Abstract
Tissue immunostaining is critically important in clinical applications, and antibodies have been used extensively as the molecular probes. Recently, aptamer, as a new class of probes, have attracted much attention for their potential clinical and research value. Epithelial cell adhesion molecule (EpCAM) is a specific biomarker which is overexpressed in many cancers of epithelial origin. Here, a DNA-based EpCAM aptamer SYL3C is reported as a probe for the immunostaining of frozen and paraffin-embedded sections of colorectal cancer tissues. Commercialized EpCAM antibodies were also used as a standard control. EpCAM aptamer SYL3C specifically recognized and immunostained cancer nests of colorectal tumor sections, but it neither reacted with background cells within tumor sites nor exhibited cross-reaction to the benign lesions or inflammation of colorectal tissues. No cross-linking to EpCAM-negative malignant tumor sections occurred. Compared with standard antibody staining, our EpCAM aptamer SYL3C protocol is simpler to implement with a shorter reaction time. Moreover, SYL3C can specifically bind with either frozen or paraffin-embedded tissue sections. Since the histopathology of frozen tissue is closer to that of fresh tissue and since frozen sections can be produced more quickly than paraffin-embedded sections, SYL3C immunostaining of frozen sections is a quick protocol that is easy to implement.
Cancer histopathology is currently the preferred method for detecting microscopic anatomical changes in tissue sections, making the discovery of cancer biomarkers critical for early diagnosis and treatment, and antibodies have been used extensively as the molecular probes.1 Epithelial cell adhesion molecule (EpCAM) is a transmembrane glycoprotein known to be highly expressed in epithelial carcinomas. It is also an ideal biomarker for clinical applications in cancer diagnosis, prognosis, imaging, and therapy.2−4 Thus, EpCAM plays an important role in the diagnosis and treatment of various related malignant tumors.5 The most popular method for detection of EpCAM is immunostaining of paraffin-embedded tissue sections by antibodies,6−8 which are currently the only clinically validated and commercially available probes for this purpose. However, the production of antibodies requires a series of complicated and time-consuming processes. As such, the use of EpCAM antibodies in clinical application has been limited.9
Aptamers are single-stranded DNA, RNA, or modified nucleic acids selected through a process known as Systematic Evolution of Ligands by EXponential enrichment (SELEX).10−13 Aptamers can specifically bind to their target molecules with high affinity at pico- to nanomolar levels, very similar to antibodies.14−16 Compared with antibodies, aptamers can bind to a wider range of targets,17,18 and they have the advantages of low molecular weight, high stability, easy and reproducible synthesis, easy functionalization, lack of immunogenicity, rapid tissue penetration, and low toxicity.19−22 On the basis of their high affinity and specific binding properties, scientists are testing the use of aptamer probes for cancer cell detection23 and cancer chemotherapy.14,19,24 In 2011, Duan developed a RNA-based EpCAM aptamer.9 Unfortunately, RNA is notoriously prone to nuclease degradation, which limits its application in clinical research without expensive modification. Later, Yang and his group discovered DNA-based EpCAM aptamers, in particular SYL3C.25 Different from the RNA-based EpCAM aptamer, SYL3C is less expensive and easier to use and store, affording high potential for in vitro assays and in vivo applications.
Among in vivo applications, either frozen or paraffin-embedded sections are commonly used for histopathological examination. However, frozen tissue is closer to that of fresh tissue, and frozen sections can be produced more quickly than paraffin-embedded sections. Moreover, protein activity has maximum retention in frozen tissue sections.
We herein report the validation of EpCAM aptamer SYL3C as a probe for immunostaining the overexpressed EpCAM in colorectal cancer sections, thus providing a new, effective molecular tool for the diagnosis of cancers of epithelial origin like colorectal cancer. On the basis of its convenience and low cost, the aptamer probe is expected to replace EpCAM antibodies for use in in vitro and in vivo experiments.
Materials and Methods
Fluorescence-Labeled EpCAM Aptamer (SYL3C-CY3), DAPI
The oligonucleoide DNA aptamer probe labeled with Cy3 (SYL3C-Cy3, 48 bp) and with the following sequence was synthesized: 5′-CAC TAC AGA GGT TGC GTC TGT CCC ACG TTG TCA TGG GGG GTT GGC CTG-(PEG)3-Cy3-3′ (Sangon Biotech, Shanghai, CN). A random sequence (5′-rN (n = 48)-3′) was also generated to block nonspecific tissue staining. DAPI (Beyotime Institute of Biotechnology, Shanghai, CN) was used to label the nucleus.
Antibodies
Antihuman-EpCAM mouse monoclonal antibody MOC-31 (catalog no. Ab134301) recognizes the extracellular domain of EpCAM (EpEX), and the secondary antibody was also labeled with Cy3 (catalog no. Ab97035). Rabbit monoclonal antibody (catalog no. Ab32392) recognizes the intracellular oncogenic domain of EpCAM (EpICD), and the secondary antibody was labeled with Cy3 (catalog no. Ab97075). All antibodies were from Abcam, Cambridge, MA.
Fresh Tissue Specimens
All fresh tissues were obtained from Xiangya Hospital (Changsha, Hunan, CN) in compliance with a protocol approved by the Institutional Review Board of Central South University of Xiangya Hospital. These samples included colon cancer (n = 8), rectum cancer (n = 8), benign lesion (n = 8), and another EpCAM-negative malignant tumors, as indicated in Figure S2 in the Supporting Information.
Specimen collection was performed by surgeons, and each specimen was separated into two parts. One part was cut for EpCAM aptamer staining and hematoxylin and eosin (H&E) for frozen tissue sections; the other part was cut for EpCAM aptamer, anti-EpEX, and anti-EpICD staining for paraffin-embedded sections. The series of samples containing normal, junction, and cancer tissue were collected from the same cancer patient. All tissue specimens and slides were examined by an experienced pathologist.
Frozen Tissue Sections
The surgical specimens were immersed in a gel-like medium consisting of polyethylene glycol and poly(vinyl alcohol) (OCT gel). The specimen was positioned in the center of a disk that was placed on the cryobar in the cryostat, secured in a chuck, and frozen rapidly to about −20 to −30 °C. Sufficient OCT was removed to make a full section of the specimen visible. When in contact with the glass slide, the OCT and tissue melted, causing the tissue to adhere to the slide.
Formalin-Fixed and Paraffin-Embedded Tissue Sections
The tissues were fixed in 10% formalin for 24 h at room temperature (RT). They were then washed and embedded in paraffin blocks. After slicing, the sections were mounted on slides, air-dried for 30 min, and baked overnight at 45–50 °C.
Immunostaining of Frozen Tissue Sections by SYL3C-Cy3
Different from paraffin-embedded tissue sections, deparaffinization and antigen retrieval were not needed for frozen tissue sections. To rule out nonspecific binding, frozen sections were incubated in 1 μM random sequences for 5 min. Then, SYL3C-Cy3 was diluted in 250 nM binding buffer and incubated with the sections for 20 min before incubation with DAPI for 3 min to clearly show the position of the nucleus. Each frozen tissue section was washed with 40 mL washing buffer (DPBS with 25 mM MgCl2 and 0.045%d-glucose) three times for a total of 15 min on a shaker and then observing under a fluorescence microscope. All steps were performed at RT in the dark (Figure S1A in the Supporting Information).
Immunostaining of Paraffin-Embedded Tissue Sections by SYL3C-Cy3
For aptamer staining, tissue sections were deparaffinized three times in xylene and washed with 100% ethanol, followed by 95% ethanol and 75% ethanol. For antigen retrieval, tissue sections were boiled in Tris-EDTA buffer (pH 8.0) for 15 min, followed by incubating in binding buffer (PBS with 20% fetal calf serum and 1 mM DNA sodium salt from calf thymus) at RT for 1 h.
After two washes with binding buffer, sections were incubated with 1 μM random sequence and then incubated with 250 nM SYL3C-Cy3 for 1 h. The sections were washed three times with binding buffer, sealed, and observed by fluorescence microscopy (Figure S1B in the Supporting Information).
Immunostaining of Paraffin-Embedded Tissue Sections by EpCAM Antibodies
For antibody staining, paraffin-embedded tissue sections were baked at 62 °C for 8–24 h, then deparaffinized twice in turpentine, washed with 100% ethanol, followed by 95% ethanol and 75% ethanol. Finally, sections were washed with running water with blocking by 3% hydrogen peroxide for 10 min.
For antigen retrieval, tissue sections were boiled in citrate buffer solution (pH 6.0) for 3 min. Sections were then washed in phosphate buffer solution (PBS) three times, followed by probing with anti-EpEX and anti-EpICD (1:100 dilution) at 37 °C for 1 h, respectively. After three washes with PBS, tissue sections were incubated with secondary antibody for 30 min at 37 °C and then washed again three times with PBS. Finally, sections were washed in distilled water before examination using fluorescence microscopy (Figure S1C in the Supporting Information).
Results
Specific Immunostaining of Colorectal Cancer Tissue Sections by EpCAM Aptamer Probe SYL3C-CY3
Normal, junction, and cancer tissues from the same patient were made into a series of frozen and paraffin-embedded tissue sections. Compared with the process of producing paraffin-embedded tissue sections, the protocol for preparing frozen tissue sections is simpler and less time-consuming. Using SYL3C-CY3 to incubate the frozen tissue sections at RT, only 20 min is required before the results can be viewed by fluorescence microscopy. For comparison, the paraffin-embedded tissue sections were probed by SYL3C-CY3 and EpCAM antibodies (anti-EpEX or anti-EpICD), respectively. Before incubation, the paraffin-embedded tissue sections had to be deparaffinized and antigen retrieved for 24 h at 100 °C. Using SYL3C-CY3 as the probe, incubation time was 1 h at RT before examining sections by fluorescence microscopy. When using anti-EpEX or anti-EpICD, we first needed to incubate the paraffin-embedded tissue sections at 37 °C to probe out the EpCAM, followed by an additional incubation with CY3-labeled secondary antibody for another 30 min at 37 °C before submitting to microscopy (Figure S1 in the Supporting Information).
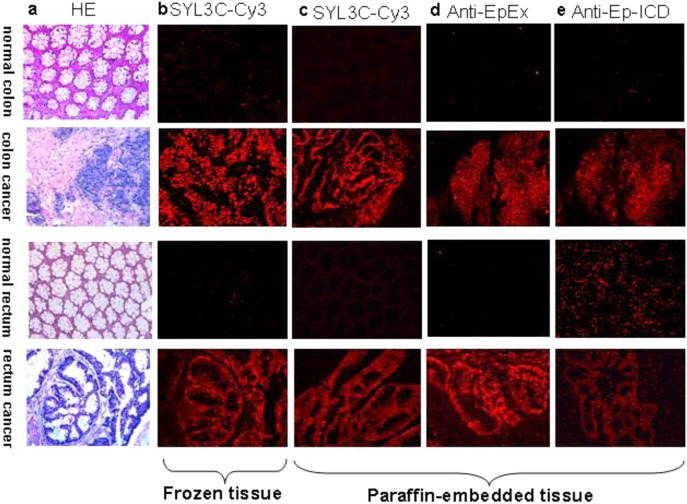
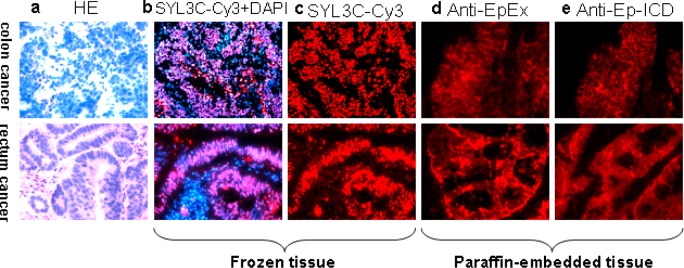
Cancer cases were stained with H&E for morphological confirmation (Figure 1a). In both frozen (Figure 1b) and paraffin-embedded tissue sections (Figure 1c), SYL3C-CY3 could probe out the cancer nest with high specificity, without any cross-linking with normal colon or rectal tissue. Similarly, both anti-EpEX (Figure 1d) and anti-EpICD (Figure 1e) could immunostain the cancer with high specificity, with only very weak staining in normal tissues.
Figure 1.
Tissue immunostaining of colon cancer and rectal cancer by EpCAM Aptamer probe and EpCAM antibodies: (a) cancer cases stained with H&E for morphological confirmation, (b) frozen tissue sections probed by EpCAM aptamer-SYL3C-CY3, (c) paraffin-embedded tissue sections probed by EpCAM aptamer-SYL3C-CY3, (d) paraffin-embedded tissue sections probed by anti-EpEX, and (e) paraffin-embedded tissue sections probed by anti-EpICD. All pictures were taken under a light microscope with 200× magnification.
Figure 2 showed pictures under light microscopy with 400× magnification, using DAPI immunostaining of the nucleus. Anti-EpICD binds only with the intracellular oncogenic domain of EpCAM, while anti-EpEX binds only with the extracellular domain. For using SYL3C-CY3, we could image the cancer tissue sections in one step. When using EpCAM antibodies for imaging, anti-EpICD and anti-EpEX were both needed to probe the intracellular oncogenic domain and extracellular domain of EpCAM, respectively.
Figure 2.
(a) Cancer cases were stained with H&E for morphological confirmation: (b) DAPI (blue) stained the nucleus, (c) SYL3C-CY3 (red), (d) Anti-EpEX binds with the Ep-EX, (e) Anti-EpICD binds with the Ep-ICD. All pictures were taken under a light microscope with 400× magnification.
Specific Immunostaining of Colorectal Cancer Series Frozen Tissue Sections by EpCAM Aptamer Probe SYL3C-CY3
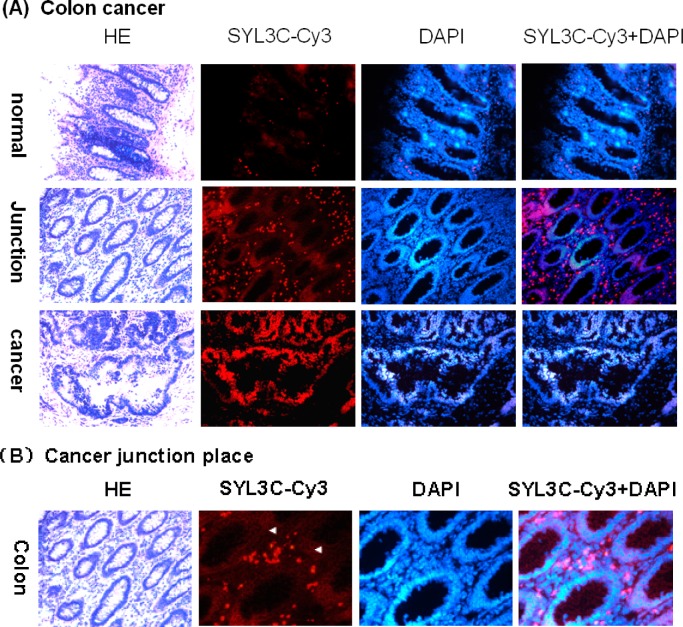
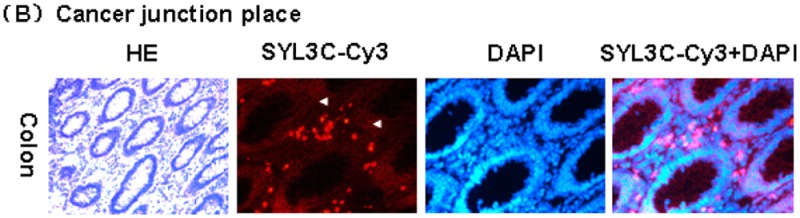
Normal, junction, and cancer tissues collected from the same colon cancer patient were prepared as a series of frozen tissue sections and immunostained by SYL3C-CY3. Normal colon showed only a weak signal. As shown in Figure 3A, the fluorescence signal from EpCAM aptamer successively increased from junction tissue to cancer tissue. In junction tissue, SYL3C-CY3 was expressed in the membrane and cytoplasm but not in nucleus (see row 2 of Figure 3A,B). However, in cancer tissue, SYL3C-CY3 mainly expressed in the nucleus (see row 3 of Figure 3A).
Figure 3.
Series of immunostained frozen tissue sections of colon cancer probed by EpCAM aptamer SYL3C-CY3: (A) pictures obtained using a light microscope with 200× magnification and (B) junction tissue images obtained using a light microscope with 400× magnification. Probing of the tissues with SYL3C-CY3 showed staining in the membrane and cytoplasm but not in the nucleus (indicated by arrows).
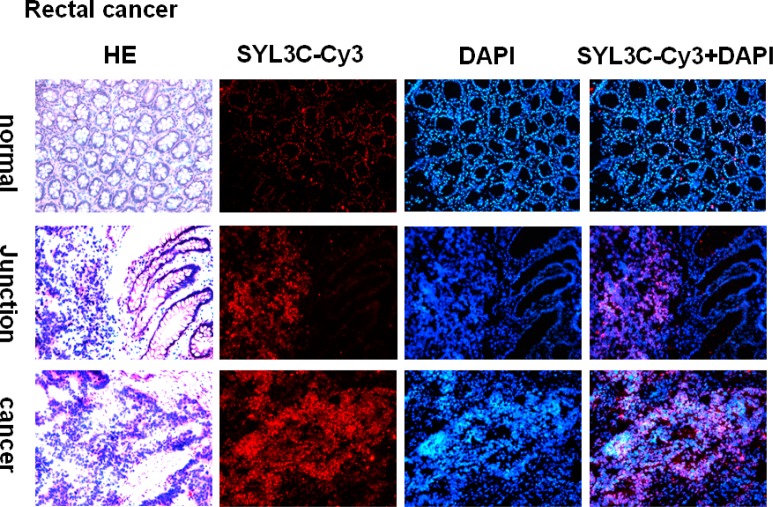
Identical results were found for the images taken from the series of rectal cancer frozen sections. The fluorescence signal from EpCAM aptamer is very weak in normal tissue (see column 2, row 1 in Figure 4). In the junction tissue section, the right side is normal rectum and the left side is rectal cancer. We can see a strong fluorescence signal from the left cancer nest but, in striking contrast, a very weak signal from the right normal rectal tissue (see column 2, row 2 of Figure 4). Also in the cancer place, the fluorescence signal is very strong (see row 3 of Figure 4).
Figure 4.
Series of immunostained frozen tissue sections of rectal cancer probed by EpCAM aptamer SYL3C-CY3. Images were obtained using a light microscope with 200× magnification.
No Cross-Reaction of the EpCAM Aptamer Probe to Non-EpCAM-Expressing Tissues
Using the SYL3C-CY3 probe, no signal was reported from frozen tissue sections exhibiting either colonitis or the presence of colon polyps. We also collected some samples of non-EpCAM-expressing malignant tumors (hypophysoma, meningioma, and renal clear cell carcinoma). No fluorescence signal output was observed after incubation (Figure S2 in the Supporting Information).
Discussion
In 1979, EpCAM, as an antigen, was found in colorectal cancer for the first time,26 and it was considered to be a biomarker for colorectal cancer. Later, research confirmed that EpCAM was widely expressed in most cancers of epithelial origin. Expression level of EpCAM is an important factor in disease pathogenesis,27 from precancerous lesions to malignant tumor to metastasis and cancer relapse.28 These findings indicate that EpCAM is overexpressed in cancers of epithelial origin, upregulated in progressive carcinogenesis, and associated with tumor metastasis. Therefore, EpCAM can be used as a molecular biomarker in the diagnosis, prognosis, and treatment of many kinds of cancer of epithelial origin.
EpCAM, also known as CD326, is a transmembrane glycoprotein that is known to be highly expressed in epithelial carcinomas. It has three main domains: EpEX, EpICD, and the transmembrane domain. In the Wnt signaling pathway, EpEX and EpICD can be transferred from the cell membrane to the nucleus and this is closely related to the occurrence and development of the tumor. Thus, EpEX and EpICD are the main antigenic sites, calling for the necessity of two different antigens by antibodies for their detection.
Yang et al.25 selected DNA-based EpCAM aptamers in 2013, with SYL3C as the most optimized. The present report is the first example applying SYL3C to the fluorescence imaging of pathological frozen and paraffin-embedded tissue sections of colorectal cancer. SYL3C specifically recognized the colorectal cancer nest, but it did not react with background cells within tumor sites. Furthermore, it exhibited no cross-reaction to benign lesion or inflammatory lesion and did not cross-link with EpCAM-negative cancer tissues. On the basis of these properties, DNA-based EpCAM aptamer SYL3C can be used as a probe for immunostaining of cancers of epithelial origin for differential diagnosis. It is also a useful molecular tool for discriminating between normal and cancerous tissue.
In this work, SYL3C was shown to specifically bind to both frozen and paraffin-embedded tissue sections but frozen sections are more similar to fresh tissue, and the preparation of frozen sections is much less time-consuming than that for paraffin-embedded sections, making the protocol of SYL3C immunostaining of frozen sections preferable by its simplicity.
As the standard control, our research utilized the traditional methods involving EpCAM antibodies and immunohistochemistry. Although the imaging effect was almost the same between aptamer and antibodies, including anti-EpEX and anti-EpICD, EpCAM aptamer SYL3C can execute the imaging of colorectal tissue sections in one simple step, while for both anti-EpEX and anti-EpICD, it is necessary to detect EpEX and EpICD separately, followed by an additional incubation with secondary antibody for fluorescence detection. Since preparation and storage of EpCAM aptamer are straightforward and cost-effective, SYL3C is expected to replace antibody-based immunostaining for the diagnosis of colorectal cancer.
EpCAM is not expressed in cancers of nonepithelial origin. Therefore, in the course of the experiment, we found no signal output for such tissue sections after incubation, indicating the ability of EpCAM aptamer to discriminate between cancers of epithelial origin and others.
Up until now, research has focused on monoclonal antibody therapy and corresponding targeted antibody development. In 2009, Catumaxomab, a rat-mouse hybrid monoclonal antibody, was approved for patients with EpCAM-positive tumors and malignant ascites.29 Many antibodies are now commonly used for the diagnosis and treatment of EpCAM-based cancers. However, the use of antibodies has been limited in clinical trials because of their instability and high costs. In the present study, we have shown that EpCAM aptamer SYL3C specifically recognized EpCAM in colorectal cancer tissue, thus providing a theoretical basis for this probe as a future target treatment for colorectal cancer.
In conclusion, we have developed a new immunofluorescence method for staining the frozen tissue sections of colorectal cancer by an EpCAM DNA-based aptamer. Thus, we have also provided a new, effective molecular tool for the diagnosis of EpCAM-positive colorectal cancer, and we anticipate that synthetic DNA-based aptamer probes will become powerful tools for pathologists in addition to currently used antibodies.
Acknowledgments
This study was supported by the Pathology Department of Xiangya Hospital and grants from the National Natural Science Foundation of China (Grants NSFC 81370983 and NSFC 21221003), the Foundation of National Key Scientific Instrument and Equipment Development Projects (Grant 2011YQ0301241403), the Hunan Province Natural Science Key Fund Project (Grant 2014SK2003), the Foundation of China Hunan Provincial Science & Technology Department (Grants 2012FJ4371 and S2014S2032), and the Special Fund for Young Teachers of Central South University (Grant 2012QNZT100). This work is also supported by the National Key Scientific Program of China (Grant 2011CB911000) and by the U.S. National Institutes of Health (Grants GM079359 and CA133086).
Supporting Information Available
Additional information as noted in text. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Zeng Z.; Zhang P.; Zhao N.; et al. Mod. Pathol. 2010, 23121553–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoecklein N. H.; Siegmund A.; Scheunemann P.; et al. BMC Cancer 2006, 6, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munz M.; Baeuerle P. A.; Gires O. Cancer Res. 2009, 69145627–5629. [DOI] [PubMed] [Google Scholar]
- Fartoux L.; Decaens T. Clin. Res. Hepatol. Gastroenterol. 2011, 35Suppl 1S21–30. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A.; Gires O. Br. J. Cancer 2007, 963417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driemel C.; Kremling H.; Schumacher S.; et al. Oncogene 2014, 33414904–4915. [DOI] [PubMed] [Google Scholar]
- Du Q.; Yan W.; Burton V. H..; et al. Am.J. Cancer Res. 2013, 34402–410. [PMC free article] [PubMed] [Google Scholar]
- Stott S. L.; Hsu C. H.; Tsukrov D. I.; et al. Proc. Natl. Acad. Sci. U.S.A. 2010, 1074318392–18397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigdar S.; Lin J.; Yu Y.; et al. Cancer Sci. 2011, 1025991–998. [DOI] [PubMed] [Google Scholar]
- Tuerk C.; Gold L. Science 1990, 2494968505–510. [DOI] [PubMed] [Google Scholar]
- Ellington A. D.; Szostak J. W. Nature 1990, 3466287818–822. [DOI] [PubMed] [Google Scholar]
- Jayasena S. D. Clin. Chem. 1999, 4591628–1650. [PubMed] [Google Scholar]
- Brody E. N.; Gold L. J. Biotechnol. 2000, 7415–13. [DOI] [PubMed] [Google Scholar]
- Bunka D. H.; Stockley P. G. Nat. Rev. Microbiol. Aug 2006, 48588–596. [DOI] [PubMed] [Google Scholar]
- Cerchia L.; Hamm J.; Libri D.; et al. FEBS Lett. 2002, 5281–312–16. [DOI] [PubMed] [Google Scholar]
- Breaker R. R. Nature 2004, 4327019838–845. [DOI] [PubMed] [Google Scholar]
- Mi J.; Liu Y.; Rabbani Z. N.; et al. Nat. Chem. Biol. 2010, 6122–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X.; Tan W. Acc. Chem. Res. 2010, 43148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.; Zhu G.; Mei L.; et al. J. Am. Chem. Soc. 2014, 13672731–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H.; Zhang X. B.; Lv Y.; et al. Acc. Chem. Res. 2014, 4761891–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkenborg J. L.; Karnowski N.; Famulok M. Nat. Chem. Biol. 2011, 78519–527. [DOI] [PubMed] [Google Scholar]
- Xiong X.; Lv Y.; Chen T.; et al. Annu. Rev. Anal. Chem. 2014, 71405–426. [DOI] [PubMed] [Google Scholar]
- Sheng W.; Chen T.; Kamath R.; et al. Anal. Chem. 2012, 8494199–4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe A. D.; Pai S.; Ellington A. Nat. Rev. Drug Discovery 2010, 97537–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y.; Zhu Z.; An Y.; et al. Anal. Chem. 2013, 8584141–4149. [DOI] [PubMed] [Google Scholar]
- Herlyn M.; Steplewski Z.; Herlyn D.; et al. Proc. Natl. Acad. Sci. U.S.A. 1979, 7631438–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M.; Petry I. B.; Bhm D. Breast Cancer Res. Treat. 2011, 1253637. [DOI] [PubMed] [Google Scholar]
- Alberti S.; Ambrogi F.; Boracchi P.; et al. Jpn. J. Clin. Oncol. 2012, 42121128. [DOI] [PubMed] [Google Scholar]
- Linke R.; Klein A.; Seimetz D. MAbs 2010, 22129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.