Abstract
Objective
To evaluate the efficacy of endovascular therapy for maintaining patency and preserving limbs among patients with failing infrapopliteal bypass grafts.
Methods
We gathered data from a registry of catheter-based procedures for peripheral artery disease. Of 1554 arteriograms performed from 2006 to 2012, 30 patients had interventions for failing bypass vein grafts to infrapopliteal target vessels. The first intervention for each patient was used in this analysis. Duplex ultrasonography was used within 30 days after intervention and subsequently at 3-6 month intervals for graft surveillance.
Results
Interventions were performed for duplex ultrasonography surveillance findings in 21 patients and for symptoms of persistent or recurrent critical limb ischemia in 9 patients. Procedural techniques included cutting balloon angioplasty (83%), conventional balloon angioplasty (7%), and stent placement (10%). Procedural success was achieved in all cases. There were no procedure-related complications, amputations, or deaths within 30 days. By Kaplan-Meier analysis, 37% were free from graft restenosis at 12 months and 31% were at 24 months. Receiver operating characteristic analysis indicated that a lesion length of 1.75 cm best predicted freedom from restenosis (C statistic: 0.74). Residual stenosis (P=0.03), patency without reintervention (P=0.01), and assisted patency with secondary intervention (P=0.02) rates were superior for short lesions compared to long lesions. The cohort had acceptable rates of adverse clinical outcomes, with 96% of patients free from amputation at both 12 and 24 months; clinical outcomes were also better in patients with short lesions.
Conclusions
In this single-center experience with endovascular therapies to treat failing infrapopliteal bypass grafts, rates of limb preservation were high, but the majority of patients developed graft restenosis within 12 months. Grafts with longer stenoses fared poorly by comparison. These data suggest that endovascular interventions to restore or prolong graft patency may be associated with maintained graft patency and that close follow up with vascular laboratory surveillance is essential.
Introduction
Critical limb ischemia (CLI) is associated with amputation rates as high as 30% at one year after presentation and mortality rates of 18% at one year and 46% at 5 years.1,2 Infrapopliteal bypass is a commonly employed part of limb salvage strategies for patients with CLI. Surgical bypass may be associated with higher rates of patency and limb salvage than endovascular therapy, especially for patients expected to live longer than two years.3,4
Despite the advantages of surgical bypass, vein grafts are at risk of late failure. While many individual series have documented excellent results for infrapopliteal grafts (grafts to the tibioperoneal trunk, posterior tibial artery, peroneal artery, anterior tibial artery, or artery below the ankle),5,6 some recent studies suggest that grafts to infrapopliteal target vessels are at higher risk than femoropopliteal grafts for perioperative death, loss of patency, or major adverse limb outcomes.7–10 Furthermore, infrapopliteal bypass grafts are generally performed for treatment of CLI, which has itself been shown to be a predictor of graft failure.10,11
Surveillance with duplex ultrasonography (DUS) is an effective strategy for maintaining patency of vein bypass grafts, and is recommended by the 2007 Inter-Society Consensus for the Management of Peripheral Arterial Disease document (TASC II).1,12,13 Surgical revision or endovascular treatment of flow-limiting stenoses can reduce the risk of graft occlusion. The role of DUS surveillance following endovascular interventions to native arteries or grafts is not as well established.14,15 The efficacy of and appropriate schedule for surveillance after peripheral artery endovascular interventions is still debated.
Historically, bypass grafts with patency-threatening stenoses have been managed with surgical revision, but endovascular treatment alternatives are now commonly used and these have yielded outcomes similar to open surgical revision in selected populations, though the efficacy and durability of these interventions in grafts to infrapopliteal target vessels has not been specifically investigated.16
In this study, we sought to evaluate the effectiveness of endovascular interventions in maintaining graft patency and preserving limbs among patients with stenoses of infrapopliteal bypass grafts.
Materials and Methods
Design
The Peripheral Arterial Disease–University of California Davis (PAD-UCD) Registry comprises all patients with a clinical diagnosis of PAD who underwent diagnostic angiography and/or therapeutic endovascular intervention at the UC Davis Medical Center from 2006 to 2013. We have previously described general characteristics and overall outcomes for patients in this registry.17,18 Three vascular surgeons and one interventional cardiologist performed all of the procedures during the study period. At the time of data analysis, the registry included 975 patients and 1554 procedures. The Institutional Review Board at the University of California, Davis Medical Center approved the study protocol and waived the requirement of informed consent.
Data Collection and Definitions
We identified patients who had at least one endovascular intervention during the study period to treat an infrapopliteal bypass graft, defined as any graft to a tibial, peroneal, pedal, or plantar artery. We retrospectively analyzed these patients’ data based on review of electronic medical record documentation. We used pre- and post-procedure hospital and clinic records to characterize patient demographics, baseline health status and medical management, clinical presentation, vascular procedures, post-procedure management, and outcomes. Mortality was verified using institutional records and the Social Security Death Index. For each patient, the first intervention during the study period was included in our analysis. Grafts with acute or chronic occlusion at baseline were excluded.
Routine practice at our institution during this period was to schedule follow-up visits within one month after endovascular graft interventions, then every 3-6 months for the first year and every 6-12 months thereafter. At these visits, patients were evaluated with DUS, ankle-brachial pressure index (ABI), and toe-brachial pressure index (TBI) measurements.
Initial procedural success after interventions was defined as target lesion stenosis of less than 30% at the conclusion of the procedure, as determined by completion angiography, without a major adverse event.19,20
Outcomes
The primary endpoint of the study was freedom from restenosis, defined as continued graft patency without development of a ≥50% stenosis after the index interventional procedure and without further endovascular or surgical intervention. Graft patency during follow-up was objectively established with DUS; criteria used to identify ≥50% stenoses included: (1) peak systolic velocity (PSV) ratio of ≥2.0; or (2) absolute peak systolic velocity of ≥180 cm/s.12,21
Secondary study endpoints included patency without reintervention, assisted patency with secondary intervention, and secondary patency; mortality; major (above ankle) amputation; major adverse cardiovascular events (MACE); major adverse limb events (MALE); and symptoms of rest pain or lower extremity ulceration.22 Residual stensosis was defined as a ≥50% stenosis detected by DUS within 30 days post-procedure. Patency was defined as the presence of detectable flow in the graft, as determined by DUS at the follow-up time point. Patency without reintervention was defined as continued patency without endovascular or surgical intervention. Assisted patency with secondary intervention was defined as continued patency after an additional intervention for restenosis. Secondary patency was defined as patency restored by intervention after graft occlusion. MACE was defined as death from any cause, myocardial infarction, or stroke. MALE was defined as major amputation, new bypass graft placement, jump/interposition graft revision, thrombectomy, or thrombolysis.7 All outcomes were adjudicated from physician documentation in the electronic medical record.
Data Analysis
Median values with interquartile ranges were used to describe continuous variables and frequencies and percentages were used for categorical variables. Outcomes were analyzed using the Kaplan-Meier estimator and log rank test for survival analyses and with raw percentages and chi squared tests for secondary patency. For outcomes evaluated with the Kaplan-Meier estimator, standard error values were verified to be below 10% for each end point. Receiver-operating characteristic analysis was used to determine the lesion length most predictive of restenosis. Continuous outcomes were compared with the Mann-Whitney U test. All analyses were performed in R version 3.0.1 and survival analyses used the survival package.23,24
Results
Early Outcomes
During the study period, 92 grafts were placed with infrapopliteal target vessels (14 to anterior tibial, seven to dorsalis pedis, 20 to peroneal, four to plantar, and 47 to posterior tibial arteries). Of these and patients with previously placed grafts, thirty patients had endovascular interventions to treat failing infrapopliteal bypass grafts; the characteristics of these patients are summarized in Table I. Most interventions were performed for abnormal graft hemodynamics detected by vascular laboratory evaluation; outcomes did not differ based upon indication for intervention. Twenty-one patients (70%) had an endovascular intervention performed after DUS detected a significant stenosis. Nine patients (30%) had an endovascular intervention performed for treatment of persistent or recurrent CLI symptoms. The median pre-procedure peak systolic velocity was 411 cm/sec, with a PSV ratio of 5.4.
Table I.
Baseline Patient and Clinical Characteristics (N=30)
| Age (years) | 72 [63,82] |
| Male | 23 (77%) |
| Diabetic | 16 (53%) |
| Insulin dependent | 9 (30%) |
| Oral medications only | 7 (23%) |
| Active smoking | 5 (17%) |
| Coronary artery disease | 18 (60%) |
| End stage renal disease | 3 (10%) |
| Statin use | 21 (70%) |
| Aspirin use | 23 (77%) |
| Clopidogrel use | 13 (43%) |
| Ankle-brachial index (ABI) | 0.78 [0.75, 0.93] |
| Toe-brachial index (TBI) | 0.33 [0.25, 0.38] |
| Indication for revascularization | |
| Critical limb ischemia | 9 (30%) |
| Duplex surveillance | 21 (70%) |
| Peak systolic velocity (cm/s) | 411 [311, 486] |
| Peak systolic velocity ratio | 5.4 [3.9, 9.3] |
| Functional status | |
| Ambulatory | 25 (83%) |
| Wheelchair | 3 (10%) |
| Not documented | 2 (7%) |
Continuous variables represented as median [interquartile range].
Categorical variables represented as count (percentage%).
Graft characteristics and interventions are summarized in Table II. The interventions occurred a median of one year after initial graft placement [IQR 4-45 months]. The median lesion length was 15 mm (interquartile range 10-30 mm). Among the 30 treated patients, 25 (83%) were treated with cutting balloon angioplasty, 3 with a stent, and two with conventional balloon angioplasty, according to operator preference. Endovascular intervention was technically successful in all cases. There were no procedure-related complications, amputations, or deaths within 30 days. The median follow-up time was 1023 days.
Table II.
Graft and Intervention Characteristics (N=30)
| Time since bypass graft operation (months) | 12 [5, 45] |
| Distal target vessel | |
| Anterior tibial artery | 3 (10%) |
| Tibioperoneal trunk | 2 (7%) |
| Peroneal artery | 8 (27%) |
| Posterior tibial artery | 16 (53%) |
| Plantar artery | 1 (3%) |
| Conduit | |
| Autogenous saphenous vein | 20 (67%) |
| Autogenous arm vein | 2 (7%) |
| Composite autogenous arm and saphenous vein | 3 (10%) |
| Undocumented | 5 (17%) |
| Lesion length (mm, by angiography) | 15 [10,30] |
| Lesion stenosis (%, by angiography) | 80 [70, 90] |
| Site of stenosis | |
| Proximal anastomosis | 5 (17%) |
| Mid-graft | 14 (47%) |
| Distal anastomosis | 11 (37%) |
| Interventions | |
| Cutting balloon angioplasty | 24 (80%) |
| Stent placement | 3 (10%) |
| Balloon angioplasty alone | 3 (10%) |
Continuous variables represented as median [interquartile range].
Categorical variables represented as count (percentage%).
The percentage of patients with residual stenosis after intervention, as detected by the initial post-procedure DUS, was estimated by Kaplan-Meier analysis to be 21%. Among the three patients treated with stents, two were found to have residual stenosis. One patient with residual stenosis had surgical graft revision within 30 days. Within three months, two more patients had re-intervention and two more had undergone surgical revision. One patient was managed without further intervention.
Continued Graft Patency
Freedom from restenosis by Kaplan-Meier analysis was 37% at 12 and 24 months. One patient's residual stenosis resolved by DUS criteria without re-intervention by one year. Patency without reintervention was observed in 43% at one year and 36% at two years. Patients who went on to have patency without reintervention had older grafts at the time of intervention than those whose grafts subsequently developed restenosis (median age of 31 vs. 5 months, P=0.01). Rates of assisted patency with secondary intervention were 70% and 58% at the same time points. At 12 and 24 months, 74% and 79% of grafts were secondarily patent.
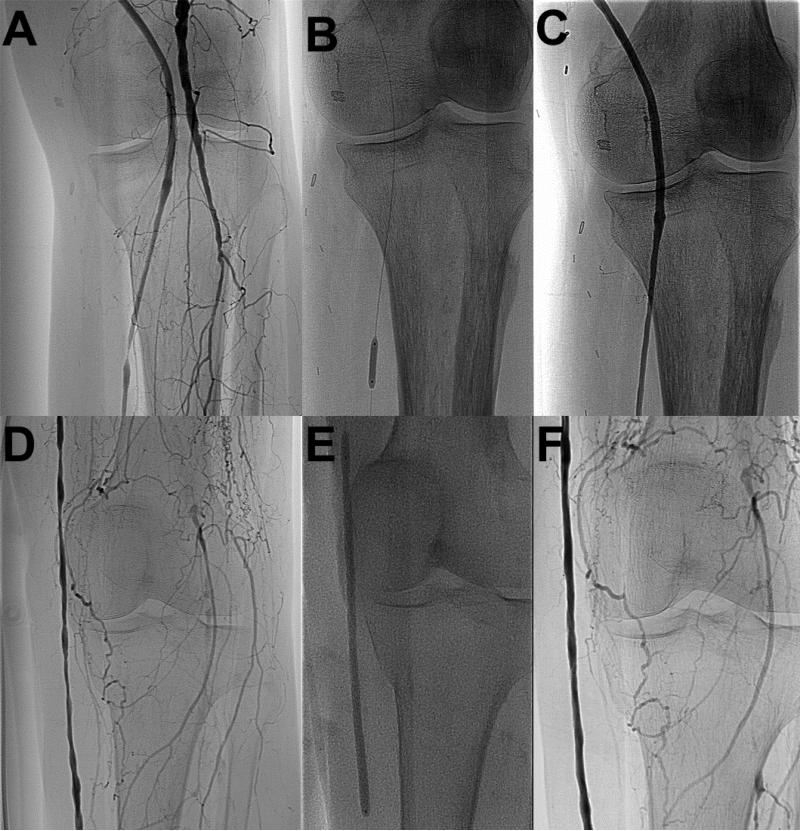
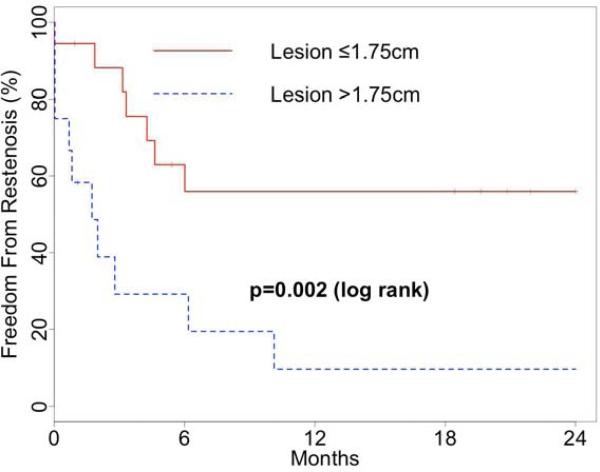
Given the established relationship between lesion length and restenosis in native vessel stenosis, we investigated the association of the length of the treated graft stenosis with subsequent restenosis risk. Receiver operating characteristic analysis indicated that a lesion length of 1.75cm maximized the sensitivity and specificity for predicting restenosis (C statistic: 0.74; representative angiographic images, Figure 1). The rate of residual stenosis was significantly lower for treated lesions ≤1.75cm (“short lesions”) than for lesions >1.75cm (“long lesions”), 6% versus 42% (p=0.03 by log rank test). The rate of freedom from restenosis for patients with short lesions was 56% at one and two years, and for long lesions was 10% at both time points (p<0.01 by log rank test, Figure 2). Patency without reintervention (59% vs. 0% at 2 years, P=0.01) and assisted patency with secondary intervention (86% vs. 0% at 2 years, P=0.02) rates were substantially better for those grafts with short lesions. Secondary patency was 92% at one and two years for short lesions, versus 50% and 57% for long lesions (P=0.07). All of the short lesions were treated with cutting balloon angioplasty.
Figure 1. Treatment of Short vs. Long Infrapopliteal Graft Lesions.
Representative angiographic images demonstrate a short (≤1.75 cm) lesion before (A), during (B), and after (C) and a long (>1.75 cm) lesion before (D), during (E), and after (F) endovascular intervention.
Figure 2. Freedom From Restenosis After Endovascular Infrapopliteal Graft Intervention by Lesion Length.
Short (≤1.75 cm) lesions had a higher rate of freedom from restenosis than longer lesions (>1.75) in the first two years after endovascular intervention for infrapopliteal graft stenosis.
Long-Term Limb and Patient Outcomes
Patients who died or required amputation during the follow-up period are summarized in Table III. Kaplan-Meier estimates of patient survival at one and two years were 96% and 81%, with a higher survival rate in patients with short lesions (P=0.02). Rates of MACE at one and two years were 16% and 29%.
Table III.
Cases of Mortality or Amputation Following Graft Intervention
| Patient # | Outcome | CLI Related? | Time to Event | Details |
|---|---|---|---|---|
| 1 | Death | No | 1.8 years | 63 year old man who died while living independently at home with a patent graft, a toe pressure of 84 mmHg, and no CLI symptoms. |
| 2 | Death | Yes | 2.5 months | 65 year old man who had a successful endovascular intervention but died 2 days after a TMA. He was not a candidate for open revision due to uncompensated CHF, and the only other option for his gangrenous toe would have been major amputation. |
| 3 | Death | No | 3.7 years | 84 year old woman who had no CLI symptoms and lived independently died two years after her procedure without any further interventions. |
| 4 | Death | No | 3.9 years | 74 year old man with no CLI symptoms who died suddenly at home with a graft that was patent at his most recent follow-up visit. |
| 5 | Death | No | 1.2 years | 81 year old woman with no CLI symptoms who died of cardiac causes. She had limited outflow and the distal graft anastomosis was below the malleolus, and as such was not a candidate for open revision. |
| 6 | Death | No | 1.9 years | 59 year old man who was living independently with a patent graft and no CLI symptoms in the treated limb. He died 2 years after his procedure from PEA arrest. |
| 7 | Death | Yes | 2.3 years | 89 year old woman who was in hospice care and was not a surgical candidate. Due to diffuse disease, she had no surgical options for graft revision other than replacement. |
| 8 | Amputation | Yes | 6 months | 84 year old man who was not a surgical revascularization candidate. His graft occluded within 3.5 months after his procedure, when he came for his first surveillance. |
| 9 | Death | Yes | 3.5 years | 60 year old morbidly obese diabetic man whose graft was patent when last evaluated before he died, 3.5 years after his procedure. He died of unknown cause two days after amputation of his contralateral foot due to gangrene. |
Abbreviations: CHF, congestive heart failure; CLI, critical limb ischemia; PEA, pulseless electrical activity; TMA, transmetatarsal amputation
The Kaplan-Meier estimate of 12-month amputation rate was 4%, and there were no major amputations performed between months 12 and 24. Rates of MALE at one and two years were 18% and 23%. At one year, median TBI had increased from a baseline of 0.33 to 0.43 (P=0.05) and 54% of patients had a TBI improved by 0.1 or more; at two years, median TBI was 0.49 (P=0.01) and 70% had improved by ≥0.1.
At one year, 13% of patients had active ulceration, none had rest pain, and 13% exhibited claudication symptoms. At two years, 26% had ulcers and 5% had claudication. None of the patients who had claudication symptoms at one year subsequently developed critical limb ischemia symptoms or received amputations. Clinical outcomes were also better in patients with short lesions than in those with longer lesions (Table IV).
Table IV.
Late Secondary Outcomes After Endovascular Graft Intervention, by Lesion Length
| Outcome | Short Lesions, 12 months (N=15) | Long Lesions, 12 months (N=12) | Short Lesions, 24 months (N=11) | Long Lesions, 24 months (N=6) |
|---|---|---|---|---|
| Mortality | 6% | 0% | 6% | 36% |
| MACE | 13% | 18% | 13% | 51% |
| MALE | 12% | 25% | 12% | 40% |
| Active ulceration | 8% | 20% | 17% | 43% |
| Median TBI increase | 0.13 | 0.07 | 0.25 | 0.15 |
Abbreviations: MACE, major adverse cardiac events; MALE, major adverse limb events; TBI, toe-brachial index
Discussion
In this single-center experience with an endovascular-first strategy (primarily using cutting balloon angioplasty, CBA) for management of stenoses in infrapopliteal bypass grafts, we report two main findings. First, we describe overall acceptable outcomes among patients with infrapopliteal bypass graft stenoses. Second, it was evident that infrapopliteal grafts treated for stenoses with shorter lesion lengths (<1.75 cm) at baseline fared significantly better than grafts treated for longer stenotic lengths.
While a few patients were treated with stenting or conventional balloon angioplasty (commonly referred to as “plain old balloon angioplasty” or POBA), the majority of patients in this series (83%) were treated with CBA. Studies of CBA for treatment of coronary artery stenoses suggested a benefit for CBA over conventional balloon angioplasty for prevention of restenosis25, although subsequent experience has led to the consensus that the two techniques lead to comparable outcomes for native vessel progressive atherosclerotic lesions.26 However, for in-stent restenosis in the coronary arteries, there does appear to be an advantage of CBA over conventional balloon angioplasty.27,28 Like the neointimal hyperplasia that causes stenosis in coronary stents, the lesions characteristic of bypass graft stenosis are typically firm, smooth, fibrous lesions that are more refractory to treatment with conventional balloon angioplasty than atherosclerotic lesions in native vessels. First reported by Engelke et al. in 2002, CBA of infrainguinal graft stenosis has subsequently been shown to have acceptable short-time patency rates, superior to standard balloon angioplasty and comparable to open surgical revision in selected patients.29,30 Positive results have not been uniformly observed, however, and even though the use of cutting balloons may provide good early results, CBA use does not eliminate the late risk of restenosis.20,31
In our cohort, restenosis rates were high, but overall clinical outcomes as assessed by limb preservation were good. Though the technical results after interventions appeared favorable by angiographic assessment, we found that one fifth of grafts had focal velocity elevations by DUS at their initial post-procedure follow up visit. This is consistent with our prior observation that the physiologic assessment provided by DUS may be more sensitive than two dimensional digital subtraction angiography for the detection of residual abnormalities.14 Because DUS was performed at a post-procedure visit rather than at the conclusion of the procedure, it is unclear whether those patients with apparent residual stenosis in fact had focal velocity elevations immediately following their intervention. While the results appeared successful angiographically, persistent stenosis may have been detectable by another modality. The use of intra-procedural intravascular ultrasound (IVUS) or DUS may be useful adjuncts to ensure treatment efficacy. The significant rate of residual stenosis or early restenosis may also indicate a degree of routine undersizing of our angioplasty balloons. IVUS may also be useful in this context, allowing more precise sizing than sizing based on angiography alone. Of note, the DUS criteria we used for identifying a ≥ 50% stenosis (adopted from established criteria) may be overly sensitive. However, clinical practice favors use of sensitive criteria so that opportunities to improve outcomes will not be missed.
Despite significant rates of residual and recurrent stenosis, patient- and limb-level outcomes were overall acceptable. The interventions were well tolerated, with procedural success in all cases and no 30-day mortality or procedure-related morbidity. We also observed that the majority of patients had sustained improvement in their hemodynamic status as measured by TBI. Despite this improvement in TBI, the median TBI was still below 0.5, which may indicate that while perfusion was sufficient to avoid amputation and heal wounds in the short term, disease progression or intimal hyperplasia significantly reduced these perfusion benefits over the long term. In addition, patients had acceptable medium-term clinical outcomes. While CLI patients have very high rates of limb loss and mortality, 96% of our patients avoided major amputation and 81% were still alive after two years. Of those who did die or require amputation, only one adverse outcome might be attributed to a failure of surveillance (patient 8, Table III). Moreover, only one quarter of the patients had symptomatic CLI at two years. Reviewing patient records, we did not identify any cases in which the patient would have been expected to have had a better outcome if surgical intervention had been offered as the initial treatment for the failing graft.
This study focused on bypass grafts to distal target vessels. Of prior studies of CBA for treatment of stenoses in lower extremity bypass grafts, only that by Garvin and Reifsnyder included a significant proportion of grafts to tibial targets.20 Their study, which limited interventions to lesions less than 2 cm in length, reported a high rate of complications (11%) and six month rates of patency without reintervention and assisted patency with secondary intervention of only 48% and 72%.
Previous studies of endovascular interventions in infrainguinal grafts have found that lesions longer than 2 cm were associated with a higher risk of failure.16,32 Studies of CBA, however, systematically excluded longer lesions.20,30,33 Our results extend the findings of CBA for treatment of stenoses in infrapopliteal grafts and, by using receiver operating characteristic analysis, we were able to more rigorously determine the lesion length associated with restenosis. Given the lower rates of mortality and MACE in patients with shorter compared to those with longer lesions, lesion length in this cohort may reflect a greater systemic burden of disease. As noted above, some of the longer lesions were also treated with techniques other than CBA, in part because long cutting balloon catheters were not available for all of the study period. Newer technologies such as long (e.g., 100 mm length) scoring balloons may be efficacious in treating such lesions. Future studies should further evaluate the efficacy of CBA and scoring balloon angioplasty in treating long lesions.
As might be expected in a cohort of patients with distal bypass grafts, patients in the present study had a high rate of diabetes – higher than in previously reported series. As the outcomes observed in the present study are comparable to (or better than) those of prior reports, the current data provide anecdotal support for the suggestion that neither diabetes nor a distal site of graft anastomosis (i.e. to a tibial artery versus a popliteal artery) predicts graft failure.16,32
This study has recognized limitations, primarily its size and observational design. It is a report of a single center experience. The size of the cohort does not allow for multivariate analysis, which could more rigorously compare the relative effects of multiple factors on outcomes. Due to the design of the study, follow-up was non-random and some differences likely represent bias from loss to follow-up or selective testing based on disease severity, such as the relatively low median TBI in follow-up. For patients predicted to have a lower risk of success with endovascular intervention, direct comparison with open surgical revascularization would be valuable. To make such comparisons, it would be ideal to conduct prospective analyses of competing surveillance and intervention strategies. Alternatively, this topic might be amenable to study using data from the Vascular Quality Initiative (VQI). In the absence of a control group followed without intervention, the natural history of patients with failing infrapopliteal bypass grafts also cannot be determined.
Conclusion
In summary, this is a report of an endovascular-first strategy to maintain infrapopliteal graft patency and preserve limbs. The majority of patients, especially those with short lesions treated with cutting balloon angioplasty, demonstrated continued graft patency and improved hemodynamic status, healed wounds, avoided new or recurrent CLI symptoms, and lived to at least two years after their procedure without losing their limb. Further study is needed to determine the optimal approach to surveillance and treatment of these grafts, limbs, and patients.
Acknowledgements
This project was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR 000002 and linked award TL1 TR 000133.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007 Jan;45(1):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 2.Rollins KE, Jackson D, Coughlin PA. Meta-analysis of contemporary short- and long-term mortality rates in patients diagnosed with critical leg ischaemia. Br J Surg. 2013 Jul;100(8):1002–8. doi: 10.1002/bjs.9127. [DOI] [PubMed] [Google Scholar]
- 3.Schamp KBC, Meerwaldt R, Reijnen MMPJ, Geelkerken RH, Zeebregts CJ. The ongoing battle between infrapopliteal angioplasty and bypass surgery for critical limb ischemia. Ann Vasc Surg. 2012 Nov;26(8):1145–53. doi: 10.1016/j.avsg.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FGR, Gillespie I, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: An intention-to-treat analysis of amputation-free and overall survival in patients randomized to a bypass surgery-first or a balloon angioplasty-first revascularization strategy. J Vasc Surg. 2010 May;51(5 Suppl):5S–17S. doi: 10.1016/j.jvs.2010.01.073. [DOI] [PubMed] [Google Scholar]
- 5.Tannenbaum GA, Pomposelli FB, Jr, Marcaccio EJ, Gibbons GW, Campbell DR, Freeman DV, et al. Safety of vein bypass grafting to the dorsal pedal artery in diabetic patients with foot infections. J Vasc Surg. 1992 Jun;15(6):982–988. discussion 989–990. [PubMed] [Google Scholar]
- 6.Marks J, King TA, Baele H, Rubin J, Marmen C. Popliteal-to-distal bypass for limb-threatening ischemia. J Vasc Surg. 1992 May;15(5):755–759. doi: 10.1067/mva.1992.36607. discussion 759–760. [DOI] [PubMed] [Google Scholar]
- 7.Conte MS. Understanding objective performance goals for critical limb ischemia trials. Semin Vasc Surg. 2010 Sep;23(3):129–37. doi: 10.1053/j.semvascsurg.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Singh N, Sidawy AN, DeZee KJ, Neville RF, Akbari C, Henderson W. Factors associated with early failure of infrainguinal lower extremity arterial bypass. J Vasc Surg. 2008 Mar;47(3):556–61. doi: 10.1016/j.jvs.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen LL, Conte MS, Menard MT, Gravereaux EC, Chew DK, Donaldson MC, et al. Infrainguinal vein bypass graft revision: factors affecting long-term outcome. J Vasc Surg. 2004 Nov;40(5):916–23. doi: 10.1016/j.jvs.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 10.Lancaster RT, Conrad MF, Patel VI, Cambria RP, LaMuraglia GM. Predictors of early graft failure after infrainguinal bypass surgery: a risk-adjusted analysis from the NSQIP. Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg. 2012 May;43(5):549–55. doi: 10.1016/j.ejvs.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 11.Goodney PP, Nolan BW, Schanzer A, Eldrup-Jorgensen J, Bertges DJ, Stanley AC, et al. Factors associated with amputation or graft occlusion one year after lower extremity bypass in northern New England. Ann Vasc Surg. 2010 Jan;24(1):57–68. doi: 10.1016/j.avsg.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Bandyk DF. Surveillance After Lower Extremity Arterial Bypass. Perspect Vasc Surg Endovasc Ther. 2007 Dec 1;19(4):376–83. doi: 10.1177/1531003507310460. [DOI] [PubMed] [Google Scholar]
- 13.Lundell A, Lindblad B, Bergqvist D, Hansen F. Femoropopliteal-crural graft patency is improved by an intensive surveillance program: a prospective randomized study. J Vasc Surg. 1995 Jan;21(1):26–33. doi: 10.1016/s0741-5214(95)70241-5. discussion 33–34. [DOI] [PubMed] [Google Scholar]
- 14.Humphries MD, Pevec WC, Laird JR, Yeo KK, Hedayati N, Dawson DL. Early duplex scanning after infrainguinal endovascular therapy. J Vasc Surg. 2011 Feb;53(2):353–8. doi: 10.1016/j.jvs.2010.08.045. [DOI] [PubMed] [Google Scholar]
- 15.Bui TD, Mills JL, Sr, Ihnat DM, Gruessner AC, Goshima KR, Hughes JD. The natural history of duplex-detected stenosis after femoropopliteal endovascular therapy suggests questionable clinical utility of routine duplex surveillance. J Vasc Surg. 2012 Feb;55(2):346–52. doi: 10.1016/j.jvs.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Avino AJ, Bandyk DF, Gonsalves AJ, Johnson BL, Black TJ, Zwiebel BR, et al. Surgical and endovascular intervention for infrainguinal vein graft stenosis. J Vasc Surg. 1999 Jan;29(1):60–71. doi: 10.1016/s0741-5214(99)70361-7. [DOI] [PubMed] [Google Scholar]
- 17.McCoach CE, Armstrong EJ, Singh S, Javed U, Anderson D, Yeo KK, et al. Gender-related variation in the clinical presentation and outcomes of critical limb ischemia. Vasc Med Lond Engl. 2013 Feb;18(1):19–26. doi: 10.1177/1358863X13475836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westin GG, Armstrong EJ, Bang H, Yeo K-K, Anderson D, Dawson DL, et al. Association Between Statin Medications and Mortality, Major Adverse Cardiovascular Event, and Amputation-Free Survival Rates in Patients with Critical Limb Ischemia. J Am Coll Cardiol. 2014 Feb 25;63(7):682–90. doi: 10.1016/j.jacc.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Söder HK, Manninen HI, Jaakkola P, Matsi PJ, Räsänen HT, Kaukanen E, et al. Prospective trial of infrapopliteal artery balloon angioplasty for critical limb ischemia: angiographic and clinical results. J Vasc Interv Radiol JVIR. 2000 Sep;11(8):1021–31. doi: 10.1016/s1051-0443(07)61332-3. [DOI] [PubMed] [Google Scholar]
- 20.Garvin R, Reifsnyder T. Cutting balloon angioplasty of autogenous infrainguinal bypasses: short-term safety and efficacy. J Vasc Surg. 2007 Oct;46(4):724–30. doi: 10.1016/j.jvs.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 21.Roth SM, Bandyk DF. Duplex imaging of lower extremity bypasses, angioplasties, and stents. Semin Vasc Surg. 1999 Dec;12(4):275–84. [PubMed] [Google Scholar]
- 22.Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia: Revised version. J Vasc Surg. 1997 Sep;26(3):517–38. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 23.R Core Team . R: A language and environment for statistical computing. [Internet] R Foundation for Statistical Computing; Vienna, Austria: 2013. Available from: http://www.R-project.org/ [Google Scholar]
- 24.Therneau T. A Package for Survival Analysis in S [Internet] 2013 Available from: http://CRAN.R-project.org/package=survival.
- 25.Kondo T, Kawaguchi K, Awaji Y, Mochizuki M. Immediate and chronic results of cutting balloon angioplasty: a matched comparison with conventional angioplasty. Clin Cardiol. 1997 May;20(5):459–63. doi: 10.1002/clc.4960200511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mauri L, Bonan R, Weiner BH, Legrand V, Bassand J-P, Popma JJ, et al. Cutting balloon angioplasty for the prevention of restenosis: results of the Cutting Balloon Global Randomized Trial. Am J Cardiol. 2002 Nov 15;90(10):1079–83. doi: 10.1016/s0002-9149(02)02773-x. [DOI] [PubMed] [Google Scholar]
- 27.Muramatsu T, Tsukahara R, Ho M, Ito Y, Hirano K, Ishimori H, et al. Efficacy of cutting balloon angioplasty for in-stent restenosis: an intravascular ultrasound evaluation. J Invasive Cardiol. 2001 Jun;13(6):439–44. [PubMed] [Google Scholar]
- 28.Adamian M, Colombo A, Briguori C, Nishida T, Marsico F, Di Mario C, et al. Cutting balloon angioplasty for the treatment of in-stent restenosis: a matched comparison with rotational atherectomy, additional stent implantation and balloon angioplasty. J Am Coll Cardiol. 2001 Sep;38(3):672–9. doi: 10.1016/s0735-1097(01)01458-9. [DOI] [PubMed] [Google Scholar]
- 29.Engelke C, Morgan RA, Belli A-M. Cutting balloon percutaneous transluminal angioplasty for salvage of lower limb arterial bypass grafts: feasibility. Radiology. 2002 Apr;223(1):106–14. doi: 10.1148/radiol.2231010793. [DOI] [PubMed] [Google Scholar]
- 30.Schneider PA, Caps MT, Nelken N. Infrainguinal vein graft stenosis: cutting balloon angioplasty as the first-line treatment of choice. J Vasc Surg. 2008 May;47(5):960–966. doi: 10.1016/j.jvs.2007.12.035. discussion 966. [DOI] [PubMed] [Google Scholar]
- 31.Vikram R, Ross RA, Bhat R, Griffiths GD, Stonebridge PA, Houston JG, et al. Cutting balloon angioplasty versus standard balloon angioplasty for failing infra-inguinal vein grafts: comparative study of short- and mid-term primary patency rates. Cardiovasc Intervent Radiol. 2007 Aug;30(4):607–10. doi: 10.1007/s00270-007-9005-x. [DOI] [PubMed] [Google Scholar]
- 32.Simosa HF, Pomposelli FB, Dahlberg S, Scali ST, Hamdan AD, Schermerhorn ML. Predictors of failure after angioplasty of infrainguinal vein bypass grafts. J Vasc Surg. 2009 Jan;49(1):117–21. doi: 10.1016/j.jvs.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Kasirajan K, Schneider PA. Early outcome of “cutting” balloon angioplasty for infrainguinal vein graft stenosis. J Vasc Surg. 2004 Apr;39(4):702–8. doi: 10.1016/j.jvs.2003.10.046. [DOI] [PubMed] [Google Scholar]