Abstract
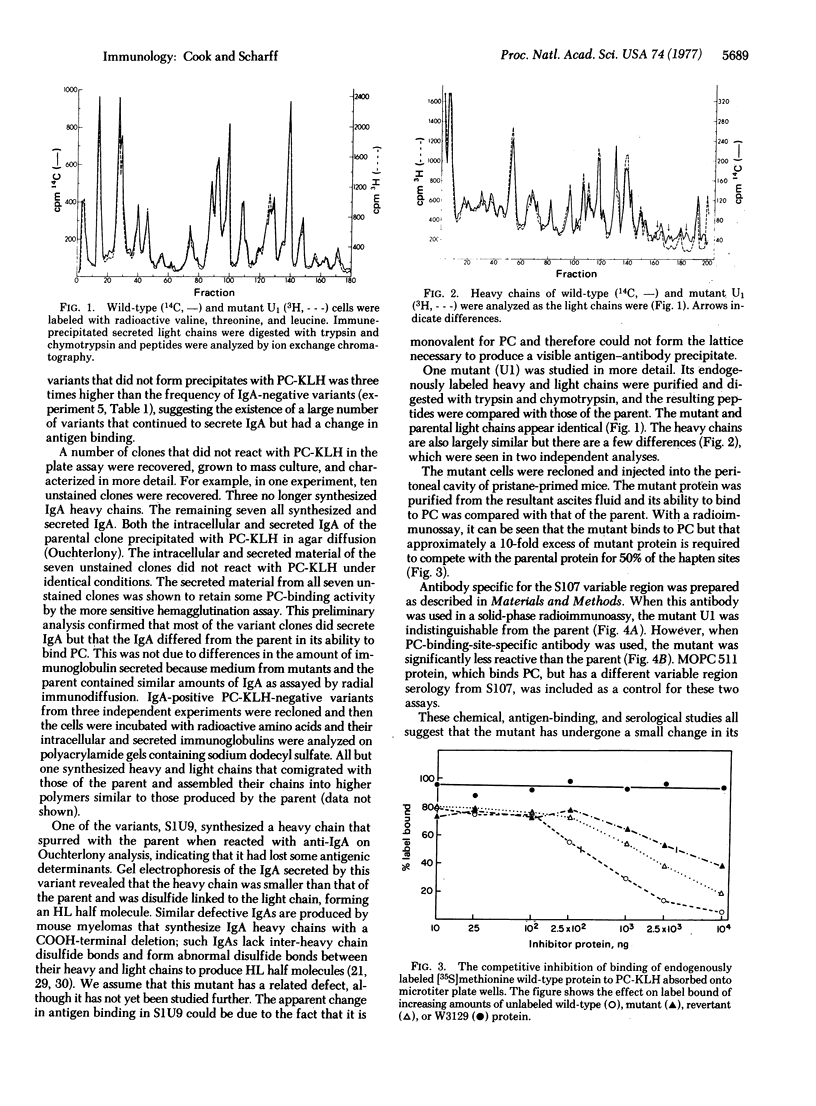
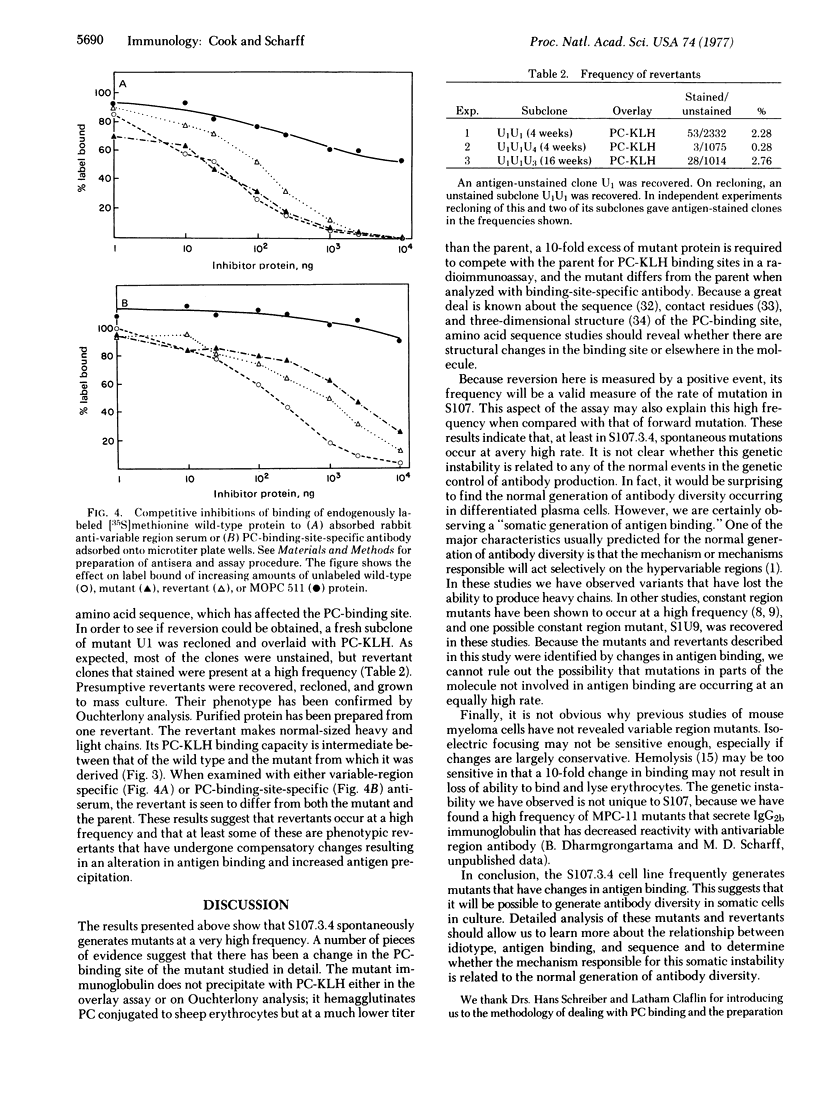
A cultured mouse myeloma cell line, S107, that secretes an IgA phosphocholine-binding immunoglobulin has been cloned in soft agar and overlaid with phosphocholine-hemocyanin. Spontaneous mutants that secrete immunoglobulin with a decreased ability to precipitate antigen were detected with this plate assay and occur at a very high frequency. From one such mutant, phenotypic revertants arise spontaneously with a frequency of 0.28-2.8%. This mutant and one of its revertants were studied, and they were found to differ from the parent and from each other serologically and in antigen binding. While it is not yet clear whether these findings bear any relationship to the normal generation of antibody diversity, they do indicate that it is possible to generate antigen binding diversity in somatic cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel C. A., Grey H. M. Studies on the structure of mouse gamma-A myeloma proteins. Biochemistry. 1968 Jul;7(7):2682–2688. doi: 10.1021/bi00847a035. [DOI] [PubMed] [Google Scholar]
- Adetugbo K., Milstein C., Secher D. S. Molecular analysis of spontaneous somatic mutants. Nature. 1977 Jan 27;265(5592):299–304. doi: 10.1038/265299a0. [DOI] [PubMed] [Google Scholar]
- Bargellesi A., Periman P., Scharff M. D. Synthesis, assembly, and secretion of globulin by mouse myeloma cells. IV. Assembly of IgA. J Immunol. 1972 Jan;108(1):126–134. [PubMed] [Google Scholar]
- Baumal R., Birshtein B. K., Coffino P., Scharff M. D. Mutations in immunoglobulin-producing mouse myeloma cells. Science. 1973 Oct 12;182(4108):164–166. doi: 10.1126/science.182.4108.164. [DOI] [PubMed] [Google Scholar]
- Birshtein B. K., Preud'homme J. L., Scharff M. D. Variants of mouse myeloma cells that produce short immunoglobulin heavy chains. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3478–3482. doi: 10.1073/pnas.71.9.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Metzger H. Affinity labeling of a phosphorylcholine binding mouse myeloma protein. Biochemistry. 1972 Feb 29;11(5):766–771. doi: 10.1021/bi00755a014. [DOI] [PubMed] [Google Scholar]
- Claflin J. L., Davie J. M. Clonal nature of the immune response to phosphorylcholine. IV. Idiotypic uniformity of binding site-associated antigenic determinants among mouse antiphosphorylcholine antibodies. J Exp Med. 1974 Sep 1;140(3):673–686. doi: 10.1084/jem.140.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claflin J. L., Davie J. M. Specific isolation and characterization of antibody directed to binding site antigenic determinants. J Immunol. 1975 Jan;114(1 Pt 1):70–75. [PubMed] [Google Scholar]
- Coffino P., Baumal R., Laskov R., Scharff M. D. Cloning of mouse myeloma cells and detection of rare variants. J Cell Physiol. 1972 Jun;79(3):429–440. doi: 10.1002/jcp.1040790313. [DOI] [PubMed] [Google Scholar]
- Cohn M., Notani G., Rice S. A. Characterization of the antibody to the C-carbohydrate produced by a transplantable mouse plasmacytoma. Immunochemistry. 1969 Jan;6(1):111–123. doi: 10.1016/0019-2791(69)90183-9. [DOI] [PubMed] [Google Scholar]
- Davies D. R., Padlan E. A., Segal D. M. Three-dimensional structure of immunoglobulins. Annu Rev Biochem. 1975;44:639–667. doi: 10.1146/annurev.bi.44.070175.003231. [DOI] [PubMed] [Google Scholar]
- Givol D. Affinity labeling and topology of the antibody combining site. Essays Biochem. 1974;10:73–103. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Laskov R., Scharff M. D. Synthesis, assembly, and secretion of gamma globulin by mouse myeloma cells. I. Adaptation of the Merwin plasma cell tumor-11 to culture, cloning, and characterization of gamma globulin subunits. J Exp Med. 1970 Mar 1;131(3):515–541. doi: 10.1084/jem.131.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushinski J. F. Gamma-A half molecules: defective heavy chain mutants in mouse myeloma proteins. J Immunol. 1971 Jan;106(1):41–50. [PubMed] [Google Scholar]
- Padlan E. A., Davies D. R., Rudikoff S., Potter M. Structural basis for the specificity of phosphorylcholine-binding immunoglobulins. Immunochemistry. 1976 Nov;13(11):945–949. doi: 10.1016/0019-2791(76)90239-1. [DOI] [PubMed] [Google Scholar]
- Pierce S. K., Klinman N. R. Allogeneic carrier-specific enhancement of hapten-specific secondary B-cell responses. J Exp Med. 1976 Nov 2;144(5):1254–1262. doi: 10.1084/jem.144.5.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poljak R. J. Three-dimensional structure, function and genetic control of immunoglobulins. Nature. 1975 Jul 31;256(5516):373–376. doi: 10.1038/256373a0. [DOI] [PubMed] [Google Scholar]
- Potter M. Immunoglobulin-producing tumors and myeloma proteins of mice. Physiol Rev. 1972 Jul;52(3):631–719. doi: 10.1152/physrev.1972.52.3.631. [DOI] [PubMed] [Google Scholar]
- Potter M., Lieberman R. Common individual antigenic determinants in five of eight BALB-c IgA myeloma proteins that bind phosphoryl choline. J Exp Med. 1970 Oct 1;132(4):737–751. doi: 10.1084/jem.132.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M., Pumphrey J. G., Walters J. L. Growth of primary plasmacytomas in the mineral oil-conditioned peritoneal environment. J Natl Cancer Inst. 1972 Jul;49(1):305–308. [PubMed] [Google Scholar]
- Prend'homme J. L., Buxbaum J., Scharff M. D. Mutagenesis of mouse myeloma cells with 'Melphalan'. Nature. 1973 Oct 12;245(5424):320–322. doi: 10.1038/245320a0. [DOI] [PubMed] [Google Scholar]
- Preud'Homme J. L., Birshtein B. K., Scharff M. D. Variants of a mouse myeloma cell line that synthesize immunoglobulin heavy chains having an altered serotype. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1427–1430. doi: 10.1073/pnas.72.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson E. A., Smith D. F., Appella E. Chemical characterization of a mouse immunoglobulin A heavy chain with a 100-residue deletion. Amino acid and carbohydrate compositions and NH2-and COOH-terminal sequences. J Biol Chem. 1974 Oct 25;249(20):6605–6610. [PubMed] [Google Scholar]
- Rudikoff S., Potter M. Size differences among immunoglobulin heavy chains from phosphorylcholine-binding proteins. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2109–2112. doi: 10.1073/pnas.73.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal D. M., Padlan E. A., Cohen G. H., Rudikoff S., Potter M., Davies D. R. The three-dimensional structure of a phosphorylcholine-binding mouse immunoglobulin Fab and the nature of the antigen binding site. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4298–4302. doi: 10.1073/pnas.71.11.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman S., Scharff M. D. Mouse myeloma mutants blocked in the assembly, glycosylation and secretion of immunoglobulin. J Mol Biol. 1976 Apr 5;102(2):237–252. doi: 10.1016/s0022-2836(76)80051-4. [DOI] [PubMed] [Google Scholar]
- Wu T. T., Kabat E. A. An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity. J Exp Med. 1970 Aug 1;132(2):211–250. doi: 10.1084/jem.132.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]


