Abstract
One well accepted functional feature of the parkinsonian state is the recording of enhanced beta oscillatory activity in the basal ganglia. This has been demonstrated in patients with Parkinson's disease (PD) and in animal models such as the rat with 6-hydroxydopamine (6-OHDA)-induced lesion and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated monkeys, all of which are associated with severe striatal dopamine depletion. Neuronal hyper-synchronization in the beta (or any other) band is not present despite the presence of bradykinetic features in the rat and monkey models, suggesting that increased beta band power may arise when nigro-striatal lesion is advanced and that it is not an essential feature of the early parkinsonian state. Similar observations and conclusions have been previously made for increased neuronal firing rate in the subthalamic and globus pallidus pars interna nuclei. Accordingly, it is suggested that early parkinsonism may be associated with dynamic changes in basal ganglia output activity leading to reduced movement facilitation that may be an earlier feature of the parkinsonian state.
Keywords: Basal ganglia, Oscillatory activity, Parkinson's disease, Beta oscillations
Introduction
The pathophysiological model of the basal ganglia that developed in the mid-1980s (DeLong, 1990; Penney and Young, 1986) fueled a large body of basic and clinical research leading to a better understanding of movement disorders and the revitalization of surgical treatments for Parkinson's disease (PD). Essentially, the model postulated that the basal ganglia integrate and process information through a series of connections from the cerebral cortex to the striatum, and via the striato-pallidal projections to the globus pallidus pars interna (GPi) and substantia nigra pars reticulata (SNpr), to provide feed-back via the ventral thalamus to the cerebral cortex.
A general functional scheme was put forward whereby activation of the “indirect circuits” leads to movement inhibition or arrest while activation of the “direct” circuit facilitates movement execution (Chevalier and Deniau, 1990; DeLong, 1990). The parkinsonian state mainly featured striatal dopamine depletion and increased neuronal activity in the subthalamic nucleus (STN) and GPi/SNpr leading to over-inhibition of the thalamocortical and brainstem motor systems, whereas the dyskinetic state featured the opposite, i.e. hypoactivity in the STN and GPi, leading to disinhibition of the thalamo-cortical projection. Accordingly, the parkinsonian state could be seen as a shift in the internal equilibrium of the basal ganglia, whereby over-activity in the excitatory STN-GPi pathway predominates, leading to excessive spontaneous firing, increased responsiveness to peripheral stimuli and decreased signal to noise ratio of the GPi, all of which go against the normal facilitation of movement (Crossman, 1990; Obeso et al., 2000b). In essence the model proposed, in physiological terms, that the degree of neuronal activity in the output nuclei determines the motor state.
Whereas the basic features of the model in the normal, parkinsonian and dyskinetic states have been supported by a large body of experimental and clinical data, including recent studies using optogenetics (Bateup et al., 2010; Kravitz et al., 2010), the “firing rate model” is known to exhibit several inconsistencies (Montgomery, 2007; Obeso et al., 2000a). Among these, we may highlight the following observations: 1. Increased firing rate in the STN and GPi (or entopeduncular nucleus of the rat) is not necessarily associated with parkinsonism in animal models of PD (Hashimoto et al., 2003; Ruskin et al., 2002; Tachibana et al., 2011). 2. Increased GPi neuronal firing induced by STN stimulation (136 Hz) in the awake 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) monkey is actually associated with motor improvement (Hashimoto et al., 2003). 3. Pallidotomy, which should reduce GPi activity towards zero leading to further disinhibition of the thalamo-cortical projection (Obeso et al., 2000a) has a profound anti-dyskinetic effect in monkeys and humans. 4. Thalamotomy, which should further reduce and impair thalamo-cortical activity, is not associated with aggravation of motor features in PD (Marsden and Obeso, 1994). These findings have led to the idea that the pathophysiology of basal ganglia movement disorders may not be solely explained by changes in the firing rate in the STN/GPi but may also involve abnormal oscillatory patterns of neuronal activity (Montgomery, 2007; Obeso et al., 2000a; Vitek et al., 1999; Wichmann et al., 2011). Thus, in the GPi of patients with hemi-ballismus and generalized dystonia abnormal discharge patterns have been described in the form of highly irregular spiking and intermittently grouped discharges separated by periods of pauses (Vitek et al., 1999). In addition, increased bursting activity at around 8-15 Hz has been recognized in the STN, globus pallidus pars externa (GPe) and GPi in the MPTP monkey model (Bergman et al., 1994; Nini et al., 1995; Tachibana et al., 2011), which was reduced after subthalamotomy (Wichmann et al., 1994).
However, definitive support for the role of oscillations and synchrony of the basal ganglia in the pathophysiology of parkinsonism arose when Brown et al. (2001) reported local field potentials (LFPs) recorded from implanted DBS macro-electrodes in the STN of PD patients. It is now well accepted that low beta activity (12-20 Hz, peak at 16 Hz) predominates in the “off medication state and is significantly attenuated during the “on” medication state, along with the appearance of higher frequencies (70-80 Hz gamma activity and 300 Hz activity) (Foffani et al., 2003; Kuhn et al., 2009; Levy et al., 2002; Lopez-Azcarate et al., 2010). Moreover, PD patients with levodopa-induced dyskinesias exhibit a peak of activity around 8 Hz in the dorsal STN (Alonso-Frech et al., 2006), while those with drug-induced impulsivity show increased activity at a slightly lower frequency (6 Hz) in the ventral STN (Rodriguez-Oroz et al., 2011). Dopaminergic medication also affects cortico-subthalamic coherence, whereby beta coherence predominates in the “off state and gamma coherence appears in the “on” state (Williams et al., 2002). Similar oscillations also occur in the GPi (Cassidy et al., 2002; Priori et al., 2002; Weinberger et al., 2012) and pedunculopontine nucleus (PPN) of PD patients (Weinberger et al., 2008).
The increased power in the low beta range is a critical feature of the dopamine depleted basal ganglia in PD patients. Such increased beta activity has been put forward as another major brake opposing movement initiation and execution in PD patients (Jenkinson and Brown, 2011; Stein and Bar-Gad, 2012). Accordingly, several studies have been carried out in animal models aiming to document the normal patterns of oscillations in the basal ganglia, data obviously not available from studies in human patients, and to foster pathophysiological research (Berke et al.,2004; Courtemanche et al., 2003; DeCoteau et al.,2007; Rodriguez et al., 2003). Indeed, a comprehensive understanding of the origin and significance of pathological oscillations in the parkinsonian brain could be very useful to monitor the effect of new therapies and ascertain putative newer potential surgical targets for PD and other movement disorders (Hariz, 2012; Jenkinson and Brown, 2011; Krack et al., 2010) and, nonetheless, to unravel the dynamic of basal ganglia-cortex interaction at the beginning and during the evolution of dopaminergic loss. However, the results obtained in experimental models have been variable and even somewhat contradictory (Israel and Bergman, 2008; Wichmann and DeLong, 2003). Here, we revisit the current understanding and significance of oscillatory activity, and the beta band in particular, throughout the onset and evolution of the parkinsonian state.
Experimental findings
Normal activity
Oscillatory activity has been established in intact animals but neuronal discharge synchrony is not a paramount feature of the basal ganglia under normal and awake conditions (Bar-Gad and Bergman, 2001; Bergman et al., 1994; Wichmann and DeLong, 2003). In monkeys for instance, the proportion of pair units firing in synchrony in the GPi/GPe or STN is very low (i.e. 10%) (Heimer et al., 2006; Leblois et al., 2007; Wichmann et al., 1994) and some authors (Bergman et al., 1998; Raz et al., 2001; Wichmann et al., 1994) have found no evidence of activities characteristic of PD within the power spectrum of resting healthy macaques. However, one study (Courtemanche et al., 2003) described the presence of 10-25 Hz activity in the monkey with chronically implanted electrodes in different portions of the striatum, in the normal state, which was modulated during behavioral tasks.
In the rat, LFPs recorded from the motor cortex and basal ganglia (STN and striatum) have revealed the presence of activity peaks in the theta (4-8 Hz) (Magill et al., 2006) and gamma (30-100 Hz) bands (Berke, 2009). However, the degree of oscillatory activity is highly influenced by the state of consciousness. Studies in the anesthetized rats under ketamine have shown cortical low frequency large amplitude oscillations of approximately 1 Hz on which a faster oscillation of 7-12 Hz (spindles) is superimposed, both of which are synchronous with STN activity (Magill et al., 2000). On the other hand, in non-anesthetized awake animals cortico-STN synchronization is very low (Brazhnik et al., 2012; Sharott et al., 2005a, 2005b) and under physiological sleep these low frequency oscillations do not correlate with STN activity (Urbain et al., 2000).
One study showed oscillatory activity in the range of 46-70 Hz in the gamma band in the STN. This gamma range activity was increased after treatment with dopaminergic drugs, mimicking to a certain extent what is observed in PD patients after receiving levodopa (Brown et al., 2002). This finding led to the suggestion that high-frequency activity in the STN of healthy rats and in levodopa-treated PD patients may represent a normal physiological feature rather than the consequence of the parkinsonian state, and that gamma oscillations play a normal role in facilitating automatic movements (Jenkinson et al., 2012). Moreover, in patients with presumed normal basal ganglia function such as in Essential Tremor, recording in the STN revealed high frequency activity (±200 Hz) (Danish et al., 2007) which might be related to the 300 Hz oscillations found in the STN of PD patients in the “on” medication state (Foffani et al., 2003; Lopez-Azcarate et al., 2010).
The parkinsonian state
The most commonly applied PD animal models to study the physiological features of the brain are the MPTP monkey and the rat with unilateral 6-hydroxydopamine (6-OHDA) lesion.
MPTP monkey model
Neuronal recordings in monkeys are typically performed with microelectrodes aiming to isolate single unit potentials, which do not convey information about neuronal population activity in a given structure. Accordingly, data regarding oscillatory activity in this model is not exactly comparable with that derived from local field recordings in patients and in the rat model. Activity in the beta range has been recognized in the STN and GPi of MPTP monkeys (Bergman et al., 1994; Tachibana et al., 2011). Several studies conducted on the African green monkey revealed that neuronal synchronization between STN and GPi and between striatal cholinergic interneurons and GPi was augmented, clearly departing from firing activity and degree of synchronization in the normal basal ganglia. The percentage of paired cells firing in synchrony increases significantly (Bergman et al., 1998; Leblois et al., 2007) once monkeys are fully parkinsonian. In this model, the peak of such synchronous spiking activity appeared between 10 and 12 Hz coinciding with the frequency of tremor typically observed in African green or vervet monkeys, which are the only ones exhibiting tremor at rest after MPTP (Bergman et al., 1998; Nini et al., 1995; Raz et al., 2001). Accordingly, the MPTP monkey model supports, by and large, the findings in PD patients (Levy et al., 2000, 2002; Zaidel et al., 2010) indicating that severe striatal dopamine depletion leads to increased synchronization in the basal ganglia in the low beta range. Indeed, synchronization between oscillatory local field potentials (LFPs) and single neuronal action potential activity within a given nucleus, i.e. STN, is enhanced in the parkinsonian state, quite unlike the normal state (Bar-Gad and Bergman, 2001; Wichmann and DeLong, 2003).
While the above findings are fairly clear for the established and advanced parkinsonian state, studies at an earlier phase of nigro-striatal dopamine depletion have produced a different picture. The group led by Boraud investigated the temporal dynamics of basal ganglia changes along with increased nigro-striatal lesion in 2 monkeys progressively intoxicated with MPTP and found some unexpected changes. Thus, by recording paired neurons from the GPi at baseline and longitudinally during MPTP administration until severe parkinsonism was obtained, they showed that the onset of initial bradykinetic features (i.e. first 15 days of intoxication) was not associated with enhanced synchrony or oscillatory activity in GPi neurons. Hyper-synchrony did appear, but only once severe parkinsonism had developed along with marked striatal dopamine depletion (Leblois et al., 2007). In this same study, an initial motor deficit during a simple upper limb movement was associated with a drastic change in the proportion of neurons responding with inhibition/excitation and the number of spikes (response amplitude) per neuron. Thus, during these first days of MPTP intoxication the proportion of neurons showing multi-spike excitation grew by around 50%, whereas the proportion of neurons exhibiting a normal inhibitory response fell significantly from 67.5 to 35.3%. This implies a general increase in excitability of GPi cells and a reduction in the number of GPi neurons capable of pausing to facilitate movement execution (DeLong, 1990).
The 6-OHDA rat model
Studies have been conducted in the 6-OHDA unilaterally lesioned rat. A peak of beta activity (essentially around 30–35 Hz in the awake animal and around 20 Hz in the anesthetized rat) has been described in the STN, SNpr, and GP and in the motor cortex (Avila et al., 2010; Sharott et al., 2005b). Recordings from the SNpr showed enhanced expression of high beta/low gamma activity (25–40 Hz) in basal ganglia output related to the transition from inattentive rest to alert, and while walking on a circular treadmill, in hemiparkinsonian animals (Avila et al., 2010; Brazhnik et al., 2012). Beta activity at around 20 Hz (15-30 Hz range) was also augmented in the GP of anesthetized hemiparkinsonian rats during stages of desynchronized cortical activity (Mallet et al., 2008) while the entopeduncular nucleus showed increased activity in a lower frequency range (4-18 Hz) (Ruskin et al., 2002) in awake immobilized and locally anesthetized rats.
A recent study using nonlinear time series analysis compared neuronal activity recorded simultaneously in STN and GPe in anesthetized rats, in order to quantify the interactions between both nuclei in 6-OHDA lesioned rats compared to control rats. Dopamine depletion altered firing rates and led to strong beta-frequency oscillations (∼20 Hz) in the STN-GPe network. This was paralleled by augmented bidirectional interactions between both nuclei as ascertained by the causal mutual information method. This has led to the consideration that such enhanced interaction could not only underlie excessive beta synchrony but also obstruct information flow and representation within the STN-GPe network and the rest of the basal ganglia (Cruz et al., 2011).
High increased coherence in the beta range (25-40 Hz) has been also found between the cortex and STN (Mallet et al., 2008), the cortex and SNpr (Brazhnik et al., 2012) and between the primary motor and somatosensory cortical areas (Degos et al., 2009; Moran et al., 2011). One recent study (Walter's laboratory at NIH) showed that increased motor cortex-SNpr (Brazhnik et al., 2010) LFP coherence precedes the enhancement of beta LFP oscillations in these structures, suggesting that tight synchronization could be an early feature of the parkinsonian state. Similarly, a previous study in the rat with 6-OHDA lesion (Dejean et al., 2012) showed that over-synchronization of neuronal activity between the cortex and basal ganglia did not signal the onset of motor impairment which was more directly correlated with a shortening in SNpr neuronal response after cortical activation. Thus, a decorrelation between the onset of motor manifestations in the rat and hyper-synchronization seems well established in this model.
The rat studies summarized above support the idea that dopamine depletion has a significant effect on synchronization and oscillatory activities in basal ganglia circuits. Accordingly, enhanced beta activity within the basal ganglia in the “indirect” circuit and between the motor cortex and the STN is considered a net characteristic of the unilaterally dopamine-depleted rat (Urbain et al., 2000). Administration of dopaminergic agents, such as apomorphine or L-dopa (Brazhnik et al., 2012; Degos et al., 2009; Sharott et al., 2005b), attenuates beta activity, further mimicking the observations in PD patients. However, most studies examining oscillatory activity in the rat model have been carried out in animals with >90% striatal dopaminergic loss, which is somewhat reminiscent of the 80-85% nigro-striatal deficit associated with advanced PD patients (Djaldetti et al., 2011; Fearnley and Lees, 1991; Nandhagopal et al., 2011). Thus, most PD patients submitted to DBS surgery have long disease evolution (mean = 14 years) and therefore are expected to have substantial striatal (and extra-striatal) dopaminergic depletion. This is unlike the typical experimental conditions. Thus, studies at early stages of nigro-striatal lesion are not very common either. One study which evaluated the effect of chronic administration of D1/D2 antagonists (Degos et al., 2009) reported a peak of cortical beta activity, paralleling that typically observed in animals with dopaminergic lesion by 6-OHDA. However, the onset of such beta activity occurred after the onset of akinetic features suggesting that the emergence of abnormal oscillatory activity reflects major but late changes in cortico-basal ganglia dynamics. In this regard, a recent study (Leventhal et al., 2012) compared neural activity during four distinct variants of a cued choice task in rats in order to analyze the functional correlates of beta oscillations. This study indicates that enhanced beta power and synchronization between cortex and basal ganglia circuits normally occur at precise brief moments of task performance and suggests that beta is associated with a post-decision change of the cortical-basal ganglia system, which decreases interference with putative competitive actions. These authors propose that enhanced beta activity in PD might replicate over-stabilization of the cortico-basal ganglia network, generating pathological persistence in the motor system. Interestingly, this study also suggests that fast reduction of dopaminergic levels is not enough for the emergence of beta oscillations in the basal ganglia.
Degree of dopaminergic striatal depletion and oscillatory activity
Experiments in the rat have been conducted under different experimental conditions, i.e. awake or under anesthesia (urethane, ketamine, etc.), immobilized (with gallamine triethiodide) or free moving and performing tasks. Similarly, the MPTP monkey model has important variability, which includes the use of different intoxication protocols and the presence or absence of rest tremor (due to differences between species). Neurophysiological studies have included the evaluation of oscillations from single unit action potential activity, pairs of neurons recorded simultaneously and LFPs. One possibly important caveat is that the peak beta band frequency recorded in the rat, monkey and PD patients differs. Whether or not this is a species-derived difference or reflects distinct pathophysiological mechanisms is not yet clear.
Despite all of these different confounding factors, some conclusions may be reached from the bibliography. Thus, rats with acute 6-OHDA lesion, as well as monkeys with severe MPTP induced parkinsonism, typically show >95% of dopamine depletion. In these conditions, beta band activity is enhanced and increased coherence between the basal ganglia and cortex is present which mimics the findings in PD patients reasonably well (Brown et al., 2001; Kuhn et al., 2004, 2005; Levy et al., 2002; Lopez-Azcarate et al., 2010; Priori et al., 2002). However, neuronal hyper-synchronization in the beta (or any other) band was not recorded in the basal ganglia and cortex in the few experimental instances when a partial and slow dopaminergic lesion was present, despite the presence of bradykinetic features in the rat and monkey models. Furthermore, stimulation with beta frequency pulses of the STN (by optogenetics) (Gradinaru et al., 2009) had no effect on rats with 6-OHDA unilateral lesions.
Our preliminary results (Fig. 1) using different types of 6-OHDA-induced lesion in the rat are in keeping with the published data. We have been recording oscillatory activity in SNpr in rats comparing the effect of a complete (6 μg of 6-OHDA HBr in 3 μl of 0.9% saline with 0.01% ascorbic acid) and partial (3 μg of 6-OHDA) 6-OHDA lesion of the medial forebrain bundle (MFB) in order to ascertain how the severity of dopaminergic depletion influences activity in the beta range. We also provoked a more progressive dopaminergic lesion by intracerebroventricular (ICV) 6-OHDA administration over 7 days (700 μg of 6-OHDA as final dose) (Rodriguez et al., 2001). Recordings from chronically implanted electrodes in SNpr show a clear peak of oscillatory activity in the high beta/low gamma frequency range (25–40 Hz) (Figs. 1A, B) only in the complete DA deprived hemisphere (Fig. 1C) (mean loss of tyrosine hydroxylase (TH+) stained cells: 97% in left hemisphere) 14 days after the unilateral 6-OHDA lesion. A similar activity is not seen in animals with partial 6-OHDA lesion through either MFB injections or ICV 6-OHDA administration (mean loss of TH+ stained cells: 72% in left hemisphere for MFB-partial lesioned and 65% for ICV-partial administration). These initial results suggest that a large dopaminergic nigro-striatal denervation is necessary for enhancing beta power in this animal model.
Fig. 1.
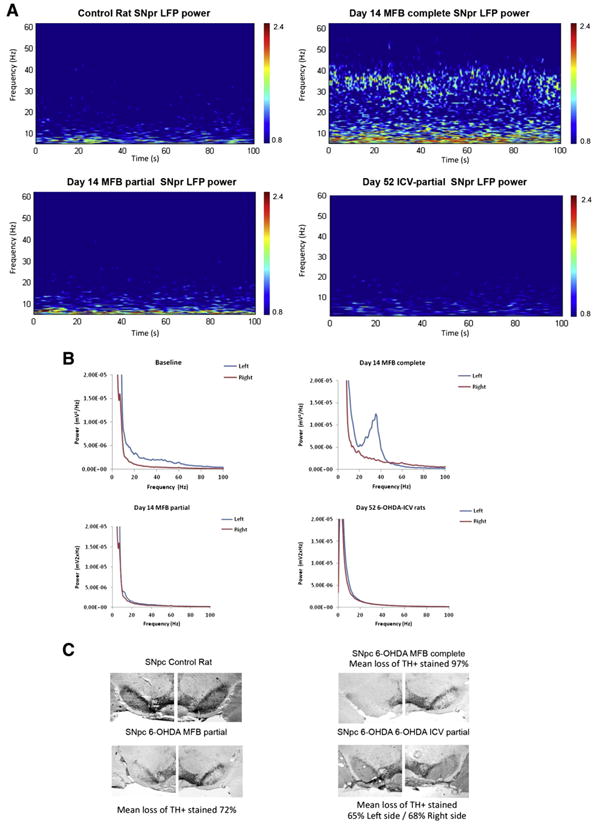
Power of SNpr LFP activity and inmunohistochemical results in intact and 6-OHDA lesioned rats, during treadmill walking. A. Representative wavelet-based scalograms represent the time–frequency plots of LFP spectral power in SNpr during treadmill walking. Spectral power was plotted on a logarithmic scale with greater power being represented by red colors, the color scales' range is from 0.8 to 2.4 mV2/Hz. Corresponding from upper left to lower right: control animal, Day 14 after 6-OHDA-MFB complete lesion (n = 1) (note the emergence of a high beta band at 25–40 Hz), Day 14 after 6-OHDA-MFB partial lesion (n = 1) and Day 52 after 6-OHDA-intraventricular partial lesion (n = 1). B. Linear graphs show averaged LFP power spectra from intact hemisphere (right, red) in baseline, and lesioned hemisphere (left, blue) complete and partial MFB lesion, at 14 days post-lesion (n = 1), note the emergence of the peak at ∼35 Hz in complete lesion. For ICV-partial lesioned animals both right and left are lesioned hemispheres (no intact side) (n = 4). C. Immunohistochemical results. Coronal sections of the substantia nigra pars compacta (SNpc) illustrating immunohistochemistry of tyrosine hydroxylase (TH). Note the deficiency of TH+ cells in the SNpc of the lesioned (left) hemisphere compared with the non-lesioned (right) hemisphere in complete MFB lesion. Mean loss of TH+ stained, in MFB-complete 97%, MFB-partial 72%, and ICV 65% in left hemisphere and 68% in right hemisphere.
Altogether, the available data suggest that the beta band enhancement and hypersynchronization throughout the motor loop are relatively late pathophysiological events associated with severe SNpc-striatal lesion (Fig. 2). This casts some doubt on its causal role as an inductor or mediator of early parkinsonian motor features.
Fig. 2.
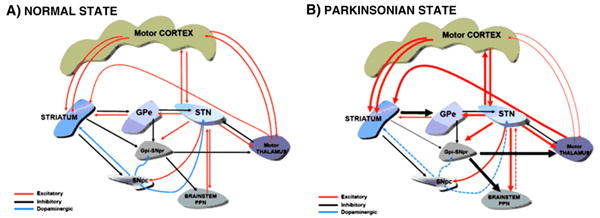
A. Schematic diagram of the main connections within the basal ganglia and major output projections. The diagram emphasizes the bilateral connections between nuclei present between several structures. B. Major changes in the functional state of the connections (indicated by thicker arrows) in the severe parkinsonian state. A net predominance of glutamatergic hyperactivity in several projections is noticed. Arrow colors indicate GABAergic inhibitory synapse in black, glutamatergic excitatory in red and dopaminergic in blue.
What is the physiological essence of the parkinsonian state?
The classic pathophysiological model of the basal ganglia was originally understood as a “go through” cortico-basal ganglia-thalamocortical system whereby dopamine modulates striatal activity, and hence neuronal firing in the output nuclei is a major determinant of the motor state. Current thinking has been somewhat modified, and emphasis is now placed on parallel interactions between cortico-striatal and cortico-subthalamic afferents on the one hand (Nambu, 2004) and re-entrant internal and external feed-back circuits (McHaffie et al., 2005) and the sub-division of cortico-basal ganglia loops into different motor, associative and emotional domains on the other. Thus, the exclusive feed-forward nature of information processing throughout the basal ganglia is no longer tenable. Several examples of reciprocal connectivity within the basal ganglia are now evident: between the STN and GPe (Shink et al., 1996), the GPe and the striatum (Mallet et al., 2012; Sato et al., 2000) and the striatum and ascending DA neurons in SNpc (Haber et al., 2000) (and also between the basal ganglia and projecting nuclei, as for example the motor cortex and the STN and, the STN and thalamus and the PPN and the STN (Fig. 2)). The basal ganglia-cortical network is better envisaged as a dynamic network engaged in movement and behavior facilitation/inhibition, switching actions, learning and motivation, which are conveyed by parallel activity in different anatomical domains. Furthermore, such a diverse spectrum of functions sustained through the basal ganglia is likely to depend upon different qualitative and quantitative degrees of multiple oscillators and frequencies as well as activation patterns. Frequency and amplitude modulation via the multiple closed and open loops of the basal ganglia provide the means to modify the excitability of different functional domains simultaneously. Severe dopaminergic striatal depletion provokes beta band hyper synchrony of the various circuits forming the motor circuit network (Fig. 2), leading to rather homogeneous output activity and reduced movement capacity. However, neither increased firing rate nor enhanced beta band power in basal ganglia output nuclei appears to be “sine-qua non” features of early parkinsonism. What are then, the physiological changes underlying the initial phase of PD?
Typically, PD is diagnosed by the onset of focal, i.e. one limb, manifestations taking the form of tremor at rest and bradykinesia with rigidity, with a particular predilection for impairing automatic movements. SNpc cell loss of around 55% and 80% striatal DA depletion in monkeys who are pre-symptomatic coincided fairly closely with that seen in PD patients with short disease evolution (Blesa et al., 2012; Damier et al., 1999; Hornykiewicz, 2001).
These early motor manifestations concur with reduced dopaminergic innervation in the posterior (motor) putamen of about 70% as evaluated “in vivo” by PET (Nandhagopal et al., 2011) and with some 50% of SNpc cell loss by post-mortem evaluation (Fearnley and Lees, 1990; Halliday et al., 1990). Because the overall degree of striatal dopaminergic depletion is relatively limited at this early PD stage, it could be thought that hyperactivity in cell firing or beta oscillations may not be detectable by current methodology, leading to a sample bias in the neurophysiological assessment. While the latter possibility cannot be ruled out, it is likely that denervation is much higher if it were possible to ascertain the specific putaminal region (leg, hand, shoulder, etc.) corresponding to the clinically most affected body part. Accordingly, LFP recordings should be sensitive to such a degree of abnormality.
In the rat and monkey with partial nigro-striatal lesion early physiological changes are the increased busting firing (Bergman et al., 1994, 1998; Tseng et al., 2005) in the STN and SNpr/GPi, increased background neuronal activity leading to reduced signal to noise ratio in GPi and augmented coherence between motor cortex and SNpr (in the rat) (Novikov et al., 2012), all of which may lead to impaired movement initiation and execution (Leblois et al., 2006). This could be more significant and important for automatic movements because the degree of cortico-striatal neuronal activation required is less than for non-habitual more complex tasks (Lehericy et al., 2005). In addition, some evidence suggests that gamma oscillatory activity is involved in movement facilitation (Alegre et al., 2013; Anzak et al., 2013; Jenkinson et al., 2012) which fits with its enhancement after administration of dopaminergic drugs. It might be therefore, that early rigid/akinetic features in PD are more associated with a reduction in the modulation of basal ganglia-cortical activity underlying “energization” (Mazzoni et al., 2007) and action selection (Redgrave et al., 2010).
For instance, it may be that it is the ratio between neuronal firing inhibition/excitation and gamma/beta activities that is faulty, and more relevant than changes in neuronal background firing and synchrony. Moreover, high frequency activity (300 Hz) present in PD patients interferes with frequency modulation of beta and gamma activities, which has been suggested as potentially impairing the fine tuning of motor control mechanisms (Lopez-Azcarate et al., 2010). However, a note of caution needs to be introduced when assessing the importance and significance of specific changes in a frequency band as the processes involved in generating synchronized activity within the basal ganglia thalamocortical circuits after loss of dopamine remain to be characterized. Indeed, the feasible existence of multiple oscillators throughout the cortico-basal ganglia loops could contribute to interactions at the cellular level which are not readily captured currently.
Tremor poses a special situation and challenge. Tremor rest is undoubtedly associated with rhythmical oscillatory activity at around 4-5 Hz in the basal ganglia and thalamic Vim which probably engages the motor cortex to create a cortico-subcortical “tremogenic” network. Importantly, while beta and gamma oscillations are modified in PD in terms of their relative power or degree of activity, the 4-5 Hz has no physiological counterpart. In tremor predominant PD this oscillation may be the only or primary pathophysiological abnormality, possibly independent of the more typical changes in neuronal firing and frequency spectrum associated with full parkinsonism. How and why this rhythm ensues in PD remains an important challenge to be unraveled in the near future.
In sum, the parkinsonian state is associated with different types of oscillatory activity. When nigro-striatal dopamine depletion is severe, the basal ganglia-cortical network becomes hyper-synchronous in the beta band and parkinsonism is profound and generalized. However, initial motor features may appear before such physiological changes, as shown in animal models. Accordingly, the emergence of enhanced beta activity could be taken as an indicator of faulty or maladaptive compensatory mechanisms and a target for experimental therapies.
References
- Alegre M, et al. The subthalamic nucleus is involved in successful inhibition in the stop-signal task: a local field potential study in Parkinson's disease. Exp Neurol. 2013;239:1–12. doi: 10.1016/j.expneurol.2012.08.027. [DOI] [PubMed] [Google Scholar]
- Alonso-Frech F, et al. Slow oscillatory activity and levodopa-induced dyskinesias in Parkinson's disease. Brain. 2006;129:1748–1757. doi: 10.1093/brain/awl103. [DOI] [PubMed] [Google Scholar]
- Anzak A, et al. Subthalamic nucleus gamma oscillations mediate a switch from automatic to controlled processing: a study of random number generation in Parkinson's disease. NeuroImage. 2013;64:284–289. doi: 10.1016/j.neuroimage.2012.08.068. [DOI] [PubMed] [Google Scholar]
- Avila I, et al. Beta frequency synchronization in basal ganglia output during rest and walk in a hemiparkinsonian rat. Exp Neurol. 2010;221:307–319. doi: 10.1016/j.expneurol.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Gad I, Bergman H. Stepping out of the box: information processing in the neural networks of the basal ganglia. Curr Opin Neurobiol. 2001;11:689–695. doi: 10.1016/s0959-4388(01)00270-7. [DOI] [PubMed] [Google Scholar]
- Bateup HS, et al. Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc Natl Acad Sci U S A. 2010;107:14845–14850. doi: 10.1073/pnas.1009874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman H, et al. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- Bergman H, et al. Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trends Neurosci. 1998;21:32–38. doi: 10.1016/s0166-2236(97)01151-x. [DOI] [PubMed] [Google Scholar]
- Berke JD. Fast oscillations in cortical–striatal networks switch frequency following rewarding events and stimulant drugs. Eur J Neurosci. 2009;30:848–859. doi: 10.1111/j.1460-9568.2009.06843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, et al. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron. 2004;43:883–896. doi: 10.1016/j.neuron.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Blesa J, et al. The nigrostriatal system in the presymptomatic and symptomatic stages in the MPTP monkey model: a PET, histological and biochemical study. Neurobiol Dis. 2012;48:79–91. doi: 10.1016/j.nbd.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Brazhnik E, et al. Early Developing Motor Cortex–Basal Ganglia Synchrony in High Beta Frequency Range in the Rodent Model of Parkinson's Disease. Society for Neuroscience; San Diego, CA, US.: 2010. [Google Scholar]
- Brazhnik E, et al. State-dependent spike and local field synchronization between motor cortex and substantia nigra in hemiparkinsonian rats. J Neurosci. 2012;32:7869–7880. doi: 10.1523/JNEUROSCI.0943-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, et al. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson's disease. J Neurosci. 2001;21:1033–1038. doi: 10.1523/JNEUROSCI.21-03-01033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, et al. Oscillatory local field potentials recorded from the subthalamic nucleus of the alert rat. Exp Neurol. 2002;177:581–585. doi: 10.1006/exnr.2002.7984. [DOI] [PubMed] [Google Scholar]
- Cassidy M, et al. Movement-related changes in synchronization in the human basal ganglia. Brain. 2002;125:1235–1246. doi: 10.1093/brain/awf135. [DOI] [PubMed] [Google Scholar]
- Chevalier G, Deniau JM. Disinhibition as a basic process in the expression of striatal functions. Trends Neurosci. 1990;13:277–280. doi: 10.1016/0166-2236(90)90109-n. [DOI] [PubMed] [Google Scholar]
- Courtemanche R, et al. Synchronous, focally modulated beta-band oscillations characterize local field potential activity in the striatum of awake behaving monkeys. J Neurosci. 2003;23:11741–11752. doi: 10.1523/JNEUROSCI.23-37-11741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossman AR. A hypothesis on the pathophysiological mechanisms that underlie levodopa- or dopamine agonist-induced dyskinesia in Parkinson's disease: implications for future strategies in treatment. Mov Disord. 1990;5:100–108. doi: 10.1002/mds.870050203. [DOI] [PubMed] [Google Scholar]
- Cruz AV, et al. Effects of dopamine depletion on information flow between the subthalamic nucleus and external globus pallidus. J Neurophysiol. 2011;106:2012–2023. doi: 10.1152/jn.00094.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damier P, et al. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain. 1999;122(Pt 8):1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- Danish SF, et al. High-frequency oscillations (>200 Hz) in the human non-parkinsonian subthalamic nucleus. Brain Res Bull. 2007;74:84–90. doi: 10.1016/j.brainresbull.2007.05.006. [DOI] [PubMed] [Google Scholar]
- DeCoteau WE, et al. Oscillations of local field potentials in the rat dorsal striatum during spontaneous and instructed behaviors. J Neurophysiol. 2007;97:3800–3805. doi: 10.1152/jn.00108.2007. [DOI] [PubMed] [Google Scholar]
- Degos B, et al. Chronic but not acute dopaminergic transmission interruption promotes a progressive increase in cortical beta frequency synchronization: relationships to vigilance state and akinesia. Cereb Cortex. 2009;19:1616–1630. doi: 10.1093/cercor/bhn199. [DOI] [PubMed] [Google Scholar]
- Dejean C, et al. Evolution of the dynamic properties of the cortex–basal ganglia network after dopaminergic depletion in rats. Neurobiol Dis. 2012;46:402–413. doi: 10.1016/j.nbd.2012.02.004. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Djaldetti R, et al. Residual striatal dopaminergic nerve terminals in very longstanding Parkinson's disease: a single photon emission computed tomography imaging study. Mov Disord. 2011;26:327–330. doi: 10.1002/mds.23380. [DOI] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Striatonigral degeneration. A clinicopathological study. Brain. 1990;113(Pt 6):1823–1842. doi: 10.1093/brain/113.6.1823. [DOI] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Foffani G, et al. 300-Hz subthalamic oscillations in Parkinson's disease. Brain. 2003;126:2153–2163. doi: 10.1093/brain/awg229. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, et al. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, et al. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday GM, et al. Neuropathology of immunohistochemically identified brainstem neurons in Parkinson's disease. Ann Neurol. 1990;27:373–385. doi: 10.1002/ana.410270405. [DOI] [PubMed] [Google Scholar]
- Hariz M. Twenty-five years of deep brain stimulation: celebrations and apprehensions. Mov Disord. 2012;27:930–933. doi: 10.1002/mds.25007. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, et al. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer G, et al. Dopamine replacement therapy does not restore the full spectrum of normal pallidal activity in the 1-methyl-4-phenyl-1,2,3,6-tetra-hydropyridine primate model of Parkinsonism. J Neurosci. 2006;26:8101–8114. doi: 10.1523/JNEUROSCI.5140-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornykiewicz O. Chemical neuroanatomy of the basal ganglia–normal and in Parkinson's disease. J Chem Neuroanat. 2001;22:3–12. doi: 10.1016/s0891-0618(01)00100-4. [DOI] [PubMed] [Google Scholar]
- Israel Z, Bergman H. Pathophysiology of the basal ganglia and movement disorders: from animal models to human clinical applications. Neurosci Biobehav Rev. 2008;32:367–377. doi: 10.1016/j.neubiorev.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Jenkinson N, Brown P. New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci. 2011;34:611–618. doi: 10.1016/j.tins.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Jenkinson N, Kühn AA, Brown P. Gamma oscillations in the human basal ganglia. Exp Neurol. 2012;245:72–76. doi: 10.1016/j.expneurol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Krack P, et al. Deep brain stimulation: from neurology to psychiatry? Trends Neurosci. 2010;33:474–484. doi: 10.1016/j.tins.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn AA, et al. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain. 2004;127:735–746. doi: 10.1093/brain/awh106. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, et al. The relationship between local field potential and neuronal discharge in the subthalamic nucleus of patients with Parkinson's disease. Exp Neurol. 2005;194:212–220. doi: 10.1016/j.expneurol.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, et al. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson's disease relates to both bradykinesia and rigidity. Exp Neurol. 2009;215:380–387. doi: 10.1016/j.expneurol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Leblois A, et al. Temporal and spatial alterations in GPi neuronal encoding might contribute to slow down movement in Parkinsonian monkeys. Eur J Neurosci. 2006;24:1201–1208. doi: 10.1111/j.1460-9568.2006.04984.x. [DOI] [PubMed] [Google Scholar]
- Leblois A, et al. Late emergence of synchronized oscillatory activity in the pallidum during progressive Parkinsonism. Eur J Neurosci. 2007;26:1701–1713. doi: 10.1111/j.1460-9568.2007.05777.x. [DOI] [PubMed] [Google Scholar]
- Lehericy S, et al. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci U S A. 2005;102:12566–12571. doi: 10.1073/pnas.0502762102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal DK, et al. Basal ganglia beta oscillations accompany cue utilization. Neuron. 2012;73:523–536. doi: 10.1016/j.neuron.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, et al. High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor. J Neurosci. 2000;20:7766–7775. doi: 10.1523/JNEUROSCI.20-20-07766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, et al. Dependence of subthalamic nucleus oscillations on movement and dopamine in Parkinson's disease. Brain. 2002;125:1196–1209. doi: 10.1093/brain/awf128. [DOI] [PubMed] [Google Scholar]
- Lopez-Azcarate J, et al. Coupling between beta and high-frequency activity in the human subthalamic nucleus may be a pathophysiological mechanism in Parkinson's disease. J Neurosci. 2010;30:6667–6677. doi: 10.1523/JNEUROSCI.5459-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill PJ, et al. Relationship of activity in the subthalamic nucleus–globus pallidus network to cortical electroencephalogram. J Neurosci. 2000;20:820–833. doi: 10.1523/JNEUROSCI.20-02-00820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill PJ, et al. Delayed synchronization of activity in cortex and subthalamic nucleus following cortical stimulation in the rat. J Physiol. 2006;574:929–946. doi: 10.1113/jphysiol.2006.110379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, et al. Parkinsonian beta oscillations in the external globus pallidus and their relationship with subthalamic nucleus activity. J Neurosci. 2008;28:14245–14258. doi: 10.1523/JNEUROSCI.4199-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, et al. Dichotomous organization of the external globus pallidus. Neuron. 2012;74:1075–1086. doi: 10.1016/j.neuron.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden CD, Obeso JA. The functions of the basal ganglia and the paradox of stereotaxic surgery in Parkinson's disease. Brain. 1994;117(Pt 4):877–897. doi: 10.1093/brain/117.4.877. [DOI] [PubMed] [Google Scholar]
- Mazzoni P, et al. Why don't we move faster? Parkinson's disease, movement vigor, and implicit motivation. J Neurosci. 2007;27:7105–7116. doi: 10.1523/JNEUROSCI.0264-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHaffie JG, et al. Subcortical loops through the basal ganglia. Trends Neurosci. 2005;28:401–407. doi: 10.1016/j.tins.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Montgomery EB., Jr Basal ganglia physiology and pathophysiology: a reappraisal. Parkinsonism Relat Disord. 2007;13:455–465. doi: 10.1016/j.parkreldis.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Moran RJ, et al. Alterations in brain connectivity underlying beta oscillations in Parkinsonism. PLoS Comput Biol. 2011;7:e1002124. doi: 10.1371/journal.pcbi.1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A. A new dynamic model of the cortico-basal ganglia loop. Prog Brain Res. 2004;143:461–466. doi: 10.1016/S0079-6123(03)43043-4. [DOI] [PubMed] [Google Scholar]
- Nandhagopal R, et al. Longitudinal evolution of compensatory changes in striatal dopamine processing in Parkinson's disease. Brain. 2011;134:3290–3298. doi: 10.1093/brain/awr233. [DOI] [PubMed] [Google Scholar]
- Nini A, et al. Neurons in the globus pallidus do not show correlated activity in the normal monkey, but phase-locked oscillations appear in the MPTP model of parkinsonism. J Neurophysiol. 1995;74:1800–1805. doi: 10.1152/jn.1995.74.4.1800. [DOI] [PubMed] [Google Scholar]
- Novikov N, et al. Lack of Correlation Between Progressive Emergence of Motor Cortex–Basal Ganglia Synchrony in the High Beta/Low Gamma Frequency Range and Motor Deficits in Rodent Models of Parkinson's Disease. Society for Neuroscience; New Orleans (US): 2012. [Google Scholar]
- Obeso JA, et al. Pathophysiology of levodopa-induced dyskinesias in Parkinson's disease: problems with the current model. Ann Neurol. 2000a;47:S22–S32. discussion S32-4. [PubMed] [Google Scholar]
- Obeso JA, et al. Pathophysiology of the basal ganglia in Parkinson's disease. Trends Neurosci. 2000b;23:S8–S19. doi: 10.1016/s1471-1931(00)00028-8. [DOI] [PubMed] [Google Scholar]
- Penney JB, Jr, Young AB. Striatal inhomogeneities and basal ganglia function. Mov Disord. 1986;1:3–15. doi: 10.1002/mds.870010102. [DOI] [PubMed] [Google Scholar]
- Priori A, et al. Movement-related modulation of neural activity in human basal ganglia and its L-DOPA dependency: recordings from deep brain stimulation electrodes in patients with Parkinson's disease. Neurol Sci. 2002;23(Suppl. 2):S101–S102. doi: 10.1007/s100720200089. [DOI] [PubMed] [Google Scholar]
- Raz A, et al. Activity of pallidal and striatal tonically active neurons is correlated in MPTP-treated monkeys but not in normal monkeys. J Neurosci. 2001;21:RC128. doi: 10.1523/JNEUROSCI.21-03-j0006.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, et al. Goal-directed and habitual control in the basal ganglia: implications for Parkinson's disease. Nat Rev Neurosci. 2010;11:760–772. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, et al. Dopamine cell degeneration induced by intraventricular administration of 6-hydroxydopamine in the rat: similarities with cell loss in Parkinson's disease. Exp Neurol. 2001;169:163–181. doi: 10.1006/exnr.2000.7624. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, et al. Firing regulation in dopaminergic cells: effect of the partial degeneration of nigrostriatal system in surviving neurons. Eur J Neurosci. 2003;18:53–60. doi: 10.1046/j.1460-9568.2003.02723.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Oroz MC, et al. Involvement of the subthalamic nucleus in impulse control disorders associated with Parkinson's disease. Brain. 2011;134:36–49. doi: 10.1093/brain/awq301. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, et al. Nigrostriatal lesion and dopamine agonists affect firing patterns of rodent entopeduncular nucleus neurons. J Neurophysiol. 2002;88:487–496. doi: 10.1152/jn.00844.2001. [DOI] [PubMed] [Google Scholar]
- Sato F, et al. Axonal branching pattern of neurons of the subthalamic nucleus in primates. J Comp Neurol. 2000;424:142–152. doi: 10.1002/1096-9861(20000814)424:1<142::aid-cne10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Sharott A, et al. Directional analysis of coherent oscillatory field potentials in the cerebral cortex and basal ganglia of the rat. J Physiol. 2005a;562:951–963. doi: 10.1113/jphysiol.2004.073189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharott A, et al. Dopamine depletion increases the power and coherence of beta-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. Eur J Neurosci. 2005b;21:1413–1422. doi: 10.1111/j.1460-9568.2005.03973.x. [DOI] [PubMed] [Google Scholar]
- Shink E, et al. The subthalamic nucleus and the external pallidum: two tightly interconnected structures that control the output of the basal ganglia in the monkey. Neuroscience. 1996;73:335–357. doi: 10.1016/0306-4522(96)00022-x. [DOI] [PubMed] [Google Scholar]
- Stein E, Bar-Gad I. Beta oscillations in the cortico-basal ganglia loop during parkinsonism. Exp Neurol. 2012;245:52–59. doi: 10.1016/j.expneurol.2012.07.023. [DOI] [PubMed] [Google Scholar]
- Tachibana Y, et al. Subthalamo-pallidal interactions underlying parkinsonian neuronal oscillations in the primate basal ganglia. Eur J Neurosci. 2011;34:1470–1484. doi: 10.1111/j.1460-9568.2011.07865.x. [DOI] [PubMed] [Google Scholar]
- Tseng KY, et al. Consequences of partial and severe dopaminergic lesion on basal ganglia oscillatory activity and akinesia. Eur J Neurosci. 2005;22:2579–2586. doi: 10.1111/j.1460-9568.2005.04456.x. [DOI] [PubMed] [Google Scholar]
- Urbain N, et al. Unrelated course of subthalamic nucleus and globus pallidus neuronal activities across vigilance states in the rat. Eur J Neurosci. 2000;12:3361–3374. doi: 10.1046/j.1460-9568.2000.00199.x. [DOI] [PubMed] [Google Scholar]
- Vitek JL, et al. Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus. Ann Neurol. 1999;46:22–35. doi: 10.1002/1531-8249(199907)46:1<22::aid-ana6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Weinberger M, et al. Pedunculopontine nucleus microelectrode recordings in movement disorder patients. Exp Brain Res. 2008;188:165–174. doi: 10.1007/s00221-008-1349-1. [DOI] [PubMed] [Google Scholar]
- Weinberger M, et al. Oscillatory activity in the globus pallidus internus: comparison between Parkinson's disease and dystonia. Clin Neurophysiol. 2012;123:358–368. doi: 10.1016/j.clinph.2011.07.029. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Pathophysiology of Parkinson's disease: the MPTP primate model of the human disorder. Ann N Y Acad Sci. 2003;991:199–213. doi: 10.1111/j.1749-6632.2003.tb07477.x. [DOI] [PubMed] [Google Scholar]
- Wichmann T, et al. The primate subthalamic nucleus. III. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:521–530. doi: 10.1152/jn.1994.72.2.521. [DOI] [PubMed] [Google Scholar]
- Wichmann T, et al. Milestones in research on the pathophysiology of Parkinson's disease. Mov Disord. 2011;26:1032–1041. doi: 10.1002/mds.23695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D, et al. Dopamine-dependent changes in the functional connectivity between basal ganglia and cerebral cortex in humans. Brain. 2002;125:1558–1569. doi: 10.1093/brain/awf156. [DOI] [PubMed] [Google Scholar]
- Zaidel A, et al. Subthalamic span of beta oscillations predicts deep brain stimulation efficacy for patients with Parkinson's disease. Brain. 2010;133:2007–2021. doi: 10.1093/brain/awq144. [DOI] [PubMed] [Google Scholar]


