Abstract
Objectives:
The co-existence of diabetes mellitus (DM) and sickle cell disease (SCD) is rare. This study aimed to explore whether SCD patients have the same DM prevalence as the general population in a country with a high prevalence of DM.
Methods:
This cross-sectional study included all SCD adult patients admitted to Salmaniya Medical Complex, Bahrain, between 2003 and 2010 (n = 2,204). A random sample (n = 520) was taken to establish the prevalence of DM. Laboratory records were examined to determine the presence of DM.
Results:
There were 376 SCD patients with complete records; of these, 24 (6.4%) had DM. The age- and sex-standardised prevalence of DM was 8.3%.
Conclusion:
While the prevalence of DM in SCD patients in Bahrain was high, it was lower than expected in this population. SCD may have a protective effect towards DM development. However, the impact of these two conditions on vascular diseases suggest a need for screening and aggressive treatment in this population.
Keywords: Diabetes Mellitus, Sickle Cell Disease, Prevalence, Epidemiology, Comorbidity, Vascular Diseases, Bahrain
Sickle cell disease (scd) is one of the most common inherited haemoglobinopathies.1 The leading cause of death among patients with SCD has changed from infections to chronic cardiopulmonary complications.2 Diabetes mellitus (DM) is a major risk factor for cardiovascular diseases, accounting for an increased risk of myocardial infarctions or strokes.3 In addition, DM patients are predisposed to complications that are also associated with SCD, including infections, renal failure and retinopathy. Fortunately, research has indicated that the co-existence of type 1 and type 2 DM with SCD is rare; several studies have found no cases of DM at all among SCD patients.4,5 A large multi-centre study, carried out in 31 centres in the USA, Canada and the UK, revealed that only 2% of blood-transfused SCD patients also had DM.6
Bahrain has a population of approximately 1.2 million, although around 50% are expatriates.7 The country has a high prevalence of both DM and SCD, ranking fifth in the world for DM at 15.8%.8 The prevalence of SCD in Bahrain was 2.1% in 1987,9 but this prevalence had decreased by 1.3% in 2008.10 Both diseases present considerable challenges to the national healthcare system.11,12 The co-existence of these two conditions in a population where both are highly prevalent has not been previously studied. Therefore, this preliminary study aimed to explore whether patients with SCD have the same prevalence of DM as the general population in Bahrain.
Methods
The medical records of all patients with SCD aged 18 years and over who were admitted to the Salmaniya Medical Complex in Manama, Bahrain, between January 2003 and December 2010 were included in this study (n = 2,204). The Salmaniya Medical Complex is the single government sickle cell service available in the country. The majority of patients attending this clinic are of Arab descent.
Based on the population size, a random sample of 327 patients was calculated as the minimum sample required to establish the prevalence of DM with a 5% margin of error. In order to allow for missing medical records and data, a computer-generated random sample of 520 subjects was taken. However, 26 patients (5%) were subsequently found not to have SCD based on their haemoglobin electrophoresis results. These patients were therefore removed from the sample (n = 494).
Data from the patients’ medical records were collected, including age, gender, fasting blood sugar levels, random blood sugar levels, glucose tolerance test results and haemoglobin electrophoresis (haemoglobin S and fetal haemoglobin percentages). Laboratory data from other governmental health centres and hospitals in Bahrain were accessed through a centralised repository. Patients were categorised into the following four groups according to the World Health Organization’s (WHO) diagnostic criteria for DM: diagnosed or undiagnosed DM; impaired fasting glucose and/or impaired glucose tolerance (also known as pre-diabetes); no DM, or unknown DM status due to insufficient or missing blood results.13
Among the patients included in the study, DM was ruled out by two normal readings of fasting or random blood glucose levels on two separate days. Haemoglobin A1c levels were not used to either establish or rule out a diagnosis of DM, as these measurements are unreliable in patients with haemoglobinopathies such as SCD.14 A total of 118 patients (22.7%) had insufficient data in their medical records to establish their diabetic status. These patients were therefore excluded from the analysis, resulting in a final sample of 376.
The study sample was statistically compared to data from the 2010 census of the Bahraini population in order to establish the age- and sex-standardised prevalence of DM in the sample.7 Data were analysed using JMP-IN®, Version 9 (SAS Institute Inc., Cary, North Carolina, USA) with descriptive statistics and non-parametric tests of association.
Ethical approval for this study was granted by the Research Ethics Committee of the Royal College of Surgeons in Ireland (RCSI) Medical University of Bahrain, as well as the Research Technical Support Team of the Bahraini Ministry of Health and the Research Committee of Salmaniya Medical Complex.
Results
There were 376 SCD patients with complete records. The mean age was 33.5 ± 11.2 years (range: 18–79 years). There were 196 male patients (52.1%) among the cohort, which was consistent with the gender ratio of the general Bahraini population (1.07 versus 1.01, respectively; P = 0.47).7 However, the sample had a lower proportion of individuals aged >55 years in comparison to the general Bahraini population (4.4% versus 9.8%, respectively; P <0.001). The mean number of hospital admissions was 14.5 ± 24.4 with a range of 1–195 admissions.
Of the final sample, 24 SCD patients (6.4%) were determined to have either type 1 or type 2 DM. A total of 11 patients had previously been diagnosed with DM and their diagnosis was confirmed by recent glucose measurements. A further 13 patients had sufficiently raised blood glucose levels to establish a diagnosis of DM according to WHO criteria. In addition, 32 patients (8.5%) were found to have impaired fasting glucose or impaired glucose tolerance.
As expected, the prevalence of DM rose with age (χ2 = 42.9; degrees of freedom [df ] = 2; P <0.0001). The prevalence of DM in patients aged ≥40 years was 16.4% (17 out of 103 patients). The age- and sex-standardised prevalence of DM was 8.3% while impaired glucose tolerance/impaired fasting glucose was 11.0%. No association was found between DM and gender (χ2 = 1.5; df = 2; P = 0.47) or number of hospital admissions (χ2 = 2.2; df = 2; P = 0.34).
Discussion
Previous studies have found low or non-existent rates of DM in patients with SCD.4–6 This has led to the hypothesis that SCD may inhibit the inheritance or the development of DM through a genetic mechanism.15 Alternative explanations include the lowered life expectancy of SCD patients, which reduces the likelihood of overt DM developing over time,15 or the increased frequency of illnesses among SCD patients population, which leads to an underweight population with a lower predisposition to DM.16
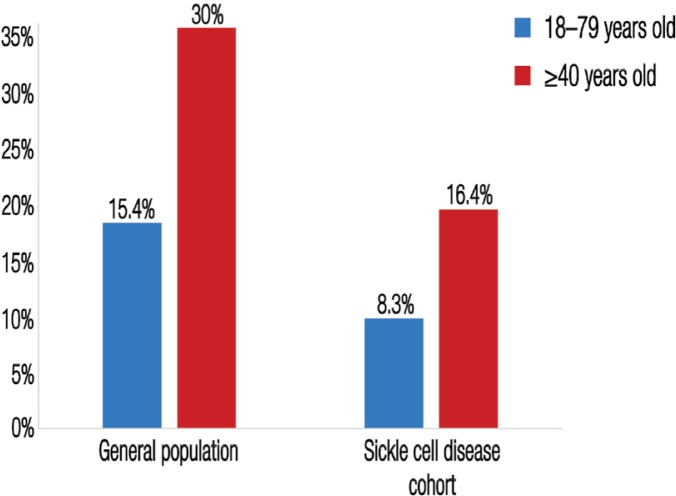
A high age- and sex-standardised prevalence of DM was found among the studied Bahraini patients with SCD (8.3%). However, this was still lower than the expected prevalence (15.4%) among the general population of Bahrain [Figure 1].8,17 Despite this, the prevalence of DM among the studied cohort was not as uncommon as other studies based outside of Bahrain have indicated.4–6 The prevalence of DM in the general Bahraini population aged ≥40 years is approximately 30%,17 as can be seen in Figure 1, this is almost double the rate of DM that was observed among SCD patients of the same age category in the current study. Lower life expectancy was excluded as an explanation for the lower prevalence of DM in these SCD patients by statistical adjustments of age. However, decreased rates of obesity in the SCD population may be responsible for the low observed prevalence of DM. Subsequent matched case-control or cohort studies are recommended in order to establish an age-, sex- and body mass index-adjusted prevalence for DM in patients with SCD in Bahrain and the Gulf.
Figure 1:
Comparison of the age- and sex-adjusted prevalence of diabetes found among the studied sickle cell disease cohort in Bahrain (N = 376) with the prevalence of diabetes among the general population in Bahrain.
*Data sourced from International Diabetes Federation. IDF Diabetes Atlas: Fourth edition8 and al-Mahroos, McKeigue PM. High prevalence of diabetes in Bahrainis: Associations with ethnicity and raised plasma cholesterol.17
Although the prevalence of DM among the studied cohort was lower than expected, the rate of DM found among the studied SCD patients is still high enough to raise concerns about the potential impact of these two co-existing conditions on the development of cardiovascular disease. To the best of the authors’ knowledge, no published studies have yet observed that the occurrence of SCD alongside DM leads to poorer outcomes; however, the coexistence of vascular risk factors (e.g. hypertension or smoking) could theoretically lead to further increased vascular risk. As a result, there may be a need for both structured screening programmes and the aggressive treatment of vascular risk factors in this population. Furthermore, additional research is recommended to identify the potential factors of SCD which may have a protective effect against the development of DM.
This study was undertaken as a preliminary study to determine whether the lower prevalence of DM in patients with SCD established in several previous studies would be reflected in this highly prevalent Bahraini population.4–6 The results of this rapid and cost-effective study shed light on the potential protective effect of SCD over DM. Nonetheless, the results should be interpreted in light of several limitations. While the selection of a nationally-representative sample was assured due to the existence of a single centre in Bahrain serving all patients with SCD, the study was retrospective in nature and therefore limited to patients with hospital admissions—however, it is probable that most adults with SCD would have been admitted at least once during the seven-year study period. As a result, a portion of the population with milder SCD may have been excluded, leading to a selection bias. This bias may have led to an underestimation of the DM prevalence.
In addition, this study was also dependent on available laboratory glucose results; any recently developed symptoms of DM may have been missed, thereby potentially resulting in an underestimation of the prevalence. Furthermore, since it was not possible to obtain sufficient data to establish patients’ body mass indexes, a link between lower rates of obesity and a lower prevalence of DM among the SCD could not be excluded. Data regarding any medications were also not obtained and this may have led to an additional underestimation of the DM prevalence. Moreover, other confounding factors, including ethnicity, were not accounted for.
It would be of interest to establish whether other cardiovascular risk factors, such as hypertension and hyperlipidaemia, are less prevalent in patients with SCD. In spite of these limitations, this is the first study of its kind in a population with a high prevalence of both DM and SCD and adds to the existing literature that suggests a lower prevalence of DM among patients with SCD.
Conclusion
While the prevalence of DM among the studied SCD patients in Bahrain was high (8.3%), it was lower than expected among this population. Lower rates of obesity in this population may be responsible for the low observed prevalence of DM. Alternatively, there may be a genetic or epigenetic protective effect of SCD towards the development of DM. Nevertheless, the impact of these two conditions on the development of vascular disease suggests a need for screening and the aggressive treatment of vascular risk factors in this population.
Acknowledgments
This study was presented in abstract form as a poster at the International Diabetes Federation World Diabetes Congress in Melbourne, Australia, in December 2013 (P-1860). The abstract was also presented orally at the International Conference on Sickle Cell Disease Management & Prevention in Manama, Bahrain, in February 2013. This study received the RCSI Alumni Research Award in July 2010.
The authors would like to thank the Departments of Medical Records and Laboratory Services at Salmaniya Medical Complex for access to and help in retrieving patients’ records.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Steinberg MH. In the clinic: Sickle cell disease. Ann Intern Med. 2011;155:ITC31–15. doi: 10.7326/0003-4819-155-5-201109060-01003. [DOI] [PubMed] [Google Scholar]
- 2.Fitzhugh CD, Lauder N, Jonassaint JC, Telen MJ, Zhao X, Wright EC, et al. Cardiopulmonary complications leading to premature deaths in adult patients with sickle cell disease. Am J Hematol. 2010;85:36–40. doi: 10.1002/ajh.21569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: A population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med. 2004;164:1422–6. doi: 10.1001/archinte.164.13.1422. [DOI] [PubMed] [Google Scholar]
- 4.Morrison JC, Schneider JM, Kraus AP, Kitabchi AE. The prevalence of diabetes mellitus in sickle cell hemoglobinopathies. J Clin Endocrinol Metab. 1979;48:192–5. doi: 10.1210/jcem-48-2-192. [DOI] [PubMed] [Google Scholar]
- 5.Reid HL, Photiades DP, Oli JM, Kaine W. Concurrent sickle cell disease and diabetes mellitus. Trop Geogr Med. 1988;40:201–4. [PubMed] [Google Scholar]
- 6.Fung EB, Harmatz PR, Lee PD, Milet M, Bellevue R, Jeng MR, et al. Increased prevalence of iron-overload associated endocrinopathy in thalassaemia versus sickle-cell disease. Br J Haematol. 2006;135:574–82. doi: 10.1111/j.1365-2141.2006.06332.x. [DOI] [PubMed] [Google Scholar]
- 7.Bahrain Census Department 2010 Census. From: www.census2010.gov.bh/results_en.php Accessed: Aug 2014.
- 8.International Diabetes Federation IDF Diabetes Atlas: Fourth edition. From: www.idf.org/sites/default/files/IDF-Diabetes-Atlas-4th-edition.pdf Accessed: Apr 2014.
- 9.Al Arrayed SS, Haites N. Features of sickle-cell disease in Bahrain. East Mediterr Health J. 1995;1:112–19. [Google Scholar]
- 10.Al Arrayed SS. Prevalence of abnormal hemoglobins among students in Bahrain: A ten-year study. Bahrain Med Bull. 2011;33:19–21. [Google Scholar]
- 11.Al-Baharna MM, Whitford DL. Clinical audit of diabetes care in the Bahrain Defence Forces Hospital. Sultan Qaboos Univ Med J. 2013;13:520–6. doi: 10.12816/0003310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tawfic QA, Kausalya R, Al-Sajee D, Burad J, Mohammed AK, Narayanan A. Adult sickle cell disease: A five-year experience of intensive care management in a university hospital in Oman. Sultan Qaboos Univ Med J. 2012;12:177–83. doi: 10.12816/0003110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications: Part 1: Diagnosis and classification of diabetes mellitus, provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus. From: www.who.int/diabetes/publications/diagnosis_diabetes2011/en/ Accessed: Apr 2014.
- 15.Mohapatra MK. Type 1 diabetes mellitus in homozygous sickle cell anaemia. J Assoc Physicians India. 2005;53:895–6. [PubMed] [Google Scholar]
- 16.Bays HE, Chapman RH, Grandy S, SHIELD Investigators’ Group The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: Comparison of data from two national surveys. Int J Clin Pract. 2007;61:737–47. doi: 10.1111/j.1742-1241.2007.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.al-Mahroos F, McKeigue PM. High prevalence of diabetes in Bahrainis: Associations with ethnicity and raised plasma cholesterol. Diabetes Care. 1998;21:936–42. doi: 10.2337/diacare.21.6.936. [DOI] [PubMed] [Google Scholar]