlying parental behavior, researchers are just beginning to link animal studies of parenting with the psychology of human parenting (measured, for example, by interview or videotape assessment) and the brain circuits that underlie complex social emotions (measured, for example, by brain imaging of circuits activated by baby signals).
Table 1.
Common behavioral elements of maternal care across mammalian species
| Feature |
|---|
| Nest building and maintenance (place preference) |
| Perceptual exploration (identification of nest and/or offspring) |
| Retrieval (reciprocal calls) |
| Grooming and kissing or licking |
| Crouching or preferred nursing positions |
| Nursing and lactation and/or feeding |
| Prolonged physical contact/sleeping together |
| Aggressive behavior in response to perceived threats to their offspring |
Table 2.
Comparison of prominent features of early parent–infant bonding in humans (adapted from Leckman et al., 2006). Early parental love was rated by 21 experts as similar to infant responsiveness except where indicted by an asterisk
| Feature of love | Early parental love | Infant responsiveness |
|---|---|---|
| Selective recognition – focus exclusivity | +++/++++ | +++ |
| Altered mental state – altered autonomic and behavioral responsivity conditioned by the absence, presence, or mere cues of the other(s) |
+++/++++ | +++ a |
| Clear onset – hedonic transformation | +++ | ++ |
| Intrusive thoughts and images (preoccupations): | ||
| Longing for reciprocity | ++ | +++ a |
| Idealization of the other | +++ | +++ a |
| Heightened awareness of the other | ++++ | +++ a |
| Heightened sense of empathy, responsibility and worries about the well-being of the other |
++++ | ++ a |
| Separation distress | ++++ | ++++ |
| Upsetting aggressive thoughts focused on the self or the other | +* | +++ a |
| Altered repetitive behaviors: | ||
| Proximity seeking and direct physical contact | ++++ | +++ |
| Emotionally charged caring – talking, singing, feeding and grooming | ++++ | +++ |
| Checking to be ensure safety and security, checking that everything is ‘just right’ |
++++* | ++ |
| Dichotomous resolution, either: | ||
| Establishment of intimate mutually satisfying reciprocal patterns of interaction, usually marked by a culturally defined ritual, reorganization and ongoing development of metacognitive representations or |
+++ | +++ |
| Rejection | + | + |
Initially, the mental processes of the infant are ineffable and likely out of conscious awareness.
p > .01 Wilcoxon Signed Ranks that difference between early parental love and infant responsiveness was significant.
Our working model of the functional neuroanatomy of parenting behavior begins with rodent data that point to the importance of basal forebrain structures (Numan & Insel, 2003). For example, lesions in the vicinity of the medial preoptic area (MPOA) completely abolish all aspects of maternal behavior. Projections from the MPOA to the midbrain affect the motivational and approach pathways that normally make various pup-directed behaviors rewarding and also regulate pup retrieval after separation. Such pathways involving the MPOA may in fact regulate a broad range of ritualistic or habitual parenting behaviors such as nursing, breastfeeding and nest building through neurocircuitry that is broadly involved in the appraisal of sensory salience as well as the internal emotions and cognitions that direct attention, set arousal levels, and guide learning and memory to prepare for future behaviors. We theorize that normal brain systems, which are initially wired by evolution to handle a range of social behaviors including parenting, go awry in mental disorders. Many mental disorders may thus be considered as pathological variants of thoughts and behaviors that are important to parenting. For example, in addictive disorders, motivational pathways that normally regulate healthy responses to infant stimuli (Panksepp, Nelson, & Siviy, 1994) may be hijacked by exogenous substances. Similarly, the systems that cope with the loss of social attachment and short-term grief may be abnormally active in depression – a condition that is often triggered by social loss (Brockington, 2004; Najib, Lorberbaum, Kose, Bohning, & George, 2004). This would, in turn, interfere with social bonding and parenting itself. Along the same lines, we also speculate that human versions of rodent nest building circuits are co-opted and exaggerated in people with the hoarding symptoms of obsessive-compulsive disorder (OCD). Other brain systems that regulate normal parental preoccupations and rituals may similarly malfunction to cause other aspects of anxiety, excessive worrying, and OCD (Leckman et al., 1999).
Following this thinking, pediatrician and psychoanalyst Donald Winnicott drew attention to ‘primary maternal preoccupations’ in 1956. He observed that a mother must experience emotional parenting states constituting ‘almost an illness’ in order to meet the physical and psychological needs of her infant (Winnicott, 1960). In other words, normally adaptive thoughts and behaviors in the postpartum, such as addiction-like aspects of love, parental obsessions and hypervigilance for one’s infant’s safety, may improve infant survival and health, and promote resiliency, but may malfunction in addictive disorders and obsessive-compulsive disorder.
In addition, neural systems underlying parent–infant attachment may have substantial overlap with those underlying other forms of social bonding that share an intense, addiction-like concern and focused attention on a preferred individual, such as with romantic love (Aron et al., 2005; Bartels & Zeki, 2004b; Fisher, Aron, Mashek, Li, & Brown, 2002; Hatfield & Sprecher, 1986; Jankowiak & Fischer, 1992; Leckman et al., 2004; Leckman & Mayes, 1999). These systems appear to be sensitive to a series of important environmental and sensory inputs that shape the full expression of a parenting or social bonding type (such epigenetic phenomena are reviewed elsewhere in this issue). However, when the parent–infant bond is disrupted, ranging across situations of mother–infant separation, substance abuse and maternal depression, or dysregulated as in cases of extreme prematurity, illness or birth defects, there may be a feedback that increases risk of disturbed parenting including frank abuse or neglect (Strathearn, Gray, O’Callaghan, & Wood, 2001; Weinfield, Sroufe, & Egeland, 2000). In humans, differences in temperament, as well as socioeconomic and environmental factors may also impact the integrity of this mother–infant relationship.
As such, measurable differences in early bonding lead to long-standing patterns of thought and behavior that, in turn, contribute to individual differences in a person’s risk and resilience profiles for psychopathology in later life, parenting and social bonding that will impact the next generation.
The psychology of human parent–infant relationships
From an ethological perspective, parenting is often regarded as a subset of caregiving or social behaviors and thoughts that are evolutionarily conserved, and have a predictable time course and characteristic content (Leckman et al., 2004; Numan & Insel, 2003). Competing with each parent’s relationship with their infant and motivation to provide parental care are the needs of other children or dependants in the family, occupational duties, the needs of the marital relationship and the demands of the larger social group. Indeed, parent–infant relationships have been considered in many theoretical frameworks. Here we we particularly concencrate on attachment theory, parental motivation, and parental obsessive concern and worry for the welfare of their infants.
Attachment theory and patterns in parent–infant relationships
One of the landmarks of contemporary developmental psychology has been its focus on parent–infant attachment (Bowlby, 1969, 1973). In fact, it was after studying associations between maternal deprivation and juvenile delinquency that John Bowlby first formulated his attachment theory, postulating a universal human need to form close affect-laden bonds, primarily between mother and infant. He also strongly argued, from an evolutionary perspective, that attachment is an innate biological system promoting proximity-seeking between an infant and a specific attachment figure. This proximity then increases the likelihood of survival to a reproductive age.
Because of this powerful biological instinct, Bowlby hypothesized that all human infants attach to their caregiver – even if the care is harsh or neglectful – but that these latter children manifest different patterns of attachment ‘security.’ Infants of caregivers who are available, responsive and sensitive to their emotional and physical needs tend to manifest patterns of ‘secure attachment.’ However, if the care provided is chaotic, unpredictable, rejecting or neglectful, or if the caregiver consistently provides non-contingent responses to the child, then an anxious, insecure or disorganized pattern of attachment evolves (Shaver, Schwartz, Kirson, & O’Connor, 1987). The initial pattern of attachment security was seen as a developmental pathway of major significance throughout the child’s life course, with longitudinal research verifying many of these initial hypotheses (van IJzendoorn, 1995). This underscores how important one’s early environment is in shaping future behavior.
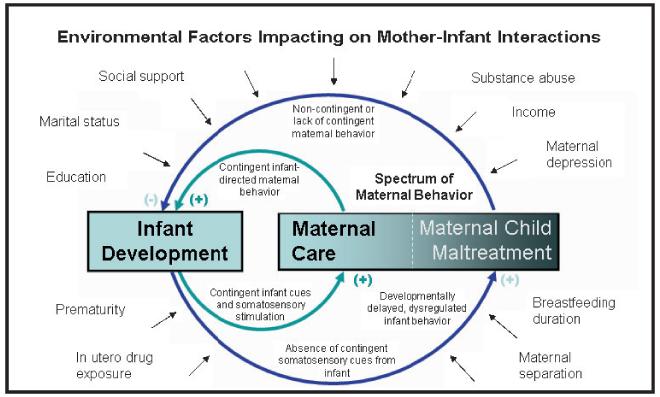
Over the past decade, a diverse spectrum of research has begun to explore the neural basis of attachment – at molecular, cellular and behavioral levels (Insel & Young, 2001; Strathearn, 2007). This research has uncovered many parallels between Bowlby’s original thesis and the biological systems which may underlie attachment and stress reactivity. Understanding the neurobiology of attachment may thus help in formulating and ameliorating pervasive and complex social problems such as child abuse and neglect, with relatively simple interventions that change one’s early environment. Bowlby’s work has made researchers think how important it is for contigent loops of interactions to take place between parents and infants in order to ensure survival and appropriate development. In rodents, in order to survive, newborns are totally dependent on the initiation of a specific set of maternal behaviors in the postpartum, such as nest maintenance, pup retrieval, licking, grooming, arched-back nursing and bold aggression toward infant threats and predators (Leckman & Herman, 2002; MacLean, 1990; Numan & Insel, 2003). These are actually reciprocal behaviors attuned to the needs of the offspring which are expressed through a series of behaviors including vocalizations, nipple-seeking and sucking, and infant odors. Such contigent loops of behavior between parents and offspring are displayed in Figure 1. Since Bowlby first published his seminal work on attachment theory, numerous research methods have been developed to systematically classify attachment styles, as observed from infancy through to adulthood. The two most accepted and empirically tested instruments are the Strange Situation Procedure in infancy (Ainsworth & Bell, 1970) and the Adult Attachment Interview (AAI) (Hesse, 1999). Understanding the neurobiological regulation of parental attachment patterns, measured for instance by the AAI, may help us understand how attachment may be transmitted across generations.
Figure 1.
Model of interdependent relationships between maternal behavior and infant development (Strathearn, 2007). Reproduced with permission
Parenting and fixations on joyous love and concern for infant’s safety
Parents may experience an anxious tension between the joyous reveries of being ‘at one’ with the child, and the intrusive worries that something terrible could happen and jeopardize the relationship. The infant’s moods may reflect this, alternating between serene contentedness and extreme fussiness. We are just beginning to quantify the frequency and intensity of joyfulness and worrying parental preoccupations (Leckman et al., 1999) and how they relate to the concepts of obsessions and compulsions, addictive disorders and romantic love (Leckman et al., 2004; Marazziti et al., 2003; Mayes, Swain, & Leckman, 2005). With regard to the joyful thoughts, many first-time parents anecdotally are themselves surprised to report similar feelings such as: ‘No one told me it was like falling in love.’ Other clear parenting themes include the tendency to be preoccupied with small details of the infant’s appearance, family similarities, and seeing the new infant as ‘perfect.’ As one mother said after her baby was born, ‘… I just can’t believe it, here she is and she’s so perfect, I can’t believe she’s really mine.’ In fact, parents state that the experience of their infant as being ‘perfect’ increases during the postpartum period, reaching a peak at three months, with 73% and 88% of mothers and fathers endorsing this experience, respectively. Future studies may tease apart the way that happy and anxious preoccupations might interact with each other and between parents and infants during bonding (Leckman et al., 1999; Swain, Leckman, Mayes, Feldman, & Schultz, 2005). To orient the reader to some aspects of parent–infant bonding and associated thoughts and behaviors, Table 2 lists prominent features of early parental love and infant responsiveness. In this table, ratings (0–4 crosses) are based on the judgments of 21 experts on bond-formation and attachment and mean values were rounded to the nearest integer. Using a Wilcoxon signed rank test, parental love compared to infant responsiveness was rated as more focused on checking and things being ‘just right’ for the other, with less aggressive thoughts toward the other. There were similar ratings in both parent and infant aspects of parent–infant bonding for awareness of the other, altered mental state, longing for reciprocity, tendency to idealize the other, emotionally charged reciprocal caring and proximity-seeking behaviors (Leckman et al., 2006).
In early parental love, initial data suggests that parents frequently feel compelled to shape their own behavior to the perceived needs of the baby (Leckman et al., 1999). Frequently, these behavioral responses have a ‘just right’ character, such that they need to exactly fit the apparent needs of the baby. This heightened sense of responsibility that usually accompanies this state may lead to increased vigilance, repeated behaviors aimed at ensuring the safety of the infant (Leckman et al., 2004) and increased sensations of reward.
In the interview studies of parents within the first four months postpartum (Leckman et al., 1999; Swain et al., 2004), parents were asked about the occurrence of specific parenting thoughts and actions for the previous week, such as how preoccupied they had been with the baby during the past week using a ten-point ordinal scale. Related interview questions included requests for time estimates such as: ‘During the past week, on average how many hours a day was your mind occupied with thoughts about your baby?’ and ‘How long can you go without having thoughts about your child?’ Two of the derived content domains were denoted as Caregiving (CARE), and Anxious Intrusive Thoughts and Harm Avoidant Behaviors (AITHAB or parental preoccupations). CARE was seen as a product of a complex interaction of the parents’ exposure to the infant’s cues (appearance, vocalizations, behavior, soothability), the nature of the current relationship, and the parent’s internal working models of their own childhood attachment figures. The content domain of AITHAB was seen as a heightened sensitivity to potential threats (imagined and real) to the well-being of the infant and repeated behaviors to remove those threats. We hypothesized that CARE and AITHAB may be considered to be normal variants of the mental states and behaviors associated with addictive diseases and obsessive-compulsive disorder, respectively. In the formulation of the latter, the performance of compulsive checking behaviors (that even the parents themselves may regard as excessive or unnecessary) relieves the intrusive thoughts that one’s baby has been harmed. Even before the child is born, parents preoccupy themselves with creating a safe and secure infant environment. Human nest-building type behaviors are common, including major cleaning and renovation projects in the postpartum. Uppermost among parental concerns and compulsions are safety (such as excessive infant cleanliness) and unimpeded access to their infant. Preliminary analysis of interview data (Swain et al., 2004) indicates significantly higher parental preoccupations in moms compared to dads (p < .001), and correlations between parental preoccupations and depression (p < .001). Later in this article, we will discuss the brain systems that underlie parents’ addictive and anxious thoughts and behaviors. In the future, studies of how these brain systems malfunction in frankly addictive or obsessive parents may offer opportunities for early detection and treatment options in vulnerable individuals.
Certain special cases of parenting bear brief consideration. In parental adjustment to the arrival of an infant, the experience and presence of other children in the home are important. Different psychological adjustments might be required to maintain close ties with existing children and the rest of the family, yet the parents may be more confident and this is partly reflected in the decreased level of parental preoccupation with a second child compared to the first (Leckman et al., 1999; Swain et al., 2004). Another special case of becoming a parent that requires different adaptations is the example of multiple births. In some cultures, twin births are regarded as unwelcome and unnatural, perhaps due to insufficient resources and increased parental preoccupations, and one of the two infants may be killed (Pector, 2002). Some parents of twins find it too difficult to establish clearly differentiated relationships with each infant and attempt to merge the twins into a single unit through similar names, dress, and perceived attributes (Robin, Kheroua, & Casati, 1992). In other instances, parents develop clear preferences, clearly favoring one twin more than the other (Mann, 1992; Minde, Corter, Goldberg, & Jeffers, 1990). There may be other effects of age and experience to consider. For example, teen mothers engaged in more instrumental activities (e.g., changing diapers, adjusting clothes), but less affectionate (e.g., stroking, kissing, patting) behavior, and older mothers showed the opposite, engaging in more affectionate and less instrumental behavior. Further, when groups were reassessed based on early life experience (consistency of care during the first 12 years of life, in which consistent care is having at least one consistent caregiver, and inconsistent care is having multiple and changing caregivers), an interaction was also found between consistency of care and type of behavior shown. Mothers who received inconsistent care engaged in more instrumental and less affectionate behavior. Also, compared to mature mothers, teen mothers who were breastfeeding also had higher salivary cortisol levels, and high cortisol in teen mothers was related to decreased fatigue and increased energy (Krpan, Coombs, Zinga, Steiner, & Fleming, 2005). Clearly, much more work is required to clarify the psychology of the postpartum, the underlying biology and the implications for infant and family outcomes.
Nursing and feeding are the parental behaviors that are perhaps most associated with a new infant. Women describe breastfeeding as a uniquely close, very physical, and sometimes sensual experience that creates a particular unity between the mother and her infant. Cleaning, grooming, play and dressing behaviors also carry a special valence inasmuch as they permit the closeness between parent and infant and provide for frequent inspection of the infant’s body and appearance (Leckman et al., 2004).
The presence of fixed behavioral patterns in human parents may at first seem to be minimal, suppressed or perhaps not as apparent as in rodents. However, detailed videotaped analysis of moment-to-moment parent–infant interaction is prompting a reconsideration of the importance of parent–infant behaviors (Feldman, 2003). In addition, special events in family life are associated ritualistic ceremonies for child naming, acceptance and guidance into the parents’ social group. We might consider that these social and parenting behaviors and rituals manifest along a continuum from adaptive vigilance and habit to pathological mood, anxiety and obsessive-compulsive disorders (Boyer & Lienard, in press; Feygin, Swain, & Leckman, 2006; Swain, in press)
Parenting is regulated by key hormones and neurotransmitters
In addition to far-reaching ‘programming’ of parents by their own early life experiences, maternal behaviors are influenced by current infant cues that activate certain interacting neurotransmitters, including oxytocin, prolactin, vasopressin and dopamine. For example, suckling, audiovisual and olfactory stimuli stimulate maternal care in rodents, even modifying pre-existing behavior patterns (Rosenblatt, 1994; Stern, 1997), at least in part through increased expression of oxytocin receptors in specific brain areas (Francis, Champagne, & Meaney, 2000). In contrast, long periods of mother–infant separation appear to inhibit maternal behavior, through oxytocin receptor modulation (Boccia & Pedersen, 2001). The oxytocinergic system is important in the formation of social and spatial memories, affiliative behavior and emotion regulation (Ferguson, Young, & Insel, 2002). Oxytocin receptors are enriched in brain areas that are significant in the manifestation of social and maternal behavior, including the bed nucleus of the stria terminalis, hypothalamic paraventricular nucleus, central nucleus of the amygdala, ventral tegmental area and lateral septum (Francis, Champagne, & Meaney, 2000). Similar systems are described in nonhuman primates (Winslow, 2005).
Some of the same processes described in animals that require oxytocin are also present in the regulation of an array of human social behaviors and cognitions (Kirsch et al., 2005), including social reduction of stress (Heinrichs, Baumgartner, Kirschbaum, & Ehlert, 2003) and mechanisms of trust (Kosfeld, Heinrichs, Zak, Fischbacher, & Fehr, 2005; Zak, Kurzban, & Matzner, 2004). Oxytocin released in mothers during breastfeeding is also associated with reduced levels of maternal anxiety and attenuated physiological stress response (Chiodera & Coiro, 1987; Legros, Chiodera, & Geenen, 1988), and more attuned patterns of maternal behavior across species (Champagne & Meaney, 2001; Uvnas-Moberg, 1998; Uvnas-Moberg & Eriksson, 1996). Perhaps among the many complex aspects of breastfeeding, oxytocin in the mother may play a role in transmitting infant cues to mothers and encouraging other parenting behaviors. This notion is consistent with the observation that the stress of prolonged mother–infant separation in humans is associated with reduced maternal sensitivity, and more negative patterns of mothering throughout the first 3 years of life (NICHD, 1999). In addition to further supporting the importance of oxytocin for maternal behaviors, rodent gene knockout studies have confirmed the importance of prolactin, estrogen, and dopamine (Leckman & Herman, 2002).
Besides broad roles in motivation and reward systems (Schultz, 2006), dopamine directly modulates oxytocinergic systems in the female prairie vole nucleus accumbens that are critical for the formation of social attachment (Liu & Wang, 2003; Young, Murphy Young, & Hammock, 2005). We would predict that neuroimaging studies of hypooxytocinergic non-breastfeeding mothers as well as non-parents will show decreased responses to parenting in areas that have oxytocin receptors or direct connections to oxytocin-sensitive areas. Understanding the links between healthy parenting and the normal modulation of anxiety, motivation and reward as well as the aberrations in these systems that may be associated with neglect or abuse will help us better prevent and treat these issues. Aberrant situations, in which cocaine abuse or mood disorders might hijack motivation and reward circuits and interfere with social bonding, is the subject of current research efforts and discussed in the following sections below.
Cocaine and maternal behavior
Maternal cocaine abuse is a significant public health issue, particularly affecting children with high rates of abuse, neglect, foster care placement (Chaffin, Kelleher, & Hollenberg, 1996) and disturbed attachment (Seifer et al., 2004). An estimated 4.6 million women use cocaine each year in the United States, with 750,000 drug-exposed births occurring annually (Porter & Porter, 2004). However, we know little about how cocaine exposure affects brain circuits involved in maternal behavior, especially in humans.
The neuropeptide hormone, oxytocin, already discussed above in normal parenting, may be affected by cocaine exposure (Johns et al., 2005a, 2005b). One human study demonstrated significant differences in peripheral oxytocin responses between cocaine exposed mothers and matched controls, in response to infant contact and a stressor (Light et al., 2004). Thus, natural infant-related reward stimuli and artificial stimulants such as cocaine may differentially affect neural development, through both dopamine and oxytocin.
For most mothers, interacting and engaging with one’s own infant is a rewarding and pleasurable experience that promotes mother–infant attachment, ensures optimal care for the developing infant, and motivates maternal behavior, even in the face of extreme fatigue and competing needs for attention. However, animal and human research suggests that cocaine-exposed mothers, even when not actively using the drug, may be less able to respond appropriately to their infants’ cues, or may find these interactions less intrinsically rewarding. Thus, cocaine effectively appropriates the motivation circuits that normally regulate parenting, resulting in increased risk of infant neglect or even abuse. In turn, many cases result in court ordered separation of mother and baby and intensification of trauma to both.
In mothers previously exposed to cocaine, a range of important, though sometimes subtle, abnormalities in maternal caregiving behaviors have also been noted, such as mothers being less attentive and more interrupting of dyadic exchanges (LaGasse et al., 2003; Mayes, Bornstein, Chawarska, & Granger, 1995; Mayes, Granger, Frank, Schottenfeld, & Bornstein, 1993; Tronick et al., 2005). Animal models support the hypothesis that maternal cocaine exposure affects dopaminergic brain pathways, which, in turn, affects early postpartum maternal care (Johns et al., 2005b). However, possible confounding influences include the mother’s own adverse childhood experience, which may also result in differences in maternal behavior (Francis, Diorio, Liu, & Meaney, 1999) and predispose to substance abuse (Kosten, Zhang, & Kehoe, 2006).
We conjecture that cocaine exposure and adverse childhood experience influence maternal responses to infant cues, perhaps interactively, as a result of neurobiological changes in mesocorticolimbic regions of the brain, and altered reward perception and salience. We also suspect that these changes may result from variations in gene expression. A recent fMRI animal study demonstrated that cocaine exposure prior to pregnancy resulted in a significantly reduced brain response to pup suckling, in the medial prefrontal cortex, associated with reduced dopamine production (Febo, Numan, & Ferris, 2005; Ferris et al., 2005). Another study showed that low levels of maternal care were associated with reduced dopamine release in the nucleus accumbens, in response to pup cues (Champagne et al., 2004). As discussed previously, cross-fostering studies in rats strongly suggest that maternal care received in infancy is causally related to subsequent maternal behavior in adulthood (Francis & Meaney, 1999; Pedersen & Boccia, 2002). Thus, maternal care in infancy may enhance the development of dopaminergic reward pathways, resulting in enhanced capacity of offspring to later provide maternal care.
Indeed, human and animal fMRI studies have shown that cocaine activates both the mesocorticolimbic and the nigrostriatal dopamine systems (Breiter et al., 1997; Kufahl et al., 2005). In lactating rats, pup suckling produces a remarkably similar pattern of brain activation, including reward-associated brain regions (Ferris et al., 2005). Studies of human mothers have demonstrated that infant cues, such as facial expressions and cries, activate similar brain reward regions to cocaine, including the ventral tegmental area/substantia nigra region, nucleus accumbens, cingulate and prefrontal cortices. Thus, in non-drug-addicted mothers, exposure to infant cues appears to be highly reinforcing (or at least invokes motivation to respond and approach behavior as in infant crying), and important in activating healthy maternal reward and motivational circuits. Healthy parent–infant interactions, which may themselves be addiction-like (Insel, 2003), are disrupted by artificial stimulants of the dopaminergic system, such as cocaine which may act as a highly reinforcing infant substitute (Meaney, Brake, & Gratton, 2002).
Parental behavior disturbances in postpartum depression
In addition to understanding normal human parenting in order to optimize health outcomes, research on parents who suffer mental health problems such as substance abuse (discussed above) and mood disorders promises to improve recognition, treatment and prevention of disturbed parenting.
Recently published follow-up data on the offspring of depressed and anxious mothers indicating increased mental health risks (Brown, Bifulco, & Harris, 1987; Heim, Owens, Plotsky, & Nemeroff, 1997; Kendler, Kessler, Neale, Heath, & Eaves, 1993; Sroufe, Carlson, Levy, & Egeland, 1999) underscores the significance of work in this area. Clearly, parental wellness (and/or the presence of other attuned caregiving adults) has long-term positive effects on resiliency and emotional well-being of children as they grow up and for decades later. Indeed, longitudinal studies of high-risk infants suggest that secure attachment in the perinatal period is associated with a degree of resiliency and protection against the development of psychopathology later in life (Werner, 2004).
Parental mental health problems in the postpartum, such as depression and anxiety, are common and contribute significantly to parent–infant attachment problems. Postpartum depression follows 10% to 15% of all deliveries (Caplan et al., 1989) and more than 60% of patients have an onset of symptoms within the first 6 weeks postpartum (Stowe & Nemeroff, 1995). While more common than problems such as pre-term delivery, postpartum depression and anxiety have received much less investigative attention and not a single fMRI study (Squire & Stein, 2003). A growing body of evidence from naturalistic longitudinal studies attests to an adverse impact of postpartum depression, with depressed mothers less sensitively attuned to their infants, less affirming and more negative in describing their infant. These disturbances in early mother–infant interactions were found to predict poorer infant cognitive outcome at 18 months (Murray & Cooper, 2003) and later time-points such as 7 years (Kim-Cohen, Moffitt, Taylor, Pawlby, & Caspi, 2005).
However, a recent study showed that maternal remission from depression within 3 months was associated with significant decreases in the mood symptoms of their children, who were 7–17 years of age (Weissman et al., 2006). We would predict an even more dramatic effect in younger children. In efforts to understand the underlying physiology, brain imaging studies are currently under way (Mayes, Swain, & Leckman, 2005) with parents at risk for postpartum depression. We predict that such work will outline future opportunities to identify families at risk for pathological attachment, assess treatments and improve parent–child attachment.
Neuroanatomical circuits of parenting
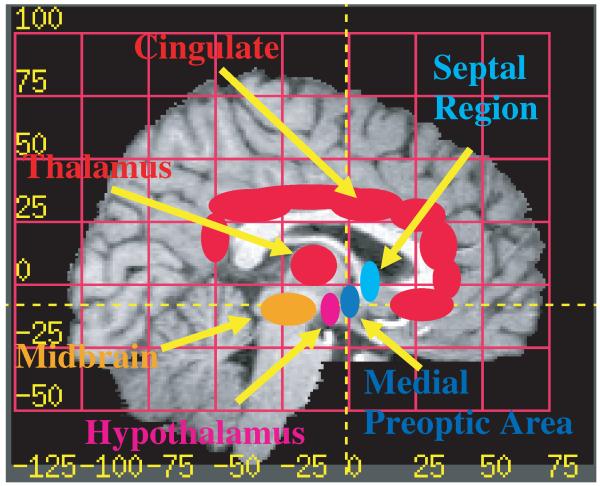
Understanding of the underlying neuroanatomy is necessary for interpreting the interplay of different neurotransmitters in health and illness. Animal models of parental behavior highlight the importance of specific brain circuits that regulate parenting per se as well general aspects of reward, motivation, sensory processing and approach vs. avoidance decision making. Please refer to Figure 2, indicating the regions that we expect to be critical to human parenting, extrapolated from work on rodent behaviors (Table 1) that we summarize below as a prelude to the human imaging studies.
Figure 2.
Human parental brain areas. Brain regions expected to be important to human parenting, based on animal studies of mother–infant behaviors. The striatum and amygdala are not shown
Maternal behavior regulation by motivational systems of the basal forebrain and midbrain
In the rat, the structures showing the most convincing evidence for a central role in maternal behavior are the medial preoptic area (MPOA) and nearby ventral part of the bed nucleus of the stria terminalis (VBNST) (Numan, 1994). These are small basal forebrain structures lying just anterior to the optic chiasm and hormone regulatory systems of the hypothalamus. Lesions of the MPOA/VBNST region or its lateral efferent connections clearly disrupt maternal behavior (Numan, 1974; Numan, Corodimas, Numan, Factor, & Piers, 1988; Numan, McSparren, & Numan, 1990; Numan, Morrell, & Pfaff, 1985; Numan & Numan, 1996) and estradiol injections into the MPOA/VBNST facilitate maternal behavior (Numan, Rosenblatt, & Komisaruk, 1977). MPOA/VBNST outputs include posterior projections to the hypothalamus and midbrain regions such as the ventral tegmental area (VTA) and retrorubral fields/substantia nigra which are rich in dopamine and important in motivated approach behavior (Mirenowicz & Schultz, 1996). Such behavior may be required in pup retrieval, motivation to care for pups, and foraging (Numan, Morrell, & Pfaff, 1985; Numan & Nagle, 1983). The VTA and substantia nigra project along the mesolimbic, mesocortical, or nigrostriatal dopaminergic pathways (midbrain–striatal–anterior cingulate/prefrontal cortex regions) (Mello & Villares, 1997), and lesions along these pathways also interfere with maternal behavior (Numan & Numan, 1997). For example, ventral striatal/nucleus accumbens lesions impair maternal behavior (Hansen, 1994), and infant cues appear to trigger dopamine release in the nucleus accumbens (Champagne et al., 2004). There are also indications that other midbrain sites are potentially important in maternal behavior. For example, MPOA projections to the peripeduncular nuclei in the lateral midbrain’s retrorubral field region may be involved in a mother’s milk letdown response (Factor, Mayer, & Rosenblatt, 1993; Hansen & Kohler, 1984). The function of the MPOA projections to the midbrain’s central gray matter, a region known to be involved in defensive behavior, is not well known. However, such projections could be potentially important for maternal aggressiveness toward intruders (Lonstein, Simmons, Swann, & Stern, 1998; Lonstein & Stern, 1997), preventing a mother’s aggression toward pups (Numan & Sheehan, 1997), or even a mother’s assuming the correct kyphotic nursing posture (Lonstein, Simmons, Swann, & Stern, 1998; Lonstein & Stern, 1997; Numan & Numan, 1997).
Maternal behavior regulation by emotion control circuits involving the amygdala and septal regions
Limbic regions such as the amygdala and the septal region also connect to the MPOA and are thought to be important for parenting. For example, the amygdala may mediate the avoidance of young pup smells by nulliparous rat females (Numan & Sheehan, 1997), since it is also known to mediate the aversive responses to foul odors (LeDoux, 1996). The hormonal changes of pregnancy might convert pup smells from an aversive to a non-aversive or perhaps even rewarding odor. Female nulliparous rats who are made anosmic (Fleming, Vaccarino, Tambosso, & Chee, 1979), undergo the hormonal changes of pregnancy (Numan, 1994), or have amygdala lesions (Fleming, Miceli, & Moretto, 1983; Numan, Numan, & English, 1993), no longer avoid pups and may even exhibit maternal behavior. These data indicate that the amygdala may inhibit maternal behavior in the rat through the olfactory system. In contrast, the amygdala has also been reported to play a role in facilitating maternal behavior in nonhuman primates (Kling & Steklis, 1976). These opposing findings may be explained by studies of sub-regions of the amygdala. In one such study, different regions of the central amygdala have been shown to contain two distinct neuronal populations, through which oxytocin modulates the integration of excitatory information from the basolateral amygdala and cerebral cortex in opposite manners (Huber, Veinante, & Stoop, 2005). Thus, different populations of cells in a small structure such as the amygdala may in fact exert opposing effects on the autonomic nervous system and parenting behavior.
The septal region of the brain may also be important in nest building, orchestrating pup retrieval, and controlling aggression toward pups (Numan & Numan, 1997). For example, nest building in mice is arrested by septal lesions (Slotnick & Nigrosh, 1975), and rodents with septal lesions are also more prone to commit infanticide (Flannelly, Kemble, Blanchard, & Blanchard, 1986; Novakova, Sterc, Kuchar, & Mozes, 1993; Slotnick & Nigrosh, 1975). Moreover, in mice with septal lesions, pup retrieval becomes disorganized (Slotnick & Nigrosh, 1975) such that mothers with septal lesions often drop them and leave them scattered around the cage rather than in the nest.
Maternal behavior regulation by sensation driven thalamocingulate circuits
Several animal studies suggest that the cingulate gyrus and its connected thalamic nuclei (such as dorsomedial, medial pulvinar, midline, and anterior) also play a pivotal role in mammalian maternal behavior (Mesulam, 2000). These structures regulate selective attention through dopamine approach pathways. Cingulate lesions, which in turn cause retrograde degeneration of medial thalamic nuclei, impair maternal behavior in rats and hamsters (MacLean, 1990; Murphy, MacLean, & Hamilton, 1981; Slotnick, 1967; Stamm, 1955). For example, rat and hamster mothers with cingulate lesions (which may include the neighboring midline cortex) often have problems nest building, retrieving pups when separated, actively allowing their pups to nurse, and sustaining pups through weaning (MacLean, 1990; Murphy, MacLean, & Hamilton, 1981; Slotnick, 1967; Stamm, 1955). In fact, the degree of maternal behavior impairment appears to strongly correlate with the degree of accompanying anterior thalamic nuclei degeneration (Slotnick, 1967; Slotnick & Nigrosh, 1975). Further, motivation to care for pups appears to be present but mothering behaviors seem disorganized in a manner that is similar to that produced by septal lesions. Also, electrical stimulation of the anterior cingulate in female rabbits (Aulsebrook & Holland, 1969) can cause oxytocin release, milk ejection, and uterine contractions (Slotnick, 1967; Stamm, 1955). Even more evidence for the cingulate’s involvement in maternal behavior is that the anterior cingulate cortex is rich in opiate receptors (Wise & Herkenham, 1982). In several species, opiates influence maternal retrieval of separated young (Panksepp, Nelson, & Siviy, 1994). On the other hand, some have failed to find altered maternal behavior with cingulate lesions in mice (Slotnick & Nigrosh, 1975); and in the rat, maternal behavior is associated with prominent c-fos labeling in the basal forebrain, but not the cingulate cortex (Lonstein, Simmons, Swann, & Stern, 1998). Thus, it could be that the cingulate is important to organize a range of complex behavior types, of which parenting is one.
Integrative physiology of normal parenting behaviors
While most of the pioneering and systematic work on maternal brain behavior has been performed with rodents, there is a growing body of converging work on the human brain basis of parenting. Given our shared mammalian evolutionary heritage, it makes sense that some of the same bioactive chemicals and structures mediate parenting and social bonding through similar mechanisms across species. For example, human affiliative behaviors have been considered as part of an elaborate reward and stress-sensitive system that requires dopamine and oxytocin, and a host of other neurotransmitters including opiates as well as pituitary and gonadal hormones. It has been proposed that this system can be simplified as domains of sensation, perception, attention, learning and memory (Depue & Morrone-Strupinsky, 2005).
Altered activity of the dopaminergic system has also been associated with a wide range of human diseases and psychopathology. These include drug addiction, attention deficit hyperactivity disorder, obesity, compulsive gambling, and several personality traits (Blum et al., 2000; Comings & Blum, 2000) – arguably all of which involve malfunctioning motivation systems. We suggest that all of these may be associated with adverse early life events. A recent PET study showed that dopamine production in the human brain was associated with reduced self-reported maternal care in childhood (Pruessner, Champagne, Meaney, & Dagher, 2004). Abnormal development of the dopaminergic system may also be associated with differing patterns of adult attachment, with a relative deficit seen in ‘preoccupied’ patterns and an excess in ‘dismissing’ types. This hypothesis is currently being explored using fMRI to explore patterns of parental brain response according to attachment classification (Strathearn, 2007).
As for oxytocin, receptor binding sites measured at autopsy in humans appear in many of the regions previously mentioned as potentially important to rat maternal behavior. These include the preoptic area/hypothalamus region, midbrain and upper pons sites (especially the substantia nigra, central gray regions, and superior colliculus), and lateral septal area (Loup, Tribollet, Dubois-Dauphin, & Dreifuss, 1991; Loup, Tribollet, Dubois-Dauphin, Pizzolato, & Dreifuss, 1989). There are also human oxytocin binding sites in other brain regions including the basal nucleus of Meynert, diagonal band of Broca, and lower pons/medulla/upper spinal cord sites (facial nucleus, nucleus of the solitary tract, spinal trigeminal nucleus, rostral nucleus ambiguus, hypoglossal nucleus, area postrema, and dorsal horn of the upper spinal cord) suggesting an extended range of functions.
Evidence for the importance of stress hormones in parenting includes the work of Fleming and co-workers (Fleming, Steiner, & Corter, 1997), who found that first-time mothers with high levels of circulating cortisol were better able to identify their own infant’s odors. In these same primiparous mothers, the level of affectionate infant contact (affectionate burping, stroking, poking and hugging) by the mother was related to levels of salivary cortisol.
A key question for research on human parenting is which infant stimuli elicit parental thoughts and feelings most potently and meaningfully. MacLean (1990), a pioneer in neuroethological approaches to brain research, hypothesized that the brain’s thalamocingulate division (the cingulate cortex and its connected medially located thalamic nuclei) is important in mammalian mother–infant attachment behavior such as infant crying (a caretaking elicitor in all studied mammals) and a mother’s caretaking response. MacLean (1990) reasoned that the thalamocingulate division is likely involved in parenting behavior and attachment behavior, since it is present in mammals but not in lizard-like reptiles, who, unlike mammals, do not cry, exhibit significant parental care, or even hear well. In fact, lizard-like reptiles are likely to eat their young if they find them. Alligators and crocodiles that provide some maternal care are more evolutionarily related to birds and dinosaurs and have a rudimentary anterior cingulate. Further, lesioning the thalamocortical circuit appears to impair executive control of maternal behavior and produces disorganized pup retrieval, rather than a lack of motivation to respond. MacLean’s evolutionary theories have been a major inspiration in our field, including insights about the importance of the universally present mammalian caretaking cue of infant vocalizations.
Thus far, however, there is not strong evidence for acoustically distinct infant cry types in humans, in the way that hunger and separation cries have been found in animals (Newman, 2003). It has been suggested that human infant cries may function and be characterized rather as graded signals (Soltis, 2004). During pain-induced autonomic nervous system arousal, for example, neural input to the vocal cords increases cry pitch in a graded fashion. Caregivers may use this acoustic information, together with other cues, to guide caregiving behavior. In one study of normal parents, controlled for extraneous cues, 80% of mothers were able to recognize their infants’ cries, as were 45% of fathers at 30 days postpartum (Green & Gustafson, 1983). Serious pathology, on the other hand, results in chronically and severely abnormal cry acoustics. Such abnormal crying may be a proximate cause of infant mal-treatment in circumstances in which parents reduce or withdraw investment from infants with low survival chances. An increase in the amount of crying during the first few months of life is universal in humans, and excessive crying, or colic, represents the upper end of this normal increase. Potential signal functions of excessive crying include manipulation of parents to acquire additional resources, honest signaling of need, and honest signaling of vigor (Soltis, 2004). Manipulation in the context of infant behavior refers to signaling for more resources than might be necessary for survival. Infant cry-care loops may thus be thought of as part of an elaborate, dynamic and interactive communication system that maintains proximity to and elicits care from caregivers (MacLean, 1990; Swain, Mayes, & Leckman, 2004).
Fathers have also been studied for physiological markers of parenting. In one set of studies, Fleming and colleagues found that fathers hearing baby cry stimuli felt more sympathetic and more alert compared to groups who did not hear the cries or to non-fathers who heard the cries, and testosterone and prolactin were key mediators of paternal physiology. Fathers and non-fathers with lower testosterone levels had higher sympathy and/or need to respond to the infant cries than fathers with higher testosterone levels. In addition, fathers hearing the cry stimuli showed a greater percentage increase in testosterone than fathers not hearing the cry stimuli, and both experience and testosterone contributed to the variance in fathers’ affective responses to infant cries. Prolactin levels were higher with paternal alertness and positive response to the cries, and experienced fathers hearing the cries showed a greater percentage increase in prolactin levels compared to first-time fathers or to any group of fathers hearing control stimuli (Fleming, Corter, Stallings, & Steiner, 2002). These results are particularly interesting in light of the convergent findings that men and women have similar stage-specific differences in hormone levels, including higher concentrations of prolactin and cortisol in the period just before the births and lower postnatal concentrations of sex steroids (testosterone or estradiol). Men with more pregnancy symptoms (couvade) and men who were most affected by the infant reactivity test had higher prolactin levels and greater post-test reduction in testosterone. Hormone concentrations were correlated between partners. This pattern of hormonal change in men and other, paternal mammals, and its absence in nonpaternal species, suggests that certain hormones also play key roles in priming males to provide care for their young (Storey, Walsh, Quinton, & Wynne-Edwards, 2000).
Another potentially important maternal behavior concerns the infant carrying hypothesis (Salk, 1960), which is based on the observation that most women (whether they were right- or left-handed) carry their infants with their left arm so that the infant’s head lies against the left breast. Many higher primates appear to do so as well (Sieratzki & Woll, 1996). While it could be true that a major evolutionary force guiding the left-sided infant carrying might be that this places the infant near the mother’s heartbeat, an important effect of placing a child on the left is that the infant lies in the mother’s left visual field with more direct communication to the right hemisphere (Sieratzki & Woll, 1996). Some have taken this to mean that the right cerebral cortex may have a specialized role in human social attachment (Henry, 1993; Horton, 1995; MacLean, 1990; Sieratzki & Woll, 1996). Horton (1995) tested whether the right hemisphere is involved in ‘giving others comfort’, by offering men and women a choice of helping one of two identical teddy bears, one on the right and the other on the left. Each bear was said to be in distress and in urgent need of help. People more often chose to comfort the teddy bear on their left with mothers more so than non-mothers and men. This result was more pronounced among females, especially mothers (Horton, 1995). This was not true for picking up a ball which most people pick up on the right side to throw. Consistent with this are observations of predominantly right-sided brain response in early brain imaging experiments described below.
Brain imaging of human parent–infant relationships
In this section, we present data on the brain basis of human maternal behavior and thoughts, using the high resolution and non-invasive technique of fMRI. This is a brain imaging technique which assays brain activity by measuring blood oxygenation. The differences between oxygenated and deoxygenated hemoglobin provide characteristic magnetic signals that are detected by scanners positioned around the head of each subject, and the signals are localized to millimeter resolution. An important caveat throughout the interpretation fMRI studies is that that brain activity measurements represent an integration of activity over blocks of several seconds. In these studies, auditory and visual baby and control stimuli are presented to parents during these blocks. Brain activity may then be measured and compared between periods of attending to different stimuli to generate maps of the brain indicating differences in brain activity that may be important for one set of thoughts versus another. For example, comparison of brain activity during baby cry vs. control noise experience may yield significant differences in certain brain regions that may then be said to relate to the experience of a baby cry, and so the associated parenting thoughts and behaviors. The experiments to date using baby sound and visual stimuli with brain fMRI are summarized in Tables 3 and 4 respectively. These inclusive reference tables are intended to suggest patterns of response across all studies and stimuli at a glance, to provide a rough model of the brain areas important for human parenting and to stimulate future studies. Parent brain areas of increased activity with baby stimuli are indicated in these tables with ‘ACT’ and a gold background, while areas of decreased activity are indicated by ‘DEACT’ and a blue background. Also indicated are the number of subjects, age of infants at time of scan, type of study (magnet strength and block or event design), and stimuli used in each study. Statistical methods vary across studies, but all findings satisfy the criteria of fixed effects at p < .001, or random effects at p < .05. Each of these studies along with closely related research is detailed in the following sections after a brief orientation to parenting brain circuits.
Table 3.
Human parent brain responses to infant cries. Anatomical brain regions with increased activity (ACT) during infant cry are indicated in gold, and areas of decreased activity (DEACT) are indicated in blue. Empty boxes indicate no significant changes in brain activity with exposure to baby sounds
| Author (year): | Lorberbaum et al. (1999) | Lorberbaum et al. (2002) | Seifritz et al. (2003) | Swain et al. (2003, 2004) | Swain et al. (2003, 2004) | ||
|---|---|---|---|---|---|---|---|
| Number of subjects: | n = 4 | n = 10 | n = 20 | n = 7–14 | n = 7–8 | ||
| Age of infants at time of scan: | 3 weeks – 3.5 years | 1–2 months | <3 years | time 1: 2–4 weeks, time 2: 3–4 months |
time 1: 2–4 weeks, time 2: 3–4 months |
||
| Parental groups: | mothers only | mothers only | mothers + fathers + non-parents |
novice + multiparous mothers |
novice + multiparous fathers |
||
| Type of study: | 1.5T, 30 s blocks, fixed effects |
1.5T, 30 s blocks random effects |
1.5T, 6 s events random effects |
3T, 30 s blocks fixed effects |
3T, 30 s blocks fixed effects |
||
| Baby cry used: | other cry>white noise |
other cry>control noise |
other cry + laugh | own cry >control |
other cry >control |
own cry >control |
other cry >control |
| Septal regions (MPOA/VBNST/ caudate head) |
ACT | ACT | ACT | ||||
| Hypothalamus | ACT | ACT | ACT | ||||
| Thalamus | ACT | ACT | ACT | ||||
| Striatum/Putamen/Nucleus accumbens |
ACT | ACT | ACT | ACT | |||
| Anterior cingulate | ACT | ACT | DEACT | ACT | ACT | ACT | ACT |
| Middle cingulate | ACT | ACT | |||||
| Posterior cingulate | ACT | ||||||
| Amygdala | ACT (cry-rest) | ACT | ACT | ||||
| Lentiform nucleus Globus pallidus | ACT | ACT | ACT | ||||
| Hippocampus | ACT | ACT | |||||
| Midbrain | ACT | ACT | ACT | ACT | |||
| Insula | ACT | ACT | ACT | ACT | ACT | ||
| Orbitofrontal/Inferior frontal gyri | ACT | ACT | ACT | ACT | ACT | ||
| Medial frontal Gyrus | ACT | ACT | DEACT | DEACT | ACT | ||
| Ventral prefrontal cortex | ACT | ACT | |||||
| Temporoparietal cortex | ACT | ACT | ACT | ACT | ACT | ACT | |
| Parahippocamal/Limbic lobe | ACT | ACT | |||||
| Occipital cortex | Not examined | Not examined | ACT | ACT | ACT | ||
| Fusiform gyrus | ACT | ACT | ACT | ACT | |||
| Temporal/Auditory cortex | ACT | ACT | ACT | ||||
| Cerebellum | Not examined | Not examined | ACT | ACT | ACT | ||
Glossary for Table 3: Activations and deactivations, measured by functional magnetic resonance imaging, satisfied significance criteria of random effects analysis at p < .05 or fixed effects analysis at p < .001 at a minimum; T = Tesla (unit of magnetic field strength); blocks = periods of stimulus exposure and fMRI data acquisition; events = brief exposures to infant stimuli during fMRI experiments; other cry = ciy of an unfamiliar baby; own cry = cry of the subject’s own baby; MPOA = medial preoptic area; BNST = bed nucleus of the stria terminalis.
Table 4.
Human parent brain responses to infant pictures. Anatomical brain regions with increased activity (ACT) during infant cry are indicated in gold, and areas of decreased activity (DEACT) are indicated in blue. Empty boxes indicate no significant change in brain activity with exposure to baby pictures
| Author (year): | Bartels & Zeki (2004) |
Nitschke et al. (2004) | Ranote et al. (2004) | Strathearn & McClure (2002) |
Swain et al. (2003, 2004, 2005) | Swain et al. (2003, 2004, 2005) | ||
|---|---|---|---|---|---|---|---|---|
| Number of subjects: | n = 19 | n = 6 | n = 10 | n = 8 | n = 9–14 | n = 4–9 | ||
| Age of infants at time of scan: | 9 months–3.5 years | 2–4 months | 4–8 months | 3–18 months | time 1: 2–4 weeks time 2: 3–4 months |
time 1: 2–4 weeks time 2: 3–4 months |
||
| Parental groups: | mothers only | mothers only | mothers only | mothers only | novice+multiparous mothers |
novice+multiparous fathers |
||
| Type of study: | 2T, 15 s blocks random effects |
1.5T, 30 s blocks fixed effects |
1.5T, 20–40 s blocks random effec |
3T, 6 s events fixed effects |
3T, 30 s blocks fixed effects |
3T, 30 s blocks fixed effects |
||
| Baby picture used: | own>other | own>other | own>other videos | own>other | own >other |
baby >house |
own >other |
baby >house |
| Septal regions (MPOA/VBNST/ caudate head) |
ACT | |||||||
| Hypothalamus | ACT | |||||||
| Thalamus | ACT | ACT | ACT | ACT | ACT | ACT | ACT | |
| Striatum/putamen/nucleus accumbens |
ACT | ACT | ACT | ACT | ||||
| Anterior cingulate | ACT | ACT | ACT | ACT | ACT | |||
| Middle cingulate | ACT | ACT | ACT | ACT | ||||
| Posterior cingulate | DEACT | |||||||
| Amygdala | DEACT | ACT | ||||||
| Lentiform nucleus Globus pallidus |
ACT | ACT | ACT | |||||
| Hippocampus | ACT | ACT | ||||||
| Midbrain | ACT | ACT | ACT | ACT | ACT | |||
| Insula | ACT | |||||||
| Orbitofrontal/inferior frontal | ACT | ACT | ACT | ACT | ACT | ACT | ||
| Medial frontal gyrus | DEACT | DEACT | ACT | ACT | ||||
| Ventral prefrontal | ACT | |||||||
| Temporoparietal | DEACT | ACT | ACT | ACT | ACT | |||
| Parahippocamal/Limbic lobe | ACT | |||||||
| Occipital cortex | ACT | ACT | ACT | ACT | ACT | ACT | ACT | |
| Fusiform gyrus | ACT | ACT | ACT | ACT | ACT | ACT | ACT | |
| Temporal/Auditory cortex | ACT | |||||||
| Cerebellum | ACT | ACT | ACT | ACT | ACT | |||
Glossary for Table 4: Activations and deactivations, measured by functional magnetic resonance imaging, satisfied significance criteria of random effects analysis at p < .05 or fixed effects analysis at p < .001 at a minimum; T = Tesla (unit of magnetic field strength); blocks = periods of stimulus exposure and fMRI data acquisition; events = brief exposures to infant stimuli during fMRI experiments; other cry = cry of an unfamiliar baby; own cry = cry of the subject’s own baby; MPOA = medial preoptic area; BNST = bed nucleus of the stria terminalis.
First, based on animal studies of parenting behaviors in animals reviewed in previous sections, we expect that human parenting brain responses will include motivation circuits of the midbrain and basal forebrain, emotion control circuits involving the amygdala and other limbic regions and sensation driven emotion and decision-making thalamocingulate circuits (Figure 2). In humans, we would also expect that regions involved in the appraisal of parenting context and memory would require hippocampal and parahippocampal circuits. Finally, we suppose that higher order emotion and cognition areas facilitate parental empathy and caregiving for the infant, especially in humans. Empathy in general requires forming a model of another’s mind that predicts their behavior and influences emotions (Baron-Cohen & Wheelwright, 2004). Parental empathy toward an infant would require the understanding and predicting of one’s infant’s mental states and behaviors as well as the experiencing of appropriate emotions. Candidate brain circuits that could support parental empathy include a variety of cortical regions including inferior frontal, premotor, insular, temporo-parietal and cingulate cortices (Decety & Grezes, 2006; Saxe, 2006a).
In order to explicitly study the biological bases of human attachment, brain activity can be measured during tasks designed to activate the underlying systems. An example of this innovative approach used the projective measure of broad aspects of adult attachment (the adult attachment projective) during brain scanning (Buchheim et al., 2006). In this pilot study of eleven women, line drawings meant to activate the attachment system (illness, solitude, separation and abuse) were presented to subjects during brain imaging. The authors reported that subjects with organized compared to disorganized attachment patterns showed increased activity in the right amygdala, left hippocampus and right inferior frontal gyrus – areas hypothesized to be important in the attachment system. Allied research on the brain basis of thinking about other minds (mentalization) is also beginning to dissect the brain basis of complex social emotional thinking (Pelphrey, Morris, Michelich, Allison, & McCarthy, 2005; Saxe, 2006b), and this research suggests that specific regions in the medial prefrontal cortex and temporal cortex mediate aspects of emotional empathy and collaborative behaviors. In the following section, we describe attempts to specifically understand the brain basis of parental attachment by presenting emotionally charged infant stimuli during brain imaging. We hypothesize that ‘parenting’ brain circuits, which are activated by baby stimuli, share much with circuits that regulate other social attachments, and might be even more active in parents during the early postpartum than at other times of life.
Parental brains and baby cry stimuli
The first experiments using the pioneering approach of studying brain activity in mothers while they listen to infant cries was done by Lorberbaum and colleagues. Building on the thalamocingulate theory of maternal behavior in animals developed by MacLean (1990), they initially predicted that baby cries would selectively activate cingulate and thalamus in mothers (ranging from 3 weeks to 3.5 years postpartum) exposed to an audio-taped 30-second standard baby cry, not from their own infant (Lorberbaum et al., 1999), although they later expanded their hypotheses to include the MPOA/BNST and its connections including its indirect connections to motivational circuitry (Lorberbaum et al., 2002). In their first study (Lorberbaum et al., 1999), a group of 4 mothers were studied for their response to 30 seconds of a standard cry compared with 30 seconds of a control sound consisting of white noise that was shaped to the temporal pattern and amplitude of the cry. With cry versus control sound, the 4 mothers showed increased activity in the subgenual anterior cingulate and right mesial prefrontal/orbitofrontal using a fixed effects data analysis. In a methodologically more stringent follow-up study, brain activity was measured in 10 healthy, breastfeeding, first-time mothers with infants 1–2 months old. While they listened to standard infant cry recordings compared to similarly cry-shaped control sounds, brain activity in many candidate parenting centers was revealed using a random effects imaging analysis, in which posterior regions were not imaged (Lorberbaum et al., 2002). Activated regions included the anterior and posterior cingulate, thalamus, midbrain, hypothalalamus, septal regions, dorsal and ventral striatum, medial prefrontal cortex, right orbitofrontal/insula/temporal polar cortex region, and right lateral temporal cortex and fusiform gyrus. Additionally, when cry response was compared with the inter-stimulus rest periods, instead of the control sound (which some mothers judged to be aversive), the amygdala was active. The fusiform gyrus activity is interesting because this structure has been implicated in human face and voice recognition along with related social cognitions that might be impaired in autism (Schultz, 2005).
These initial studies fit with the regions thought to be involved in animal parenting behavior. In this study, brain activations occurred for these cries even though they did not originate from the parent’s own infant and the control sounds were emotionally negative (sounded like static on the television). Perhaps then, this activity might partly represent increased attention to cries compared to control sounds, rather than ‘parenting’ responses per se. This is suggested by related research on auditory event-related brain potentials (ERPs). For example, Tzourio and colleagues showed that auditory attention requires anterior cingulate and temporal cortices (Tzourio et al., 1997). In another study, women responded significantly more to a baby cry than to an emotionally neutral vocalization in these regions (Purhonen, Paakkonen, Ypparila, Lehtonen, & Karhu, 2001) and in a third study, mothers responded more than control women to infant cries (Purhonen et al., 2001). These results suggest a general increase in alertness and arousal for baby signals and for mothers in particular, perhaps assisting them in their ability to be continuously alert or be attuned to the infant’s needs. It is not clear yet how much the N100 signal represents general arousal versus selective parenting attention per se. In the end, the argument here might be merely semantic as we would expect attention and arousal to be important elements of response to infant crying. Support for this view might be found in studying parents who abuse or neglect their children and might be having difficulty sustaining or appropriately modulating their attention and arousal in response to infant cries. In one such physiological study of parents who maltreat their children (Frodi & Lamb, 1980), audiovisual infant stimuli elicited exaggerated physiological responses. Indeed, infant crying is a proximate risk factor for infanticide (Soltis, 2004), perhaps due to parents’ failure to regulate their arousal. Future work may shed light on this question: What is unique about a healthy parent’s brain compared to a parent at risk for neglect and abuse? One might think that healthy parents would attend to infant cues and respond appropriately, but not be so aroused as to make an impulsive, disinhibited decision. We hypothesize that this capacity to assume a caretaking role in the face of ostensibly aversive stimuli may have measurable brain activity signals.
Hypothesizing that gender and experience would affect the neural responses to baby sounds including baby cry and laughter, Seifritz and colleagues (Seifritz et al., 2003) studied four groups: mothers and fathers of children under age 3, and non-parent males and females, with 10 subjects in each group. They used an event-related fMRI design, which measures brain response to brief 6-s events. Over the entire sample, intensity-matched baby sounds of crying and laughing compared to ‘neutral’ sounds (white noise pulsed at 5-Hz with an averaged frequency spectrum similar to the infant vocalizations) produced more brain activity in bilateral temporal regions. These regions might be important for hearing processes (Heshyl’s gyrus and temporal poles), processing human vocalizations, and empathic emotion processing (see below). They also reported that women, as a group including parents and non-parents (but not males), had a decrease in activity in response to both baby cry and laughter in the subgenual anterior cingulate cortex. This finding is, however, contrary to the other studies (Lorberbaum et al., 1999, 2002; Swain et al., 2003, 2004; Swain, Leckman, Mayes, Feldman, & Schultz, 2005), which highlights the importance of the choice of stimuli in these experiments as well as not viewing the anterior cingulate as one structure without subdivisons. Perhaps also, 6-second vs. 30-second stimuli have very different meanings to new parents and there may be non-linear or biphasic anterior cingulate responses. Finally, within-group analyses showed that parents activated more to infant crying than laughing in the right amygdala, while non-parent response was greater for infant laughing then crying (Seifritz et al., 2003). These within-group results suggest a potential change in amygdala function with being a parent, although there was no direct comparison of parents to non-parents. Inclusion of psychological measures of parenting parameters will make future studies more practically insightful. These data do, however, represent the first attempts to extend the previous work on parental brain circuits to include gender and experience-dependant aspects of human parenting.
Relevant to parent responses to infant sounds, other fMRI research has been exploring brain responses to emotionally laden human vocalizations, such as having non-parents listen to adult cries and laughter. Some of the brain responses overlap with those found in the parent–infant studies. To reveal emotion circuits, subjects were asked to self-induce happy or sad emotions to correspond with the laughing or crying stimuli respectively. For pitch detection, subjects were asked to detect pitch shifts. Both conditions led to bilateral activation of the amygdala, insula and auditory cortex with a right-hemisphere advantage in the amygdala, and larger activation during laughing than crying in the auditory cortex with a slight right-hemisphere advantage for laughing, both likely due to acoustic stimulus features. These results suggest that certain brain regions, including the amygdala, activate to emotionally meaningful sounds like laughing and crying independent of the emotional involvement, suggesting the pattern recognition aspect of these sounds is crucial for this activation and that emotional valence might be represented elsewhere in the brain (Sander, Brechmann, & Scheich, 2003). Frontal areas may be good candidates as suggested by more recent work by Sander and colleagues, in which they found a correlation between activity in the orbitofrontal cortex in response to angry utterances and an emotional sensitivity scale across a group of young adults (Sander et al., 2005).
In an attempt to further this research on the neurocircuitry underlying emotionally laden parenting behavior and parent–infant attachment, the authors and their colleagues have been gathering datasets on groups of new parents across a range of experience, temperament and parent–infant interaction styles using own baby cries. For example, Swain and colleagues (Swain et al., 2003) reported on a comprehensive interview and self-report assessment and fMRI brain imaging (using own infant cry stimuli) of postpartum mothers and fathers, across experience from novice to multiple pregnancy families. In this design, inspired by Lorberbaum and colleagues (described above), parents underwent brain fMRI during 30-second blocks of infant cries generated by their own infant as well as a ‘standard’ cry and control noises matched for pattern and intensity. In addition, they added a longitudinal component with scans and interviews done at 2 time points: 2–4 weeks and 12–16 weeks postpartum. These times were chosen to coincide with the transforming experience of having a baby known to be associated with increased tendency for parents to be highly preoccupied in the early postpartum (Leckman et al., 1999). They hypothesized that parental responses to own baby cries would include specific activations in thalamo–cortico–basal ganglia circuits believed to be involved in human ritualistic and obsessive-compulsive thoughts and behaviors (Baxter, 2003; Leckman et al., 2004). Swain and colleagues also reasoned that emotional alarm, arousal and salience detection centers including amygdala, hippocampus and insula (Britton et al., in press; LeDoux, 2003) would be activated by baby cry stimuli. The experimental block design was used in order to give parents a chance to reflect on their experience of parenting and, according to our hypothesis, become more preoccupied with their infants’ well-being and safety. In a group of first-time mothers (n = 9) at 2–4 weeks postpartum, own baby cry stimuli compared with other baby cry regions of relative activation included midbrain, basal ganglia, cingulate, amygdala and insula (Swain et al., 2003). Preliminary analysis of the parenting interview data shows that mothers were significantly more pre-occupied than fathers, which was reflected in the relative lack of activation for fathers in the amygdala and basal ganglia (Swain et al., 2004). In the group of primiparous mothers, given the same stimuli at 3–4 months postpartum, amygdala and insular activations were not evident; and instead, medial prefrontal cortical and hypothalamic (hormonal control) regions were active (Swain et al., 2004). This may reflect a change in regional brain responses as the parent–infant relationship develops, and the mother learns to associate her infant cries more with social behaviors and habit systems, and less with alarm and anxiety. Manuscripts are in preparation to include data grouped across different variables, and include correlations between brain activity in regions of interest with measures of parental preoccupations and parent–infant behaviors.
Parental brains and baby visual stimuli
Several groups are using baby visual stimuli to activate parental brain circuits (Bartels & Zeki, 2004b; Leibenluft, Gobbini, Harrison, & Haxby, 2004; Nitschke et al., 2004; Ranote et al., 2004; Strathearn, 2002; Strathearn, Li, & Montague, 2005; Swain et al., 2003) with a variety of designs, parent populations and infant age.
Hypothesizing that reward and emotion circuits, which are important for aspects of romantic love (Bartels & Zeki, 2000), might also be involved in maternal love, Bartels and Zeki used photographs of own, familiar and unfamiliar infants (9 months to 3.5 years of age) as stimuli for parental brain circuits (Bartels & Zeki, 2004b). They measured brain activity in 20 healthy mothers while viewing still-face photographs of their own child compared to age-matched photographs of other children. There was increased activity in the midbrain (periaqueductal gray and substantia nigra regions), dorsal and ventral striatum, thalamus, left insula, orbitofrontal cortex, sub-, pre-, and supra-genual anterior cingulate, and superior medial prefrontal cortex. There were also increases in the cerebellum, left fusiform, and left occipital cortex, but decreases in the left amygdala. Bartels and Zeki also compared mother brain responses of own child vs. familiar child to the best friend vs. familiar friend in order to control for familiarity and positive affect, and they argue that responses were unique to the own child stimuli. They suggested that parent–infant attachment may be regulated by a push–pull mechanism that selectively activates motivation and reward systems, while at the same time suppressing circuits responsible for critical social assessment and negative emotions (Bartels & Zeki, 2004b).
Using a similar approach, but focusing on early stage romantic love, attachment and mate selection (Fisher et al., 2002; Fisher, Aron, Mashek, Li, & Brown, 2002), Aron, Fisher and colleagues conducted fMRI studies of brain response to photographs of beloved and familiar individuals (Aron et al., 2005; Fisher, Aron, & Brown, 2005). They replicated the findings of Bartels and Zeki (Bartels & Zeki, 2000) and also reported activations specific to the beloved in the midbrain (right ventral tegmental area) and the caudate nucleus (right postero-dorsal body and medial parts). The activation in these dopamine-rich areas associated with mammalian reward and motivation were correlated with facial attractiveness scores. Further, activation in the right anteromedial caudate was correlated with questionnaire scores that quantified intensity of romantic passion for the individuals whose photographs were used as stimuli. Also, activity in the left insula-putamen-globus pallidus correlated with trait affect intensity, wheras activity in limbic cortical regions, including insula, cingulate parietal, inferior temporal and middle temporal cortex was correlated with the length of time in love. Taken together, these studies suggest that romantic love uses subcortical reward and motivation systems to focus on a specific individual, while limbic cortical regions process individual emotion factors. The inverse approach to attachment circuits was taken by Najib, Lorberbaum, Kose, and colleagues (Najib, Lorberbaum, Kose, Bohning, & George, 2004). In this study of women whose romantic relationship had ended within the 4 months preceding the experiment, they found that acute grief related to the loss of a romantic attachment figure modulated activity in some of the same areas implicated in social attachment and parenting. This included activations in temporal cortex, insula and prefrontal cortex. In contrast to the romance-studies which found activations in the anterior cingulate, they also found that romantic grief was consistently associated with deactivations in this region. Finally, they found that activity in the anterior cingulate, insula, and amygdala was inversely related to the grief inventory score.
Returning to the focus of parent–infant relations, Swain and colleagues presented blocks of own and other baby photographs (aged 0–2 weeks) to groups of mothers and fathers with similar block design for pictures as was used for cries (Swain et al., 2003). Photographs were chosen by the parents themselves in order to provide the most potent and ethologically appropriate signals to evoke their own parenting emotions involving motivation and reward. In these studies, there were also activations in frontal and thalamo-cortical circuits to own vs. other baby pictures at 2–4 weeks postpartum. Specific characterization of these regions according to differences by gender, experience and postpartum time of assessment are under way.
In a related study using photographs of much older children (5–12 years), mothers viewed pictures of their own and other children’s faces during brain fMRI measurements, while being asked to press a button to indicate identity (Leibenluft, Gobbini, Harrison, & Haxby, 2004). Some social cognition regions that were not activated in the Bartels and Zeki study (2004b) were significantly activated in this study, including the anterior paracingulate, posterior cingulate and the superior temporal sulcus. This may be explained by the use of much older children, which might involve a different set of circuits relevant to those particular relationships. It may also be that the cognitive task interacts with affective responses to face images in some way (Gray, 2001). Differences in child photo affective facial expressions (happy vs. neutral vs. sad) may also constitute a confounding factor. Another possible reason for differences between studies is that sample populations and their relationships likely differ in important ways. Although all of the studies were of ‘normative’ parent populations, most studies only screened for clinical psychiatric disease. It appears that different populations may process infant cues in different ways. Perhaps studies involving more specific tasks and correlations between brain activations and relationship-specific variables will be able to tease apart the particular roles of different brain regions in different aspects of those relationships.
Across auditory and visual sensory stimuli thus far used in parent imaging studies, a convergence of brain responses is emerging to include many regions. Although baby cries may be aversive compared with baby pictures, considerable overlap in activation of motivation, arousal and reward circuits may not be too surprising since, for example, parents are still generally compelled to approach a crying infant – perhaps in anticipation of reward. It also makes sense that common social cognition circuits would be involved. In particular, it is interesting to consider the common activation of the precuneus cortex in parents responding to own child stimuli across visual and auditory stimuli (Leibenluft, Gobbini, Harrison, & Haxby, 2004; Swain, Leckman, Mayes, Feldman, & Schultz, 2005). This fits with the rapidly expanding literature on the importance of this region for episodic memory retrieval necessary for recognizing familiar auditory and visual social stimuli, as well as self-referential mental imagery (Cavanna & Trimble, 2006; Gobbini & Haxby, in press; Lundstrom, Ingvar, & Petersson, 2005; Lundstrom et al., 2003; Nakamura et al., 2001; Todorov, Gobbini, Evans, & Haxby, in press).
In another study focusing on parents’ brains using visual stimuli, Nitschke and colleagues studied six healthy, primiparous mothers’ brains at 2–4 months postpartum as they viewed smiling pictures of their own and unfamiliar infants. They reported orbitofrontal cortical activations that correlated positively with pleasant mood ratings. In contrast, areas of visual cortex that also discriminated between own and unfamiliar infants were unrelated to mood ratings (Nitschke et al., 2004). Perhaps, as they suggest, activity in the orbitofrontal cortex – which may vary across individuals – is involved with high order dimensions of maternal attachment. Perhaps the complex aspects of parenting may be quantified using fMRI of frontal brain areas to help predict the risks of mood problems in parents.
With the innovative and perhaps more realistic and ethologically appropriate use of videotape infant stimuli, Ranote and colleagues conducted a similar experiment (Ranote et al., 2004). In their study, 10 healthy mothers viewed alternating 40-second blocks of their own infant’s video, a neutral video, and an unknown infant. For these women, there was significant activation in the ‘own’ versus ‘unknown’ infant comparison in the left amygdala and temporal pole. They interpreted this circuit as regulating emotion and theory-of-mind regions relating to the ability to predict and explain other people’s behaviors. Certainly, this fits with fMRI experiments on biological motion, which activate similar regions (Morris, Pelphrey, & McCarthy, 2005). It is important to note that all of these visual paradigms used to examine differences between one’s own infant and unfamiliar infants employ a complex set of brain systems necessary for sensory perception, identification, and emotional response. Yet, it now appears from a number of studies that despite the multisensory complexities of audiovisual stimuli, meaningful analysis of fMRI data is possible. For example, there seems to be a striking inter-subject synchronization among emotion regulating brain areas responding to audiovisual cues during observation of the same scenes of an emotionally powerful movie (Hasson, Nir, Levy, Fuhrmann, & Malach, 2004). Also, the intensity with which subjects perceive different features in a movie (color, faces, language, and human bodies) was correlated with activity in separate brain areas (Bartels & Zeki, 2004a). Finally, regional activity between brain areas that are known to be anatomically connected has been shown to be simultaneously active during movie viewing (Bartels & Zeki, 2005). This work suggests the use of movies as more naturalistic baby stimuli for parents may also be used to develop a functional architecture of brain parenting brain systems. Perhaps related decision-making can also be studied with interactive stimuli in future parent–infant brain imaging work.
Finally, Strathearn and colleagues have also been studying healthy mother–infant dyads using fMRI to examine maternal brain regions activated in response to visual infant facial cues of varying affect (smiling, neutral and crying). They have completed a pilot study of eight healthy right-handed mothers, without a history of psychiatric impairment or child maltreatment along with their infants aged between 3 and 8 months. They assessed serum oxytocin levels sequentially from the mothers during a standardized period of mother–infant interaction, during which they acquired infants’ facial expression videotapes. Maternal brain activity was then assayed with fMRI in response to 6-second exposures to the facial images of their own infant compared with familiar and unknown infant facial images (Strathearn, 2002). Areas of significant activation (uncorrected p < .005) unique to own infant viewing included brain reward areas with dopaminergic projections (ventral striatum, thalamus and nucleus accumbens), areas containing oxytocin projections (amygdala, bed nucleus of the stria terminalis and hippocampus), the fusiform gyrus (involved in face processing), and bilateral hippocampi (involved in episodic memory processing). Further, a positive, but non-significant trend in this small sample was seen in serum oxytocin concentration before and after mother–infant interaction (prior to scanning), suggesting a possible correlation between brain activation and peripheral affiliative hormone production. A further study, which was limited to the presentation of crying infant faces, revealed activation of the anterior cingulate and insula bilaterally (Strathearn, Li, & Montague, 2005).
Careful use of a variety of baby stimuli to activate parent brains, along with correlations of parental brain activity with psychometric parameters, will help in the understanding of these circuits. It may also be helpful to include comprehensive measurements of parent physiology during infant response. In addition to understanding normal parental behavior, this field promises to elucidate abnormalities of parental circuitry that may be manifest in postpartum depression and anxiety. Such understanding may suggest optimal detection and treatment strategies for these conditions that have profound deleterious effects on the quality of parent–infant interactions, and the subsequent long-term health risks and resiliencies of infants. These studies will also inform our understanding of social circuits important for empathy across a range of relationships.
The neurobiology of empathy and parenting
Empathy, defined as appropriate perception, experience and response to another’s emotion, is especially relevant to parenting in which the infant’s needs are great, yet most communication is exclusively non-verbal. The growing field of cognitive neuroscience, propelled by modern brain imaging techniques, is revealing networks of brain activity relating to empathy and emotional mirroring (Gallese, Keysers, & Rizzolatti, 2004) that seem to overlap significantly with parenting brain responses reviewed in this paper, and relevant to the brain basis of social cognition. Two of these overlapping regions are the cingulate and insular cortices. Indeed, empathy has become one of the central interests of psychodynamic clinicians, particularly since the writings of Kohut (Kohut, 1982), and we are now in a position to explore the neuroanatomy.
In one fascinating study, focusing on the neuroanatomy of empathy using fMRI techniques, Singer and colleagues measured brain activity while volunteers experienced a painful stimulus or observed a signal indicating that their loved one (‘other’), present in the same room, had received a similar pain stimulus (Singer et al., 2004). They found a separation of circuits responding to the sensory-discriminitative components of pain from the autonomic-affective aspects. Specifically, posterior insula, the sensorimotor cortex, and the caudal anterior cingulate, brainstem and cerebellum were active while receiving pain stimuli, yet for the emotional aspects of experiencing the pain of a loved one, the rostral anterior cingulate and anterior insula were specifically active. Such decoupled representations, which may even be independent of the sensory inputs of the outside world, have been postulated to be necessary for our empathic abilities to mentalize, that is, to understand the thoughts, beliefs, and intentions of others (Frith & Frith, 2003). It may well be that humans use separate circuits to decouple representations of the external world to understand physical properties and assess personal emotional values. This framework may be of great importance to those studying the brain substrates of relationships, as well as traumatic stress disorder, dissociation, and our imagination – which may occur without any real sensory experience.
In another relevant study of the cingulate in mediating the brain basis of social behavior, Eisenberger and colleagues utilized virtual reality to simulate shunning. In this study (Eisenberger, Lieberman, & Williams, 2003), the subject is involved in a virtual game of Cyberball which includes three players. Suddenly, the subject player is excluded from the virtual game and there is a rapid change in the anterior cingulate cortex. Perhaps the cingulate mediates the separation/attachment system, which may be so important to parenting, the development of the individual and in the work of the psychoanalyst. Thus, in addition to registering pain, anterior cingulate may also be an important circuit in thinking about a range of emotional signals (pain of oneself or social pain such as in witnessing the pain of a loved one, social rejection, or stimuli of one’s child or romantic love) in order to shift attention, make decisions, recruit memory, regulate mood, or direct behavior.
The insula has also been raised as an important center for integrating emotional information (Carr, Iacoboni, Dubeau, Mazziotta, & Lenzi, 2003) with connections to mirror areas in the posterior parietal, inferior frontal, and superior temporal cortices also of interest. In one study subjects were shown pictures of standard emotional faces (happy, sad, angry, surprised, disgusted, and afraid) and fMRI was used to measure responses to two behavioral tasks: (i) mere observation and (ii) observation as well as internal simulation of the emotion observed. As expected, imitation produced greater activity in frontotemporal areas in the mirror network, including the premotor face area, the dorsal pars opercularis of the inferior frontal cortex, and the superior temporal sulcus. Imitation also produced greater activity in the right anterior insula and right amygdala. This is particularly intriguing in light of evidence that the anterior insula responds to pleasant ‘caress-like touch’ (Olausson et al., 2002) and that the insula plays a crucial role in emotional and interpersonal interaction in health and mental illness such as autism (Dapretto et al., 2006). A further confirmation of the insula’s role in emotion recognition comes from the study of patients with strokes. Stroke patients with insular lesions showed a significantly greater deficit in emotion recognition than other stroke patients (Bodini, Iacoboni, & Lenzi, 2004). We speculate that cingulate and insula will continue to emerge as key areas of importance during the transformations that are typical in the initial formation of a new family. Perhaps studies of high-risk families may fail to show this pattern of activation, while early intervention programs shown to have beneficial long-term effects on child development (Olds et al., 2004) will be associated with changes in the activation patterns seen in these limbic cortical regions.
Conclusions and critical summary
Forming strong interpersonal bonds involves understanding the needs of the other, providing care and protection, and a preoccupation with the interests and wants of the other. The human transition to parenthood involves a set of highly conserved behaviors and mental states, reflecting both genetic endowment and early life experience – including the intrauterine environment not covered in this review. Indeed, while we have focused on parenting, there are many other forms of interpersonal relationship – adoption, foster care, step-parenting, teaching, mentoring, grandparenting as well as friendship and romantic love – each involving similar genetic, neurobiological and experiential systems that have the potential to inform clinical practice, particularly early intervention programs for high-risk expectant parents. To paraphrase Winnicott (1960), ‘good enough’ genes combined with good enough parental care ensure positive outcomes in childhood and beyond.
Unfortunately, ‘good enough’ circumstances are often not available. Each year in the United States, over 900,000 children become victims of abuse or neglect, with the biological mother identified as a perpetrator in two-thirds of these cases (U.S. Dept. of Health and Human Services, 2001, 2003). Abuse and neglect perpetrated by a child’s biological mother represents a fundamental breakdown in this important attachment relationship, resulting in serious long-term consequences for the offspring. Accumulating evidence from basic, clinical and epidemiological research indicates that the mother–infant relationship may be a critical target in optimizing developmental outcomes and preventing child maltreatment (Olds et al., 1997; Sanchez, Ladd, & Plotsky, 2001). Measures of ‘primary parental preoccupations’ will be useful in future early intervention programs as an index of change within a key domain of functioning. Viewing parenting as an interaction among genes, past parenting, current experience, psychological state, neurobiological systems, and environmental constraints brings many disciplines to the study of parenting. Future multidisciplinary studies should permit the examination of how successful early intervention programs influence brain development, problem-solving abilities, stress response, as well as later parenting ability and vulnerability to psychopathology. This may have far-reaching consequences for human mental health. In fact, we expect that with a better understanding of the neurobiological processes underlying this reciprocal attachment relationship, we will be better able to understand – and ultimately help to prevent – child abuse and neglect.
Functional MRI experiments on parenting using baby stimuli are just beginning to make a meaningful contribution. This selective review of the physiology of parenting across species predicts many brain areas that are likely important in regulating human parenting. For this review, virtually all of the studies involving infant stimuli to study parent brains with fMRI are summarized and contrasted in Table 3 and 4 (baby cry stimuli), (baby picture stimuli). So far, it appears that a set of brain circuits of parental response to baby stimuli, whether picture or cry, is emerging. This appears to center on the cingulate with feedback loops involving midbrain, basal ganglia regions and thalamus for motivation and reward. More complex planning and social emotional/empathy responses may involve frontal, insular, fusiform and occipital areas. Other important aspects of parenting may be contributed by context and memory processing regions including the hippocampus, parahippocampus and amygdala. Clearly, baby pictures and cries can be used to selectively activate brain circuits related to arousal, mood, and social and habitual behaviors. However, different groups have used a mixture of stimuli including baby cries, laughter and child pictures of very different ages and different facial affect and experience. A clearer picture of the specificity of different brain areas may emerge as brain responses in these areas are linked to specific aspects of parenting, by adding sophisticated interviews, naturalistic assessments of parent–infant interaction and bonding.
This review is an attempt to synthesize our current understanding of parent–infant bonding, largely from the perspective of the parent’s brain physiology. The parent–infant bond, so central to the human condition, may also determine risks for mood and anxiety disorders, and potential for resiliency and protection against the development of psychopathology later in life, not to mention the far-reaching aspects of human attachment across individual behaviors and between cultures. Efforts to characterize this reciprocal interaction between caregiver and infant and to assess its impact have provided a powerful theoretical and empirical framework in the fields of social and emotional development.
Future directions
Likely, the stimuli and populations will be expanded and refined in parental brain research to include the use of more movie stimuli and the different sensory systems such as the olfactory system. This will require careful consideration and study of how these patterns of brain activation may differ between attachment groups. Do mothers with insecure patterns of attachment respond differently to their infant cues? Are neglecting mothers unresponsive to these cues or do they fail to receive reward signals in the brain? Longitudinal research designs may help in this regard. In addition, it will be important to clarify the role of different neuroendocrine pathways and different genetic variations in mediating parenting brain activations.
A helpful approach to these questions will include systematic studies of well-characterized but different populations of parents using a variety of infant stimuli paradigms and psychometric tools. As in other areas of cognitive neuroscience, there will be debates about whether to use more ethologically sound but poorly controlled versus more tightly controlled, but less generalizable stimuli. Both types of experiment will be needed to tease apart the basic apparatus of baby responsiveness and bond formations as well as the parts of the circuit that are actually at work in normal day-to-day-parenting. This work will also require joint study of parents and infants to understand how their interactions contribute to their bond and infant outcomes.
In the near future, we expect that differences in parental response patterns will be reported in specific clinical populations, such as those with postpartum depression and substance abuse. This may lead to future assessments of parent mental health risk and resilience profiles using standardized imaging techniques and to improvements in the detection, treatment and prevention of mental illness that interferes with parenting.
Acknowledgements
The authors would like to acknowledge the generous support of colleagues, research assistants and research participants at our respective institutions:
JES was supported by grants from the Institute for Research on Unlimited Love (unlimitedloveinstitute.org), the National Alliance for Research on Schizophrenia and Depression (narsad.org), the Yale Center for Risk, Resilience and Recovery, and Associates of the Yale Child Study Center. Dr. Swain would especially like to acknowledge the mentorship of Drs. James F. Leckman, Linda C. Mayes, and Robert T. Schultz.
JPL was supported by the child neglect consortium RFA grant, NINDS R01 NS40259–01, NICHD R-03 HD49422–01, the Center for Advanced Imaging Research (CAIR) at the Medical University of South Carolina, the National Alliance for Research on Schizophrenia and Depression (narsad.org), and Penn State’s Child Youth and Family Consortium. Dr. Lorberbaum would especially like to acknowledge the ideas of Drs. Paul Maclean and Mark S. George in getting started in this line of research.
SK was supported by the child neglect consortium RFA grant R-01 NINDS R01 NS40259–01, and the National Alliance for Research on Schizophrenia and Depression (narsad.org)
LS was supported by grants from the National Institute of Child Health and Human Development (K23 HD043097) and the Baylor CHRC: Pediatrics Mentored Research Program (K12 HD41648), and the South Central MIRECC.
We gratefully acknowledge that Figure 1 and portions of the text were reproduced with permission from the book, Developmental Science and Psychoanalysis: Integration and Innovation, © 2007 Linda Mayes, Peter Fonagy, Mary Target, London, UK. Published by Karnac, London, UK.
Abbreviation
- fMRI
functional magnetic resonance imaging
References
- Ainsworth MD, Bell SM. Attachment, exploration, and separation: Illustrated by the behavior of one-year-olds in a strange situation. Child Development. 1970;41:49–67. [PubMed] [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. Journal of Neurophysiology. 2005;94:327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Aulsebrook LH, Holland RC. Central regulation of oxytocin release with and without vasopressin release. American Journal of Physiology. 1969;216:818–829. doi: 10.1152/ajplegacy.1969.216.4.818. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S. The empathy quotient: An investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. Journal of Autism and Developmental Disorders. 2004;34:163–175. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural basis of romantic love. NeuroReport. 2000;11:3829–3834. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. Functional brain mapping during free viewing of natural scenes. Human Brain Mapping. 2004a;21:75–85. doi: 10.1002/hbm.10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. NeuroImage. 2004b;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. Brain dynamics during natural viewing conditions—a new guide for mapping connectivity in vivo. NeuroImage. 2005;24:339–349. doi: 10.1016/j.neuroimage.2004.08.044. [DOI] [PubMed] [Google Scholar]
- Baxter LR., Jr Basal ganglia systems in ritualistic social displays: Reptiles and humans; function and illness. Physiology and Behavior. 2003;79:451–460. doi: 10.1016/s0031-9384(03)00164-1. [DOI] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, Lubar JO, Chen TJ, Comings DE. Reward deficiency syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. Journal of Psychoactive Drugs. 2000;32(Suppl.):i–iv. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Pedersen CA. Brief vs. long maternal separations in infancy: Contrasting relationships with adult maternal behavior and lactation levels of aggression and anxiety. Psychoneuroendocrinology. 2001;26:657–672. doi: 10.1016/s0306-4530(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Bodini B, Iacoboni M, Lenzi GL. Acute stroke effects on emotions: An interpretation through the mirror system. Current Opinion in Neurology. 2004;17:55–60. doi: 10.1097/00019052-200402000-00010. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment and loss, Vol 1. Attachment. Hogarth Press; London: 1969. [Google Scholar]
- Bowlby J. Attachment and loss, Vol 2. Separation: Anxiety and anger. Basic Books; London: 1973. [Google Scholar]
- Boyer P, Lienard P. Why ritualized behavior?. Precaution Systems and action parsing in developmental, pathological and cultural rituals. Behavioral and Brain Sciences. doi: 10.1017/s0140525x06009332. in press. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Welsh RC, Berridge KC, Liberzon I. Neural correlates of social and nonsocial emotions: An fMRI study. NeuroImage. doi: 10.1016/j.neuroimage.2005.11.027. in press. [DOI] [PubMed] [Google Scholar]
- Brockington I. Postpartum psychiatric disorders. Lancet. 2004;363:303–310. doi: 10.1016/S0140-6736(03)15390-1. [DOI] [PubMed] [Google Scholar]
- Brown GW, Bifulco A, Harris TO. Life events, vulnerability and onset of depression: Some refinements. British Journal of Psychiatry. 1987;150:30–42. doi: 10.1192/bjp.150.1.30. [DOI] [PubMed] [Google Scholar]
- Buchheim A, Erk S, George C, Kachele H, Ruchsow M, Spitzer M, Kircher T, Walter H. Measuring attachment representation in an fMRI environment: A pilot study. Psychopathology. 2006;39:144–152. doi: 10.1159/000091800. [DOI] [PubMed] [Google Scholar]
- Caplan HL, Cogill SR, Alexandra H, Robson KM, Katz R, Kumar R. Maternal depression and the emotional development of the child. British Journal of Psychiatry. 1989;154:818–822. doi: 10.1192/bjp.154.6.818. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proceedings of the National Academy of Sciences U S A. 2003;100:5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chaffin M, Kelleher K, Hollenberg J. Onset of physical abuse and neglect: Psychiatric, substance abuse, and social risk factors from prospective community data. Child Abuse and Neglect. 1996;20:191–203. doi: 10.1016/s0145-2134(95)00144-1. [DOI] [PubMed] [Google Scholar]
- Champagne F, Meaney MJ. Like mother, like daughter: Evidence for non-genomic transmission of parental behavior and stress responsivity. Progress in Brain Research. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. Journal of Neuroscience. 2004;24:4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodera P, Coiro V. Oxytocin reduces metyrapone-induced ACTH secretion in human subjects. Brain Research. 1987;420:178–181. doi: 10.1016/0006-8993(87)90257-5. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH. The evolution of parental care. Princeton University Press; Princeton: 1991. [Google Scholar]
- Comings DE, Blum K. Reward deficiency syndrome: Genetic aspects of behavioral disorders. Progress in Brain Research. 2000;126:325–341. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, Iacoboni M. Understanding emotions in others: Mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Grezes J. The power of simulation: Imagining one’s own and other’s behavior. Brain Research. 2006;1079:4–14. doi: 10.1016/j.brainres.2005.12.115. [DOI] [PubMed] [Google Scholar]
- Depue RA, Morrone-Strupinsky JV. A neurobehavioral model of affiliative bonding: Implications for conceptualizing a human trait of affiliation. Behavioral and Brain Sciences. 2005;28:313–350. doi: 10.1017/S0140525X05000063. discussion, 350–395. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Ellison K. The mommy brain: How motherhood makes us smarter. 2nd edn Perseus Books Group; New York: 2006. [Google Scholar]
- Factor EM, Mayer AD, Rosenblatt JS. Peripeduncular nucleus lesions in the rat: I. Effects on maternal aggression, lactation, and maternal behavior during pre- and postpartum periods. Behavioral Neuroscience. 1993;107:166–185. doi: 10.1037//0735-7044.107.1.166. [DOI] [PubMed] [Google Scholar]
- Febo M, Numan M, Ferris CF. Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. Journal of Neuroscience. 2005;25:11637–11644. doi: 10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. Infant–mother and infant–father synchrony: The coregulation of positive arousal. Infant Mental Health Jounal. 2003;24:1–23. [Google Scholar]
- Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Front Neuroendocrinology. 2002;23:200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Kulkarni P, Sullivan JM, Jr, Harder JA, Messenger TL, Febo M. Pup suckling is more rewarding than cocaine: Evidence from functional magnetic resonance imaging and three-dimensional computational analysis. Journal of Neuroscience. 2005;25:149–156. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feygin DL, Swain JE, Leckman JF. The normalcy of neurosis: Evolutionary origins of obsessive-compulsive disorder and related behaviors. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2006;30:854–864. doi: 10.1016/j.pnpbp.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Fisher H, Aron A, Brown LL. Romantic love: An fMRI study of a neural mechanism for mate choice. Journal of Comparative Neurology. 2005;493:58–62. doi: 10.1002/cne.20772. [DOI] [PubMed] [Google Scholar]
- Fisher H, Aron A, Mashek D, Li H, Strong G, Brown LL. Review. The neural mechanisms of mate choice: A hypothesis. Neuro Endocrinology Letters. 2002;23(Suppl. 4):92–97. [PubMed] [Google Scholar]
- Fisher HE, Aron A, Mashek D, Li H, Brown LL. Defining the brain systems of lust, romantic attraction, and attachment. Archives of Sexual Behavior. 2002;31:413–419. doi: 10.1023/a:1019888024255. [DOI] [PubMed] [Google Scholar]
- Flannelly KJ, Kemble ED, Blanchard DC, Blanchard RJ. Effects of septal-forebrain lesions on maternal aggression and maternal care. Behavioral and Neural Biology. 1986;45:17–30. doi: 10.1016/s0163-1047(86)80002-4. [DOI] [PubMed] [Google Scholar]
- Fleming A, Vaccarino F, Tambosso L, Chee P. Vomeronasal and olfactory system modulation of maternal behavior in the rat. Science. 1979;203:372–374. doi: 10.1126/science.760196. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Corter C, Stallings J, Steiner M. Testosterone and prolactin are associated with emotional responses to infant cries in new fathers. Hormones and Behavior. 2002;42:399–413. doi: 10.1006/hbeh.2002.1840. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Miceli M, Moretto D. Lesions of the medial preoptic area prevent the facilitation of maternal behavior produced by amygdala lesions. Physiology and Behavior. 1983;31:503–510. doi: 10.1016/0031-9384(83)90073-2. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Steiner M, Corter C. Cortisol, hedonics, and maternal responsiveness in human mothers. Hormones and Behavior. 1997;32:85–98. doi: 10.1006/hbeh.1997.1407. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. Journal of Neuroendocrinology. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Francis DD, Meaney MJ. Maternal care and the development of stress responses. Current Opinion in Neurobiology. 1999;9:128–134. doi: 10.1016/s0959-4388(99)80016-6. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London B Biological Science. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodi AM, Lamb ME. Child abusers’ responses to infant smiles and cries. Child Development. 1980;51:238–241. [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends in Cognitive Science. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Gerhardt S. Why love matters: How affection shapes a baby’s brain. Brunner-Routledge; New York: 2006. [Google Scholar]
- Gobbini MI, Haxby JV. Neural systems for recognition of familiar faces. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2006.04.015. in press. [DOI] [PubMed] [Google Scholar]
- Gray JR. Emotional modulation of cognitive control: Approach–withdrawal states double-dissociate spatial from verbal two-back task performance. Journal of Experimental Psychology: General. 2001;130:436–452. doi: 10.1037//0096-3445.130.3.436. [DOI] [PubMed] [Google Scholar]
- Green JA, Gustafson GE. Individual recognition of human infants on the basis of cries alone. Developmental Psychobiology. 1983;16:485–493. doi: 10.1002/dev.420160604. [DOI] [PubMed] [Google Scholar]
- Hansen S. Maternal behavior of female rats with 6-OHDA lesions in the ventral striatum: Characterization of the pup retrieval deficit. Physiology and Behavior. 1994;55:615–620. doi: 10.1016/0031-9384(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Hansen S, Kohler C. The importance of the peripeduncular nucleus in the neuroendocrine control of sexual behavior and milk ejection in the rat. Neuroendocrinology. 1984;39:563–572. doi: 10.1159/000124038. [DOI] [PubMed] [Google Scholar]
- Hasson U, Nir Y, Levy I, Fuhrmann G, Malach R. Intersubject synchronization of cortical activity during natural vision. Science. 2004;303:1634–1640. doi: 10.1126/science.1089506. [DOI] [PubMed] [Google Scholar]
- Hatfield E, Sprecher S. Measuring passionate love in intimate relationships. Journal of Adolescence. 1986;9:383–410. doi: 10.1016/s0140-1971(86)80043-4. [DOI] [PubMed] [Google Scholar]
- Heim C, Owens MJ, Plotsky PM, Nemeroff CB. The role of early adverse life events in the etiology of depression and posttraumatic stress disorder. Focus on corticotropin-releasing factor. Annals of the New York Academy of Sciences. 1997;821:194–207. doi: 10.1111/j.1749-6632.1997.tb48279.x. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Henry JP. Psychological and physiological responses to stress: The right hemisphere and the hypothalamo–pituitary–adrenal axis, an inquiry into problems of human bonding. Integrative Physiological and Behavioral Science. 1993;28:369–387. doi: 10.1007/BF02690935. discussion, 368. [DOI] [PubMed] [Google Scholar]
- Hesse E. The Adult Attachment Interview: Historical and current perspectives. In: Cassidy JA, Shaver PR, editors. Handbook of attachment: Theory, research, and clinical applications. Guilford Press; New York: 1999. pp. 395–433. [Google Scholar]
- Horton PC. The comforting substrate and the right brain. Bulletin of the Menninger Clinic. 1995;59:480–486. [PubMed] [Google Scholar]
- Hrdy SB. Mother Nature: Maternal instincts and how they shape the human species. Ballantine Books; New York: 2000. [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Insel TR. Is social attachment an addictive disorder? Physiology and Behavior. 2003;79:351–357. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nature Reviews Neuroscience. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Jankowiak WR, Fischer EF. A cross-cultural perspective on romantic love. Ethnology. 1992;31:149–155. [Google Scholar]
- Johns JM, Elliott DL, Hofler VE, Joyner PW, McMurray MS, Jarrett TM, Haslup AM, Middleton CL, Elliott JC, Walker CH. Cocaine treatment and prenatal environment interact to disrupt intergenerational maternal behavior in rats. Behavioral Neuroscience. 2005a;119:1605–1618. doi: 10.1037/0735-7044.119.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Joyner PW, McMurray MS, Elliott DL, Hofler VE, Middleton CL, Knupp K, Greenhill KW, Lomas LM, Walker CH. The effects of dopaminergic/serotonergic reuptake inhibition on maternal behavior, maternal aggression, and oxytocin in the rat. Pharmacology, Biochemistry and Behavior. 2005b;81:769–785. doi: 10.1016/j.pbb.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kessler RC, Neale MC, Heath AC, Eaves LJ. The prediction of major depression in women: Toward an integrated etiologic model. American Journal of Psychiatry. 1993;150:1139–1148. doi: 10.1176/ajp.150.8.1139. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Moffitt TE, Taylor A, Pawlby SJ, Caspi A. Maternal depression and children’s antisocial behavior: Nature and nurture effects. Archives of General Psychiatry. 2005;62:173–181. doi: 10.1001/archpsyc.62.2.173. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. Journal of Neuroscience. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling A, Steklis HD. A neural substrate for affiliative behavior in nonhuman primates. Brain Behavior and Evolution. 1976;13:216–238. doi: 10.1159/000123811. [DOI] [PubMed] [Google Scholar]
- Kohut H. Introspection, empathy, and the semi-circle of mental health. International Journal of Psychoanalysis. 1982;63(Pt 4):395–407. [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY, Kehoe P. Heightened cocaine and food self-administration in female rats with neonatal isolation experience. Neuropsychopharmacology. 2006;31:70–76. doi: 10.1038/sj.npp.1300779. [DOI] [PubMed] [Google Scholar]
- Krpan KM, Coombs R, Zinga D, Steiner M, Fleming AS. Experiential and hormonal correlates of maternal behavior in teen and adult mothers. Hormones and Behavior. 2005;47:112–122. doi: 10.1016/j.yhbeh.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Li Z, Risinger RC, Rainey CJ, Wu G, Bloom AS, Li SJ. Neural responses to acute cocaine administration in the human brain detected by fMRI. NeuroImage. 2005;28:904–914. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- LaGasse LL, Messinger D, Lester BM, Seifer R, Tronick EZ, Bauer CR, Shankaran S, Bada HS, Wright LL, Smeriglio VL, Finnegan LP, Maza PL, Liu J. Prenatal drug exposure and maternal and infant feeding behaviour. Archives of Disease in Childhood: Fetal and Neonatal Edition. 2003;88:0F391–399. doi: 10.1136/fn.88.5.F391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, Carter CS, Hennessy MB, Hrdy SB, Kervene EB, Klann-Delius G, Schradin C, Todt D, Von Holst D. Biobehavioral Processes in Attachment and Bonding. In: Carter CS, Ahnert L, Klaus E, Grossman SB, Hrdy SB, Lamb SW, et al., editors. Attachment and bonding: A New Synthesis, Dahlem Workshop Report 92. MIT Press; Cambridge, Mass: 2005. pp. 301–347. [Google Scholar]
- Leckman JF, Feldman R, Swain JE, Eicher V, Thompson N, Mayes LC. Primary parental preoccupation: Circuits, genes, and the crucial role of the environment. Journal of Neural Transmission. 2004;111:753–771. doi: 10.1007/s00702-003-0067-x. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Herman AE. Maternal behavior and developmental psychopathology. Biological Psychiatry. 2002;51:27–43. doi: 10.1016/s0006-3223(01)01277-x. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Mayes LC. Understanding developmental psychopathology: How useful are evolutionary accounts? Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:1011–1021. doi: 10.1097/00004583-199810000-00010. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Mayes LC. Preoccupations and behaviors associated with romantic and parental love. Perspectives on the origin of obsessive-compulsive disorder. Child and Adolescent Psychiatric Clinics of North America. 1999;8:635–665. [PubMed] [Google Scholar]
- Leckman JF, Mayes LC, Feldman R, Evans DW, King RA, Cohen DJ. Early parental preoccupations and behaviors and their possible relationship to the symptoms of obsessive-compulsive disorder. Acta Psychiatrica Scandinavica Suppl. 1999;396:1–26. doi: 10.1111/j.1600-0447.1999.tb10951.x. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Emotional networks and motor control: A fearful view. Progress in Brain Research. 1996;107:437–446. doi: 10.1016/s0079-6123(08)61880-4. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cellular and Molecular Neurobiology. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros JJ, Chiodera P, Geenen V. Inhibitory action of exogenous oxytocin on plasma cortisol in normal human subjects: Evidence of action at the adrenal level. Neuroendocrinology. 1988;48:204–206. doi: 10.1159/000125009. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Gobbini MI, Harrison T, Haxby JV. Mothers’ neural activation in response to pictures of their children and other children. Biological Psychiatry. 2004;56:225–232. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Light KC, Grewen KM, Amico JA, Boccia M, Brownley KA, Johns JM. Deficits in plasma oxytocin responses and increased negative affect, stress, and blood pressure in mothers with cocaine exposure during pregnancy. Addictive Behaviors. 2004;29:1541–1564. doi: 10.1016/j.addbeh.2004.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscienceence. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Simmons DA, Swann JM, Stern JM. Forebrain expression of c-fos due to active maternal behaviour in lactating rats. Neuroscience. 1998;82:267–281. doi: 10.1016/s0306-4522(97)00283-2. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Stern JM. Role of the midbrain periaqueductal gray in maternal nurturance and aggression: C-fos and electrolytic lesion studies in lactating rats. Journal of Neuroscience. 1997;17:3364–3378. doi: 10.1523/JNEUROSCI.17-09-03364.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Dubno JR, Horwitz AR, Nahas Z, Teneback CC, Bloomer CW, Bohning DE, Vincent D, Johnson MR, Emmanuel N, Brawman-Mintzer O, Book SW, Lydiard RB, Ballenger JC, George MS. Feasibility of using fMRI to study mothers responding to infant cries. Depression and Anxiety. 1999;10:99–104. doi: 10.1002/(sici)1520-6394(1999)10:3<99::aid-da2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, Bohning DE, George MS. A potential role for thalamocingulate circuitry in human maternal behavior. Biological Psychiatry. 2002;51:431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Research. 1991;555:220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Pizzolato G, Dreifuss JJ. Localization of oxytocin binding sites in the human brainstem and upper spinal cord: An autoradiographic study. Brain Research. 1989;500:223–230. doi: 10.1016/0006-8993(89)90317-x. [DOI] [PubMed] [Google Scholar]
- Lundstrom BN, Ingvar M, Petersson KM. The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. NeuroImage. 2005;27:824–834. doi: 10.1016/j.neuroimage.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Lundstrom BN, Petersson KM, Andersson J, Johansson M, Fransson P, Ingvar M. Isolating the retrieval of imagined pictures during episodic memory: Activation of the left precuneus and left prefrontal cortex. NeuroImage. 2003;20:1934–1943. doi: 10.1016/j.neuroimage.2003.07.017. [DOI] [PubMed] [Google Scholar]
- MacLean PD. The triune brain in evolution: Role in paleocerebral functions. Plenum Press; New York: 1990. [DOI] [PubMed] [Google Scholar]
- Mann J. Nurturance or negligence: Maternal psychology and behavior preference among pre-term twins. In: Barkow J, et al., editors. The adapted mind. Oxford University Press; New York: 1992. pp. 367–390. [Google Scholar]
- Marazziti D, Di Nasso E, Masala I, Baroni S, Abelli M, Mengali F, Mungai F, Rucci P. Normal and obsessional jealousy: A study of a population of young adults. European Psychiatry. 2003;18:106–111. doi: 10.1016/s0924-9338(03)00024-5. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Bornstein MH, Chawarska K, Granger RH. Information processing and developmental assessments in 3-month-old infants exposed prenatally to cocaine. Pediatrics. 1995;95:539–545. [PubMed] [Google Scholar]
- Mayes LC, Granger RH, Frank MA, Schottenfeld R, Bornstein MH. Neurobehavioral profiles of neonates exposed to cocaine prenatally. Pediatrics. 1993;9:778–783. [PubMed] [Google Scholar]
- Mayes LC, Swain JE, Leckman JF. Parental attachment systems: Neural circuits, genes, and experiential contributions to parental engagement. Clinical Neuroscience Research. 2005;4:301–313. [Google Scholar]
- Meaney MJ, Brake W, Gratton A. Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology. 2002;27:127–138. doi: 10.1016/s0306-4530(01)00040-3. [DOI] [PubMed] [Google Scholar]
- Mello LE, Villares J. Neuroanatomy of the basal ganglia. Psychiatric Clinics of North America. 1997;20:691–704. doi: 10.1016/s0193-953x(05)70340-3. [DOI] [PubMed] [Google Scholar]
- Mesulam M. Brain, mind, and the evolution of connectivity. Brain and Cognition. 2000;42:4–6. doi: 10.1006/brcg.1999.1145. [DOI] [PubMed] [Google Scholar]
- Minde K, Corter C, Goldberg S, Jeffers D. Maternal preference between premature twins up to age four. Journal of the American Academy of Child and Adolescent Psychiatry. 1990;29:367–374. doi: 10.1097/00004583-199005000-00006. [DOI] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Morris JP, Pelphrey KA, McCarthy G. Regional brain activation evoked when approaching a virtual human on a virtual walk. Journal of Cognitive Neuroscience. 2005;17:1744–1752. doi: 10.1162/089892905774589253. [DOI] [PubMed] [Google Scholar]
- Murphy MR, MacLean PD, Hamilton SC. Species-typical behavior of hamsters deprived from birth of the neocortex. Science. 1981;213:459–461. doi: 10.1126/science.7244642. [DOI] [PubMed] [Google Scholar]
- Murray L, Cooper PJ. The impact of postpartum depression on child development. In: Goodyer I, editor. Aetiological mechanisms in developmental psychopathology. Oxford University Press; Oxford: 2003. [Google Scholar]
- Najib A, Lorberbaum JP, Kose S, Bohning DE, George MS. Regional brain activity in women grieving a romantic relationship breakup. American Journal of Psychiatry. 2004;161:2245–2256. doi: 10.1176/appi.ajp.161.12.2245. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kawashima R, Sugiura M, Kato T, Nakamura A, Hatano K, Nagumo S, Kubota K, Fukuda H, Ito K, Kojima S. Neural substrates for recognition of familiar voices: A PET study. Neuropsychologia. 2001;39:1047–1054. doi: 10.1016/s0028-3932(01)00037-9. [DOI] [PubMed] [Google Scholar]
- Newman JD. Vocal communication and the triune brain. Physiology and Behavior. 2003;79:495–502. doi: 10.1016/s0031-9384(03)00155-0. [DOI] [PubMed] [Google Scholar]
- NICHD Child care and mother–child interaction in the first 3 years of life. Developmental Psychology. 1999;35:1399–1413. [PubMed] [Google Scholar]
- Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, Davidson RJ. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. NeuroImage. 2004;21:583–592. doi: 10.1016/j.neuroimage.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Novakova V, Sterc J, Kuchar S, Mozes S. Maternal behaviour in septal rat females. Physiological Research. 1993;42:351–360. [PubMed] [Google Scholar]
- Numan M. Medial preoptic area and maternal behavior in the female rat. Journal of Comparative Physiology and Psychology. 1974;87:746–759. doi: 10.1037/h0036974. [DOI] [PubMed] [Google Scholar]
- Numan M. A neural circuitry analysis of maternal behavior in the rat. Acta Paediatrica Suppl. 1994;397:19–28. doi: 10.1111/j.1651-2227.1994.tb13261.x. [DOI] [PubMed] [Google Scholar]
- Numan M, Corodimas KP, Numan MJ, Factor EM, Piers WD. Axon-sparing lesions of the preoptic region and substantia innominata disrupt maternal behavior in rats. Behavioral Neuroscience. 1988;102:381–396. doi: 10.1037//0735-7044.102.3.381. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. The neurobiology of parental behavior. Springer-Verlag; New York: 2003. [Google Scholar]
- Numan M, McSparren J, Numan MJ. Dorsolateral connections of the medial preoptic area and maternal behavior in rats. Behavioral Neuroscience. 1990;104:964–979. doi: 10.1037//0735-7044.104.6.964. [DOI] [PubMed] [Google Scholar]
- Numan M, Morrell JI, Pfaff DW. Anatomical identification of neurons in selected brain regions associated with maternal behavior deficits induced by knife cuts of the lateral hypothalamus in rats. Journal of Comparative Neurology. 1985;237:552–564. doi: 10.1002/cne.902370411. [DOI] [PubMed] [Google Scholar]
- Numan M, Nagle DS. Preoptic area and substantia nigra interact in the control of maternal behavior in the rat. Behavioral Neuroscience. 1983;97:120–139. doi: 10.1037//0735-7044.97.1.120. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan M. A lesion and neuroanatomical tract-tracing analysis of the role of the bed nucleus of the stria terminalis in retrieval behavior and other aspects of maternal responsiveness in rats. Developmental Psychobiology. 1996;29:23–51. doi: 10.1002/(SICI)1098-2302(199601)29:1<23::AID-DEV2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ. Projection sites of medial preoptic area and ventral bed nucleus of the stria terminalis neurons that express Fos during maternal behavior in female rats. Journal of Neuroendocrinology. 1997;9:369–384. doi: 10.1046/j.1365-2826.1997.t01-1-00597.x. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, English JB. Excitotoxic amino acid injections into the medial amygdala facilitate maternal behavior in virgin female rats. Hormones and Behavior. 1993;27:56–81. doi: 10.1006/hbeh.1993.1005. [DOI] [PubMed] [Google Scholar]
- Numan M, Rosenblatt JS, Komisaruk BR. Medial preoptic area and onset of maternal behavior in the rat. Journal of Comparative Physiology and Psychology. 1977;91:146–164. doi: 10.1037/h0077304. [DOI] [PubMed] [Google Scholar]
- Numan M, Sheehan TP. Neuroanatomical circuitry for mammalian maternal behavior. Annals of the New York Academy of Sciences. 1997;807:101–125. doi: 10.1111/j.1749-6632.1997.tb51915.x. [DOI] [PubMed] [Google Scholar]
- Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, Ekholm S, Strigo I, Worsley K, Vallbo AB, Bushnell MC. Unmyelinated tactile afferents signal touch and project to insular cortex. Nature Neuroscience. 2002;5:900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Olds DL, Eckenrode J, Henderson CR, Jr, Kitzman H, Powers J, Cole R, Sidora K, Morris P, Pettitt LM, Luckey D. Long-term effects of home visitation on maternal life course and child abuse and neglect. Fifteen-year follow-up of a randomized trial. Journal of the American Medical Association. 1997;278:637–643. [PubMed] [Google Scholar]
- Olds DL, Kitzman H, Cole R, Robinson J, Sidora K, Luckey DW, Henderson CR, Jr, Hanks C, Bondy J, Holmberg J. Effects of nurse home-visiting on maternal life course and child development: Age 6 follow-up results of a randomized trial. Pediatrics. 2004;114:1550–1559. doi: 10.1542/peds.2004-0962. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Nelson E, Siviy S. Brain opioids and mother–infant social motivation. Acta Paediatrica Suppl. 1994;397:40–46. doi: 10.1111/j.1651-2227.1994.tb13264.x. [DOI] [PubMed] [Google Scholar]
- Pector EA. Twin death and mourning worldwide: A review of the literature. Twin Research. 2002;5:196–205. doi: 10.1375/136905202320227853. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Boccia ML. Oxytocin links mothering received, mothering bestowed and adult stress responses. Stress. 2002;5:259–67. doi: 10.1080/1025389021000037586. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, Michelich CR, Allison T, McCarthy G. Functional anatomy of biological motion perception in posterior temporal cortex: An FMRI study of eye, mouth and hand movements. Cerebral Cortex. 2005;15:1866–1876. doi: 10.1093/cercor/bhi064. [DOI] [PubMed] [Google Scholar]
- Porter LS, Porter BO. A blended infant massage—parenting enhancement program for recovering substance-abusing mothers. Pediatric Nursing. 2004;30:363–372. [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: A positron emission tomography study using [11C]raclopride. Journal of Neuroscience. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purhonen M, Kilpelainen-Lees R, Paakkonen A, Ypparila H, Lehtonen J, Karhu J. Effects of maternity on auditory event-related potentials to human sound. NeuroReport. 2001;12:2975–2979. doi: 10.1097/00001756-200109170-00044. [DOI] [PubMed] [Google Scholar]
- Purhonen M, Paakkonen A, Ypparila H, Lehtonen J, Karhu J. Dynamic behavior of the auditory N100 elicited by a baby’s cry. International Journal of Psychophysiology. 2001;41:271–278. doi: 10.1016/s0167-8760(01)00139-8. [DOI] [PubMed] [Google Scholar]
- Ranote S, Elliott R, Abel KM, Mitchell R, Deakin JF, Appleby L. The neural basis of maternal responsiveness to infants: An fMRI study. NeuroReport. 2004;15:1825–1829. doi: 10.1097/01.wnr.0000137078.64128.6a. [DOI] [PubMed] [Google Scholar]
- Robin M, Kheroua H, Casati I. Effects of early mother–twin relationships from birth to age 3, on twin bonding. Acta Geneticae Medicae et Gemellologiae (Roma) 1992;41:143–148. doi: 10.1017/s0001566000002348. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS. Psychobiology of maternal behavior: Contribution to the clinical understanding of maternal behavior among humans. Acta Paediatrica Suppl. 1994;397:3–8. doi: 10.1111/j.1651-2227.1994.tb13259.x. [DOI] [PubMed] [Google Scholar]
- Salk L. The effects of normal heartbeat sound on the behavior of the newborn infant: Implications for mental health. World Mental Health. 1960;12:168–175. [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Developmental Psychopathology. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sander D, Grandjean D, Pourtois G, Schwartz S, Seghier ML, Scherer KR, Vuilleumier P. Emotion and attention interactions in social cognition: Brain regions involved in processing anger prosody. NeuroImage. 2005;28:848–858. doi: 10.1016/j.neuroimage.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Sander K, Brechmann A, Scheich H. Audition of laughing and crying leads to right amygdala activation in a low-noise fMRI setting. Brain Research. Brain Research Protocols. 2003;11:81–91. doi: 10.1016/s1385-299x(03)00018-7. [DOI] [PubMed] [Google Scholar]
- Saxe R. Uniquely human social cognition. Current Opinion in Neurobiology. 2006a;16:235–239. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Saxe R. Why and how to study Theory of Mind with fMRI. Brain Research. 2006b;1079:57–65. doi: 10.1016/j.brainres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Schore AN. Back to basics: Attachment, affect regulation, and the developing right brain: Linking developmental neuroscience to pediatrics. Pediatrics in Review. 2005;26:204–217. doi: 10.1542/pir.26-6-204. [DOI] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: The role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annual Review of Psychology. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Seifer R, LaGasse LL, Lester B, Bauer CR, Shankaran S, Bada HS, Wright LL, Smeriglio VL, Liu J. Attachment status in children prenatally exposed to cocaine and other substances. Child Development. 2004;75:850–868. doi: 10.1111/j.1467-8624.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- Seifritz E, Esposito F, Neuhoff JG, Luthi A, Mustovic H, Dammann G, von Bardeleben U, Radue EW, Cirillo S, Tedeschi G, Di Salle F. Differential sex-independent amygdala response to infant crying and laughing in parents versus non-parents. Biological Psychiatry. 2003;54:1367–1375. doi: 10.1016/s0006-3223(03)00697-8. [DOI] [PubMed] [Google Scholar]
- Shaver P, Schwartz J, Kirson D, O’Connor C. Emotion knowledge: Further exploration of a prototype approach. Journal of Personality and Social Psychology. 1987;52:1061–1086. doi: 10.1037//0022-3514.52.6.1061. [DOI] [PubMed] [Google Scholar]
- Sieratzki JS, Woll B. Why do mothers cradle babies on their left? Lancet. 1996;347:1746–1748. doi: 10.1016/s0140-6736(96)90813-2. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Slotnick BM. Disturbances of maternal behavior in the rat following lesions of the cingulate cortex. Behaviour. 1967;29:204–236. doi: 10.1163/156853967x00127. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Nigrosh BJ. Maternal behavior of mice with cingulate cortical, amygdala, or septal lesions. Journal of Comparative Physiology and Psychology. 1975;88:118–127. doi: 10.1037/h0076200. [DOI] [PubMed] [Google Scholar]
- Soltis J. The signal functions of early infant crying. Behavioral and Brain Sciences. 2004;27:443–458. discussion, 459–490. [PubMed] [Google Scholar]
- Squire S, Stein A. Functional MRI and parental responsiveness: A new avenue into parental psychopathology and early parent–child interactions? British Journal of Psychiatry. 2003;183:481–483. doi: 10.1192/bjp.183.6.481. [DOI] [PubMed] [Google Scholar]
- Sroufe LA. Attachment and development: A prospective, longitudinal study from birth to adulthood. Attachment and Human Development. 2005;7:349–367. doi: 10.1080/14616730500365928. [DOI] [PubMed] [Google Scholar]
- Sroufe LA, Carlson EA, Levy AK, Egeland B. Implications of attachment theory for developmental psychopathology. Developmental Psychopathology. 1999;11:1–13. doi: 10.1017/s0954579499001923. [DOI] [PubMed] [Google Scholar]
- Stamm JS. The function of the median cerebral cortex in maternal behavior of rats. Journal of Comparative Physiology and Psychology. 1955;48:347–356. doi: 10.1037/h0042977. [DOI] [PubMed] [Google Scholar]
- Stern JM. Offspring-induced nurturance: Animal–human parallels. Developmental Psychobiology. 1997;31:19–37. doi: 10.1002/(sici)1098-2302(199707)31:1<19::aid-dev3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Storey AE, Walsh CJ, Quinton RL, Wynne-Edwards KE. Hormonal correlates of paternal responsiveness in new and expectant fathers. Evolution and Human Behavior. 2000;21:79–95. doi: 10.1016/s1090-5138(99)00042-2. [DOI] [PubMed] [Google Scholar]
- Stowe ZN, Nemeroff CB. Women at risk for postpartum-onset major depression. American Journal of Obstetrics and Gynecology. 1995;173:639–645. doi: 10.1016/0002-9378(95)90296-1. [DOI] [PubMed] [Google Scholar]
- Strathearn L. A 14-year longitudinal study of child neglect: Cognitive development and head growth; Paper presented at the 14th International Congress on Child Abuse and Neglect; 2002. [Google Scholar]
- Strathearn L. Exploring the neurobiology of attachment. In: Mayes LC, Fonagy P, Target M, editors. Developmental science and psychoanalysis. Karnac Press; London: 2007. p. 450. [Google Scholar]
- Strathearn L, Gray PH, O’Callaghan F, Wood DO. Childhood neglect and cognitive development in extremely low birth weight infants: A prospective study. Pediatrics. 2001;108:142–151. doi: 10.1542/peds.108.1.142. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Li J, Montague PR. An fMRI study of maternal mentalization: Having the baby’s mind in mind. NeuroImage. 2005;26(Suppl. 1):S25. [Google Scholar]
- Swain JE. Critical developmental periods of increased plasticity program ritualized behavior. Behavioral and Brain Sciences. in press. [Google Scholar]
- Swain JE, Leckman JF, Mayes LC, Feldman R, Constable RT, Schultz RT. The neural circuitry of parent–infant attachment in the early postpartum; American College of Neuropsychopharmacology 42nd Annual Meeting, American College of Neuropsychopharmacology; San Juan, Puerto Rico. 2003.Dec 10, [Google Scholar]
- Swain JE, Leckman JF, Mayes LC, Feldman R, Constable RT, Schultz RT. Neural subatrates and psychology of human parent–infant attachment in the postpartum. Biological Psychiatry. 2004;55:153S. [Google Scholar]
- Swain JE, Leckman JF, Mayes LC, Feldman R, Schultz RT. Early Human Parent–Infant Bond Development: fMRI, thoughts and behaviors. Biological Psychiatry. 2005;57:112S. [Google Scholar]
- Swain JE, Mayes LC, Leckman JF. The development of parent–infant attachment through dynamic and interactive signaling loops of care and cry. Behavioral and Brain Sciences. 2004;27:472–473. [Google Scholar]
- Todorov A, Gobbini MI, Evans KK, Haxby JV. Spontaneous retrieval of affective person knowledge in face perception. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2006.04.018. in press. [DOI] [PubMed] [Google Scholar]
- Tronick EZ, Messinger DS, Weinberg MK, Lester BM, Lagasse L, Seifer R, Bauer CR, Shankaran S, Bada H, Wright LL, Poole K, Liu J. Cocaine exposure is associated with subtle compromises of infants’ and mothers’ social-emotional behavior and dyadic features of their interaction in the face-to-face still-face paradigm. Developmental Psychology. 2005;41:711–722. doi: 10.1037/0012-1649.41.5.711. [DOI] [PubMed] [Google Scholar]
- Tzourio N, Massioui FE, Crivello F, Joliot M, Renault B, Mazoyer B. Functional anatomy of human auditory attention studied with PET. NeuroImage. 1997;5:63–77. doi: 10.1006/nimg.1996.0252. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Administration on Children, Youth and Families . Child maltreatment 2001: Reports from the States to the National Child Abuse and Neglect Data System. U.S. Government Printing Office; Washington, DC: 2001. [Google Scholar]
- U.S. Department of Health and Human Services. Administration on Children, Youth and Families . Child maltreatment 2003: Reports from the States to the National Child Abuse and Neglect Data System. U.S. Government Printing Office; Washington, DC: 2003. [Google Scholar]
- Uvnas-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23:819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K, Eriksson M. Breastfeeding: Physiological, endocrine and behavioural adaptations caused by oxytocin and local neurogenic activity in the nipple and mammary gland. Acta Paediatrica. 1996;85:525–530. doi: 10.1111/j.1651-2227.1996.tb14078.x. [DOI] [PubMed] [Google Scholar]
- van IJzendoorn MH. Adult attachment representations, parental responsiveness, and infant attachment: A meta-analysis on the predictive validity of the Adult Attachment Interview. Psychological Bulletin. 1995;117:387–403. doi: 10.1037/0033-2909.117.3.387. [DOI] [PubMed] [Google Scholar]
- Weinfield NS, Sroufe LA, Egeland B. Attachment from infancy to early adulthood in a high-risk sample: Continuity, discontinuity, and their correlates. Child Development. 2000;71:695–702. doi: 10.1111/1467-8624.00178. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Pilowsky DJ, Wickramaratne PJ, Talati A, Wisniewski SR, Fava M, Hughes CW, Garber J, Malloy E, King CA, Cerda G, Sood AB, Alpert JE, Trivedi MH, Rush AJ. Remissions in maternal depression and child psychopathology: A STAR*D-child report. Journal of the American Medical Association. 2006;295:1389–1398. doi: 10.1001/jama.295.12.1389. [DOI] [PubMed] [Google Scholar]
- Werner EE. Journeys from childhood to mid-life: Risk, resilience, and recovery. Pediatrics. 2004;114:492. doi: 10.1542/peds.114.2.492. [DOI] [PubMed] [Google Scholar]
- Winnicott DW. The theory of the parent–infant relationship. International Journal of Psychoanalysis. 1960;41:585–595. [PubMed] [Google Scholar]
- Winslow JT. Neuropeptides and non-human primate social deficits associated with pathogenic rearing experience. International Journal of Developmental Neuroscience. 2005;23:245–251. doi: 10.1016/j.ijdevneu.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Wise SP, Herkenham M. Opiate receptor distribution in the cerebral cortex of the Rhesus monkey. Science. 1982;218:387–389. doi: 10.1126/science.6289441. [DOI] [PubMed] [Google Scholar]
- Young LJ, Murphy Young AZ, Hammock EA. Anatomy and neurochemistry of the pair bond. Journal of Comparative Neurology. 2005;493:51–57. doi: 10.1002/cne.20771. [DOI] [PubMed] [Google Scholar]
- Zak PJ, Kurzban R, Matzner WT. The neurobiology of trust. Annals of the New York Academy of Sciences. 2004;1032:224–227. doi: 10.1196/annals.1314.025. [DOI] [PubMed] [Google Scholar]