Abstract
The cardiorenal metabolic syndrome (CRS) consists of a constellation of cardiac, renal, and metabolic disorders including insulin resistance (IR), obesity, metabolic dyslipidemia, high-blood pressure, and evidence of early cardiac and renal disease. Mitochondria dysfunction often occurs in the CRS, and this dysfunction is promoted by excess reactive oxygen species, genetic factors, IR, aging, and altered mitochondrial biogenesis. Recently, it has been shown that there are important sex-related differences in mitochondria function and metabolic, cardiovascular, and renal components. Sex differences in the CRS have mainly been attributed to the estrogen’s effects that are mainly mediated by estrogen receptor (ER) α, ERβ, and G-protein coupled receptor 30. In this review, we discuss the effects of estrogen on the mitochondrial function, insulin metabolic signaling, glucose transport, lipid metabolism, and inflammatory responses from liver, pancreatic β cells, adipocytes, skeletal muscle, and cardiovascular tissue.
1. INTRODUCTION
The cardiorenal metabolic syndrome (CRS) consists of risk factors that include asatherogenic dyslipidemia, elevated blood pressure, high plasma glucose, prothrombotic disorder, and proinflammatory state (metabolic syndrome).1 There are a number of important bidirectional interactions among heart, kidney, brain, liver, muscle, and fat tissues, which cause a constellation of cardiac, renal, and metabolic disorders including insulin resistance (IR), obesity, metabolic dyslipidemia, high-blood pressure, and evidence of early cardiac and renal disease (Fig. 9.1). Epidemiological studies have shown that 36.1% of adult men and 32.4% of women had metabolic syndrome in the USA in 2010. This was a considerable increase from 21.8% to 23.7%, respectively, in 2002.2 The total number of adults with metabolic syndrome ranges from almost 77 million to almost 86 million in America.2 The first formal definition of the metabolic syndrome was proposed by the World Health Organization in 1998.3 International Diabetes Federation, American Heart Association, National Heart, Lung, and Blood Institute, World Heart Federation, International Atherosclerosis Society, and International Association for the Study of Obesity in 2009 defined the metabolic syndrome as three of the following elements: enlarged waist circumference with different population- and country-specific criteria; elevated triglycerides (TG) of ≥1.7 mmol/L (150 mg/dL); reduced high-density lipoprotein (HDL) cholesterol of <1.03 mmol/L (40 mg/dL) in men and <1.29 mmol/L (50 mg/dL) in women; elevated blood pressure, with a systolic blood pressure ≥130 mm Hg or a diastolic blood pressure ≥85 mm Hg; and elevated fasting glucose ≥5.6 mmol/L (100 mg/dL), with the inclusion of those individuals using medication to treat hypertriglyceridemia, decrease HDL, hypertension, or hyperglycemia.4
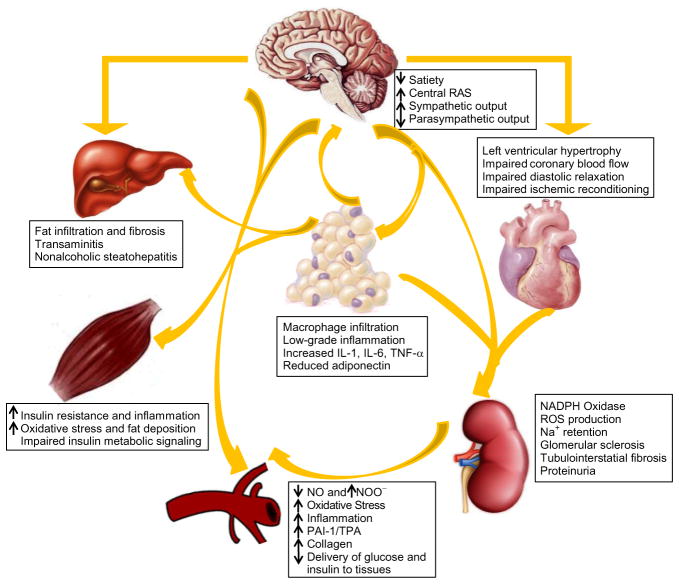
Figure 9.1.
Pathophysiological interactions between adiposity and maladaptive changes in the heart and kidney in CRS. The interactions among the environmental, behavioral, hormonal, and genes help to modify an individual’s body weight, and further show the effects of the resultant dysfunctional adipose tissue and low-grade inflammation on the structure and function of different organ systems. Abbreviations: RAS, renin–angiotensin–aldosterone system; IL, interleukin; TNF, tumor necrosis factor; NO, nitric oxide; NOO−, peroxynitrite; ROS, reactive oxygen species; PAI, plasminogen activator inhibitor; TPA, tissue plasminogen activator.
Many factors contribute to the genesis of metabolic and cardiovascular and renal abnormalities that characterize the CRS, including genetic predisposition, decreased physical activity, chronic inflammation, oxidative stress, elevated free fatty acids (FFA), hyperglycemia, aldosterone, angiotensin II, western diet as well as mitochondrial dysfunction (Fig. 9.2).5 Mitochondria function can benefit from the roles of estrogen through binding to estrogen receptor (ER) α/β, such as antioxidant ability and inhibiting renin–angiotensin system. Thus, estrogen reduces IR, diabetes, cardiovascular diseases (CVDs), and CRS (Fig. 9.2). Recently, our investigative group reported that there is a gender difference in mitochondrial function and that this difference contributes to development of the CRS in mice fed a western diet high in fat and fructose.6 Indeed, the rate of development of CVD related to CRS, such as diastolic dysfunction, coronary vascular stiffness, and impaired vasorelaxation are different in males and females. Normally women develop CVD approximately 10 years later than men, but women show a marked increase in CVD during the postmenopausal period.7 The increased risk of CVD in postmenopausal women has been linked to the decrease in plasma estrogen levels.8 However, our understanding of the biological relationship between estrogen signaling, mitochondria function, and the development CRS is still in its infancy. The objectives of this review are to provide a basic overview of the role of estrogen in regulating mitochondrial function and how abnormalities of this regulation and function contribute to the development of the CRS.
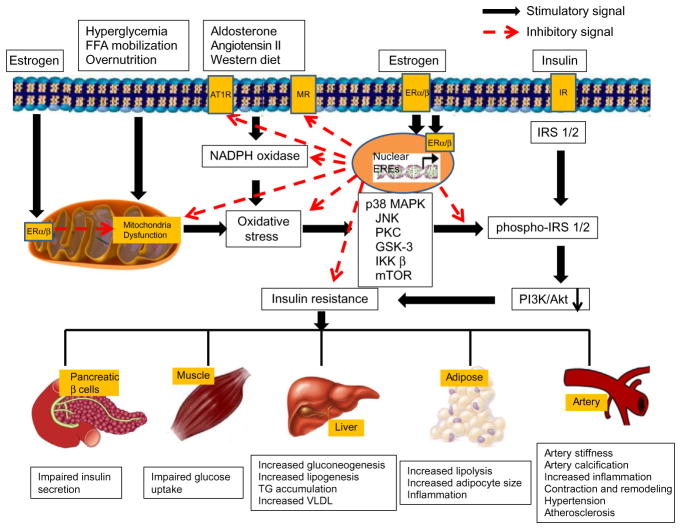
Figure 9.2.
Proposed roles of estrogen and its receptors in the maintenance of mitochondria function and reduction of insulin resistance in CRS. Overnutrients, FFA mobilization, hyperglycemia, aldosterone, angiotensin II, and western diet cause mitochondrial dysfunction and increase ROS production, resulting in insulin resistance in CRS. Estrogen binding to ERα/β protects mitochondria function, increases antioxidant ability, inhibits renin–angiotensin system, and thus reduces insulin resistance, diabetes, and cardiovascular diseases. Abbreviations: FFA, free fat acids; AT1R, angiotensin II receptor 1; MR, mineralocorticoid receptor; IR, insulin receptors; NADPH oxidase, nicotinamide adenine dinucleotide phosphate-oxidase; IRS 1/2, insulin receptor substrate 1/2; MAPK, mitogen-activated protein kinase; JNK, c-Jun N-terminal kinase; PKC, protein kinase C; GSK-3, glycogen synthase kinase 3; IKK β, IκB kinase; mTOR, mammalian target of rapamycin; PI3K/Akt, phosphatidylinositide 3-kinases/protein kinase B; VLDL, very low-density lipoprotein cholesterol.
2. MITOCHONDRIA IN CRS
Mitochondria are essential for intermediary metabolism as well as adenosine triphosphate (ATP) production, and normally provide more than 90% of cellular energy.9 It has been established that mitochondrial respiratory chain function is responsible for energy metabolism and ATP production through the tricarboxylic acid (TCA) cycle, coupling of oxidative phosphorylation (OXPHOS), and electron transfer.10 In the past decade, research has demonstrated that mitochondria play a key role in modulating cell cycle progression, cell survival, apoptosis, as well as reactive oxygen species (ROS) generation which is a “side product” in metabolism.9 Mitochondria dysfunction is recognized as playing a central role in the development of various abnormalities, including disturbed glucose homeostasis, IR, abdominal fat accumulation, dyslipidemia, hypertension and associated cardiac and renal pathology.
2.1. Mitochondria structure and function
The TCA cycle and electron transport chain (ETC) are two important mitochondrial processes in cellular ATP production. The overall pathway for TCA cycle is as follows: catabolism of glucose in the cytosol produces two molecules of pyruvate, which pass through the mitochondrion’s double membrane and enter the TCA cycle.11 When there are relatively high concentrations of ATP, pyruvate carboxylase is activated and shuttles pyruvate in the direction of gluconeogenesis. When energy demands and the concentration of ATP are relatively low, two pyruvate molecules pass through the pyruvate dehydrogenase (PDH) complex to produce two molecules of acetyl-coenzyme A (acetyl CoA), which enter the TCA cycle. Subsequently, each acetyl CoA produces three molecules of nicotinamide adenine dinuceotide (NADH) and two molecules of flavin adenine dinucleotide (FADH), for a total of six NADH and four FADH per one molecule of pyruvate.12 Additionally, acetyl CoA can be produced by oxidation of FFA, which then requires the nutrient L-carnitine to shuttle the acetyl CoA into the mitochondria to enter the TCA cycle.13 The ETC consists of five enzymatic complexes (I–V) of integral membrane proteins. They are NADH-CoQ reductase (complex I), succinate-CoQ reductase (complex II), CoQ-cytochrome c reductase (complex III), cytochrome c oxidase (complex IV), and ATP synthase (complex V).9 Electrons from NADH or FADH2 are transferred through a series of respiratory chain complexes to O2, which finally generates H2O. A proton gradient across the membrane is the driving force of F0F1-ATPase to produce ATP from adenosine diphosphate (ADP). ATP is transported to the cytosol by exchanging ADP through an adenine nucleotide translocator and is important for various biological events.14 Additionally, mitochondria generate heat by a mechanism called the “proton leak.” Proton leak from the intermembrane space to the matrix reduces proton-motive force and generates heat instead of ATP. Much of the proton leak is a catalytic property of specific molecules termed uncoupling proteins (UCPs), such as UCP1, UCP2, and UCP3. UCPs play an important role in reducing the proton gradient and mitochondrial function by regulating both heat and ROS generation.14
Oxygen consumed in mitochondrial is associated with production of oxygen free radicals (ROS) in most cells and tissues. Mitochondrial electron transport generates superoxide radical (O2•−) as an inevitable by-product in complex I and complex III.15 Biologically, ROS include O2•−, hydrogen peroxide (H2O2), and the hydroxyl radical (OH•). It has been estimated that about 0.2–2% of oxygen consumed are converted into O2•− by ETC in human cells.16 However, the mitochondria of cardiomyocytes have high respiratory rates, and approximately 90% of basal cellular ROS are from mitochondria.17 Moreover, seven other sites were also suggested to generate O2•− in the mitochondria. They are cytochrome b five reductase, monoamine oxidase, dihydroorotate dehydrogenase, dehydrogenase of α-glycerophosphate, succinate dehydrogenase, aconitase, and α-ketoglutarate dehydrogenase complex.9 Although this review is concerned with mitochondrial generation of ROS, it should be recognized that ROS are derived from outside of mitochondria, such as oxygen radicals from peroxisomal β-oxidation of fatty acids, NAD(P)H oxidase, xanthine oxidase, arachidonic acid metabolism, microsomal P-450 enzymes, and prooxidant heme molecule.18 Several enzymatic means may protect against ROS in mitochondria. These include conversion of superoxide to H2O2 by superoxide dismutase (SOD), scavenging of H2O2 by catalase, glutathione peroxidase, and peroxiredoxin III.18 Ca2+ transport can cross the mitochon-drial inner membrane and is taken up by mitochondria both through a uniporter and a pulsed or rapid mode.19 The most studied Ca2+ influx mechanism is the uniporter, which transports Ca2+ down its electrochemical gradient.20 Several important functions have been proposed for these processes, including control of the metabolic rate for cellular energy production, modulation of cytosolic Ca2+ transients, and induction of apoptosis through release of cytochrome c.21
2.2. Risk factors in mitochondria function
Mitochondrial dysfunction, such as mitochondrial loss and overproduction of oxidants, has been suggested to be involved in the development of impaired insulin metabolic signaling/IR in various tissues of skeletal muscle, liver, fat, heart, and the kidney. Sedentary lifestyle, genetic factors, oxidative stress, maladaptive immunomodulation, and aging may adversely affect mitochondrial function, leading to impaired insulin metabolic signaling and progression of the CRS (Fig. 9.2). Physical activity plays an important role in the regulation of muscle mitochondrial function because aerobic exercise potently activates mitochondrial function. On the contrary, sedentary behavior is linked with reduced activity, with obesity and other metabolic disorders.22 Thus, it is likely that some mitochondrial defects in overweight or obese insulin-resistant subjects can be explained by low levels of physical activity. Mitochondrial proteins are encoded by both nuclear and mitochondrial genomes, and there is some evidence to suggest that mitochondria DNA (mtDNA) deletions or mutations in nuclear-encoded genes are linked with various abnormalities characterizing the CRS. It has been hypothesized that the mitochondrial genome is more susceptible to various mutagenic stressors because mitochondrial genes are more proximal to the ROS source.23 Our previous research showed that a naturally occurring thymidine-to-cytidine mutation in the mitochondrial tRNAILE gene is associated with phenotypes of hypertension, hypercholesterolemia, and hypomagnesemia.14 A3243G, another mutation gene on mtDNA that encodes tRNA and causes impaired insulin secretion. In addition, patients with defects in acyl CoA dehydrogenase have phenotypes of cardiomyopathy, liver dysfunction, and neurological disorders.24 Furthermore, polymorphisms in the promoter of UCP2 are associated with decreased incidence of obesity, reduced insulin secretion, and a high prevalence of type 2 diabetes mellitus (T2DM).14 Thus, genetic factors that are inherited through nuclear or mitochondrial genes may influence the pathogenesis of the CRS and associated CVD through functional impairment of mitochondria.
ROS production occurs mainly at complex I and complex III in mitochondria.25 Under conditions of glucose and fatty acid overnutrition, nutrient overflow into cells prompts electrons transferring to oxygen without ATP production and further favors a state of increased ROS, which potentially leads to oxidative damage within mitochondria.26 Therefore, ROS generated from mitochondria damages proteins, DNA, and lipid membrane components, which results in mitochondrial dysfunction. In aging, increasing mutations in mitochondrial DNA have been found.27 Old mitochondria have changes in morphology in addition to increased ROS production and decreased ATP production. Abnormalities in aging include cumulative DNA damage, mitochondrial dysfunction, telomere loss, altered gene expression, and oxidative damages.14 In addition, mitochondrial biogenesis may be impaired by age-dependent accumulations of point mutations in human.14 Thus, increased ROS production associated with aging also contribute to mitochondrial dysfunction in the CRS.
2.3. Mitochondria dysfunction in CRS
Mitochondrial abnormalities in diabetes were initially described in the maternally inherited diabetes and deafness syndrome, which is associated with A3243G mtDNA mutation. Loss of pancreas β-cells due to increased apoptosis, influx of fatty acids, and activation of stress-related kinases have been reported in T2DM, which is also named secondary mitochondria dysfunction.28 A decrease in mitochondrial fatty acid oxidation results in increased levels of fatty acyl CoA and diacylglycerol, which in turn activate stress-related serine (ser)/threonine (thr) kinase activity and inhibit glucose transport.29 Oxidative stress contributes to impaired insulin signaling by increased UCP2 activity. Activated UCP2 induces a proton leak across the inner mitochondrial membrane; thereby resulting in the uncoupling of glucose oxidative metabolism from mitochondrial ATP production.30 Furthermore, T2DM and IR have a profound effect on lipid profiles. IR results in the increased production of FFA through loss of its inhibition of hormone-sensitive lipase of adipocytes and decreased endothelial lipoprotein lipase function. The increased influx of FFAs to the liver and insulin stimulation of hepatic lipogenesis lead to increased hepatic TG production in the form of very low-density lipoprotein (VLDL) and steatosis as the TG are stored in the liver.4 Adipocyte production of cholesterol ester transferase protein allows for the transfer of cholesteryl esters from HDL to VLDL. There is increased clearance of HDL by the kidneys, and the liver takes up HDL and produces VLDL, therefore leading to the low HDL and elevated TG levels seen in CRS.4 Moreover, it is known that an abnormal lipid profile is considered to initiate atherosclerosis by impairing the bioactivity of endothelial nitric oxide (NO) and promoting leukocyte adhesion, inflammation, thrombosis, and vascular smooth muscle cell (VSMC) proliferation.31 Taken together, these abnormalities in mitochondria are involved in the pathophysiology of lipid accumulation, IR, T2DM, and CVD.
The molecular mechanism of mitochondrial dysfunction in CRS is driven via abnormalities involving the nuclear respiratory factors (NRF) 1 and 2, the cyclic adenosine monophosphate (AMP) response element binding protein (CREB), and transcription factor A.23 Upstream, the transcriptional coactivator peroxisome proliferators activated receptor gamma coactivator 1 (PGC-1) has been known as an inducible integrator of transcriptional circuits controlling mitochondrial biogenesis and function in a variety of tissue and cell types.32 PGC-1α is a coactivator of nuclear transcription factors in NRF 1 and peroxisome proliferators activated receptors (PPARs).33 PGC-1α is also regulated by the endothelial NO synthase (eNOS)/NO/cyclic guanosine monophosphate (cGMP). The activation of eNOS plays an important role in mitochondrial biogenesis.34 Meanwhile, NRF 1 regulates the expression of many mitochondrial genes including OXPHOS genes and mitochondrial transcription factor A, which are crucial for mitochondrial gene expression and replication (Fig. 9.3).35 Another important factor related to mitochondrial biogenesis is AMP-activated protein kinase (AMPK).36 Studies have shown that decreased expression of PGC-1α-associated impairment of mitochondrial biogenesis may be responsible for various metabolic abnormalities in the CRS.32 Thus, the impairment of any of the complex steps in regulation of mitochondria biogenesis may contribute to the pathogenesis of IR and CVD in CRS.
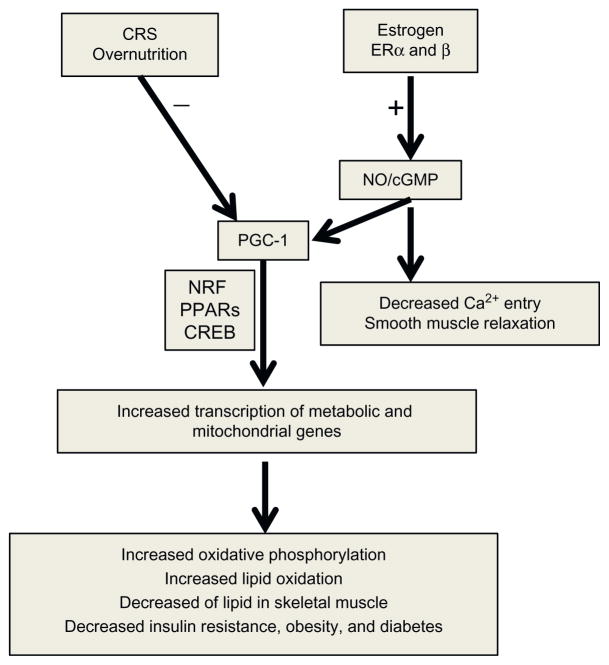
Figure 9.3.
Depiction of molecular mechanism and signaling pathways of estrogen in CRS. The molecular mechanism of mitochondrial dysfunction in CRS is involved in PGC-1, NRF 1/2, CREB, and PPARs signaling pathways. Estrogen increases PGC-1 expression and prevents the development of CRS via its receptors and NO/cGMP signaling pathways. Abbreviations: PGC-1, peroxisome proliferators activated receptor gamma coactivator 1; NRF, nuclear respiratory factors, PPARs, peroxisome proliferators activated receptors; CREB, cyclic AMP response element binding protein; NO, nitric oxide; cGMP, cyclic guanosine monophosphate.
3. ESTROGEN AND MITOCHONDRIAL FUNCTION
Normal estrogen signaling through its receptors ERα and ERβ regulates mitochondrial structure, promotes mitochondrial ETC efficiency, preserves mitochondrial integrity, and maintains normal physiological function in various tissue types, such as vascular, fat, brain, and heart.
3.1. Estrogen, ERs, and their signaling pathways
In premenopausal women, natural estrogens are synthesized in the ovaries from cholesterol. In postmenopausal women, the major precursors of estrogen from peripheral tissues are from circulating androstenedione, testosterone, and estrogen through aromatase activity.8 There are four distinct ERs: two ligand-activated transcription factors ERα and ERβ, one G-protein coupled receptor 30 (GPR30), and a less defined ER-X in the brain.37 It is remarkable that abnormalities of ER signaling in the mitochondria and nucleus can promote the pathophysiological abnormalities characteristic of the CRS (Figs. 9.2 and 9.3). The mitochondrial genome contains DNA sequences that resemble half the palindromic nuclear estrogen response element (ERE) sequence. In an early experiment, ERα and ERβ were shown to directly bind mtDNA in vitro through mitochondrial EREs and the binding response was increased with 17β-estradiol treatment.38 ERs are also widely expressed in the vascular system, specifically in endothelial cells (ECs) and VSMCs. Some studies suggest that ERβ predominates in ECs, whereas ERα expression is greater in VSMCs.8 GPR30 is widely distributed in the brain and peripheral tissues. GPR30 has also been found in human internal mammary arteries and saphenous veins.8
Classical estrogen signaling occurs through a direct binding of ER dimers to EREs in the regulatory regions of estrogen target genes. Estrogen also modulates gene expression by ERs interacting with other transcription factors, such as activating protein-1 and stimulating protein-1.39 Estrogen exerts rapid nongenomic actions by activating plasma membrane-associated ERs, leading to intracellular protein kinase-mediated phosphorylation signaling cascades, such as protein kinase A (PKA), protein kinase C (PKC), and mitogen-activated protein kinase (MAPK).40 GPR30 is structurally unrelated to ERα and ERβ. Recently, GPR30 was reported to mediate nongenomic and rapid estrogen signaling, including release of intracellular Ca2+, activation of Ca2+–calmodulin-dependent kinases, activation of MAPK, and phosphoinositide 3-kinase (PI3K) signaling pathways.41
3.2. Roles of estrogen in mitochondria function
Estrogen may exert direct or indirect effects on mitochondrial function. It has been shown that estrogen increases levels of several mitochondrial respiratory chain proteins, including cytochrome c and complex IV subunits in ECs.42 It is noted that estrogen has been recognized as antioxidants in a variety of in vivo and in vitro models, including ischemic refusion injury, heart failure, brain injury, and neurodegenerative disorders where the delivery of oxygen and glucose are inadequate and mitochondrial OXPHOS is inhibited resulting in more ROS generation.43 Mitochondria are not only a primary source of ROS, but also a main target of ROS. Mitochondria undergo several prominent alterations in the early phase of OXPHOS that may contribute to eventual cell death, including changes in production of ROS, loss of mitochondrial membrane potential, and disruption of energy metabolism.42 ERα and ERβ have been identified in mitochondria and to bind to the D-loop of mouse and human mtDNA, which encode three subunits of cytochrome oxidase, seven subunits of NADH-CoQ reductase, one subunit of cytochrome b, two subunits of ATP synthase, rRNAs, and tRNAs.44 It is not clear whether ERα and ERβ directly regulate mtDNA transcription or whether this effect is mediated through nuclear effects on nuclear-encoded genes that in turn regulate mtDNA transcription.45 Nonetheless, the presence of ERs in both nucleus and mitochondria provide a possible mechanism for estrogen coordination of the expression of mtDNA and nuclear-encoded mitochondrial respiratory complex genes.45 Mitochondria ERα and ERβ participate in the coordination of the cell’s energy requirement and in the OXPHOS biosynthesis, affecting ROS generation and induction of apoptosis (Fig. 9.2).46
In addition to roles in mitochondria, estrogen also suppresses ROS through other sites. Estrogen treatment reduces angiotensin II (Ang II)—and aldosterone signaling through the Ang II receptor 1 (AT1R) and the mineralocorticoid receptor (MR), respectively (Fig. 9.2).47 Normally stimulation of the AT1R and the MR induces free radical, superoxide production, NADPH oxidase activity, and intracellular generation of ROS in VSMCs and ECs.47 Some of our studies confirmed a critical role for increased AT1R and MR in conjunction with an altered redox-mediating impaired endothelial, cardiac, and renal function in the CRS.48 Furthermore, in VSMCs estrogen treatment increases protein levels of SOD.37 Although estrogen has only weak radical scavenging activity, estrogen is able to inhibit oxidative stress markers such as lipid peroxidation, protein oxidation, and DNA damage. In cell-free systems, estrogen inhibits iron-induced lipid peroxidation, LDL oxidation, cholesterol oxidation, and conjugated diene formation.49 These potent antioxidant activities are likely due to a novel redox cycling mechanism. Thus, it could be expected that estrogen plays a crucial role in prevention of mitochondrial dysfunction and the associated development of the CRS.
Estrogen moderates a Ca2+-induced permeability transition during excitoxicity that would clearly be beneficial in mitochondria function. Studies have found that estradiol augments sequestration of cytosolic Ca2+ in mitochondria since estrogen promotes influx of Ca2+ via L-type Ca2+ channels, which in turns activates the Src/ERK/CREB signaling pathways through activating the PI3K.50 Further, estrogen increases mitochondrial sequestration of Ca2+, preventing adverse consequences of excess cytoplasmic Ca2+, and subsequently inhibit the cell apoptosis.49 The death receptor pathway and mitochondrial pathway have been characterized to mediate apoptosis via bcl-2 family protein. Once the mitochondrial apoptotic pathway is activated, the mitochondrial permeability transition pore is open due to elevated mitochondrial calcium levels and excessive ROS. This is followed by cytochrome c release from mitochondria to cytosol with subsequent caspase activation initiating cell self-digestion and nuclear DNA fragmentation.9,51 Therefore, estrogen may prevent cell apoptosis by suppressing mitochondrial oxidative stress, maintaining Ca2+ homeostasis, and increasing bcl-2 family expression in regulation of mitochondria function.
4. ABNORMALITIES IN ESTROGEN SIGNALING PROMOTES DEVELOPMENT OF THE CRS
Our investigation has shown that there are important sex-related differences in the development of diet-induced IR and other key components of the CRS.6 Normally premenopausal women have a lower prevalence of heart and kidney disease relative to age-matched men. Estrogen participates in the modulation of insulin sensitivity, energy balance, and body composition as estrogen regulates the activity and expression of the key enzymes involved in glucose transport, glycolysis, the citric acid cycle, the mitochondrial respiratory chain, and fatty acid oxidation.52
4.1. Estrogen and ERs in CVD
Estrogen plays an important role in the regulation of vascular tone and in the pathophysiology of CVD. Physiological effects of estrogen are mediated through ERα and ERβ, which are both expressed in VSMCs and ECs.53 Estrogen may promote endothelium-dependent vasodilation by increasing the releases of NO, prostacyclin (PGI2), and endothelium-derived hyperpolarizing factor (EDHF). In comparison, estrogen decreases the release of endothelin (ET-1) and Ang II, which are potent vasoconstrictors and procoagulants.8 Furthermore, estrogen can decrease sympathetic activity, circulating levels of norepinephrine, and blood pressure.37 The mechanisms in estrogen-induced eNOS activation and NO expression on ECs are that estrogen activates PI3K/protein kinase B (Akt) pathways, which lead to phosphorylation and activation of eNOS and increases NO production.54 Estrogen also increases eNOS activity by causing rapid ER-dependent activation of MAPK.55 Furthermore, NO induced by estrogen in ECs diffuses into the smooth muscle cells of the blood vessel and interacts with soluble guanylate cyclase. NO stimulates the soluble guanylate cyclase to generate the second messenger cGMP from guanosine triphosphate (GTP). The soluble cGMP activates cyclic nucleotide-dependent protein kinase G (PKG).56 PKG, a kinase in phosphorylating a number of proteins, regulates Ca2+ concentrations and sensitization, hyperpolarizes cell through potassium channels, and causes actin filament and myosin dynamic alterations, and subsequently results in smooth muscle relaxation.57 Meanwhile, estrogen may activate ATP-sensitive K+ channels or Ca2+-activated K+ channels in VSMCs, leading to membrane hyperpolarization, and decreased Ca2+ entry.8 Additionally, estrogen may promote efflux of Ca2+ via plasmalemmal Ca2+ pump in VSMCs through inhibiting PKC signal pathway and Rho-kinase activity.8 Thus, the decrease in estrogen levels in aging women is likely to cause an increase in the expression of Ca2+ channels and decreased Ca2+ pumps, leading to increased VSMC Ca2+, vasoconstriction, and thereby increase the incidence of hypertension and CVD (Fig. 9.3).
In the context of vascular function, estrogen may play a role in the extracellular matrix (ECM) remodeling and reduction of ECM degradation.58 Matrix metalloproteinase (MMPs) are involved in the regulation of the structural integrity of the ECM. When the vessel wall is exposed to immunological stress, inflammatory cells like polymorphonuclear neutrophils (PMNs) and macrophages, produce a variety of MMPs (MMP-1, -7, -8, -13) as well as elastase. MMPs degrade the ECM by affecting the production of weaker collagen and frayed elastin fibers.59 The activity of these enzymes is regulated by augmented gene expression, posttranslational activation of cleavage of pro-MMPs, and interaction among MMPs, plasmin, thrombin and ROS.60 Increased MMP activity induces ECM degradation within the atherosclerotic plaque and may be involved in plaque instability and CVD events. However, postmenopausal women and animals with diminished estrogen function are more prone to vascular and cardiac stiffness and associated vascular disease and impaired cardiac relaxation.
Studies have suggested that the influence of estrogen on insulin sensitivity is largely mediated by estrogen signaling through ERα.61 Compared to ERα wild-type animals, knockout (KO) of the ERα in mice results in obesity at the expense of white adipose tissue expansion, reduced energy expenditure, IR, and glucose intolerance.6 It has been revealed that ERα is a positive regulator of glucose transporter type 4 (GLUT4) expressions, whereas ERβ has a suppressive role probably through regulating PPARγ activity and inhibits expression of GLUT4 in the muscle.41,62 In the cardiovascular system, ERα is found mainly in the uterine vasculature, whereas ERβ is more abundant in ECs and VSMCs from aorta, tail, and uterine vasculature.63 ERβ seems to be localized in the central nervous system cardiovascular control centers with inhibitory neurons in the brain that normally reduce sympathetic activity. ERβ depletion would be associated with withdrawal of inhibitory tone such as that imparted by the parasympathetic system, thereby increasing peripheral resistance and resulting in elevations in blood pressure and associated myocardial hypertrophy.37 Meanwhile, ERβ also plays a protective role in injured arteries by inhibiting neointima formation, which is the first step in the development of atherosclerosis in mice.64 Furthermore, both ERα and ERβ regulate inflammatory responses. ERα mediates estrogen abrogation of cytokine-induced expression of cell adhesion molecules in ECs. Alternatively, ERβ mediated the estrogenic abrogation of tumor necrosis factor α (TNF-α)-induced inflammation in cultured smooth muscle cells.37 Thus, ERα and ERβ play important roles in insulin and glucose metabolism with actions on the liver, adipose tissue, muscle, and pancreatic β cells.
4.2. Estrogen regulates glucose homeostasis and IR
Insulin is released from pancreatic β cells in response to high glucose levels and stimulates the uptake of glucose in skeletal muscles and adipose tissue as glycogen through glycogenesis in the liver.65 A recent study suggested that long term estrogen exposure increases insulin release without changing β cell mass and insulin target gene expression in mice.41 Estrogen is known to influence glucose homeostasis with dominant effects in the liver and muscle. Study has found that ERα is a positive regulator of GLUT4 expressions; whereas ERβ has a suppressive role.66 In addition to facilitating glucose transport, estrogen also promotes aerobic glycolysis by increasing activity of the glycolytic enzymes hexokinase, phosphofructokinase, and pyruvate kinase. Estrogen increases the expression of PDH which is the primary regulatory enzyme that links glycolysis to the TCA cycle.67 Further, estrogen prompts the expression of the complex I, complex IV, and complex V/ATP synthase in mitochondria.38 Our previous data are consistent with above studies and clearly show that glucose intolerance induced by high-fat diet was associated with decreased expression of ERα and GLUT4 in adipose tissue, and further confirmed systemic impairment of glucose homeostasis in the older cohort of ERαKO mice.6 Together, these findings indicate that estrogen promotes enhanced utilization of glucose and maintains glucose homeostasis.
Estrogen is known as a regulator of insulin sensitivity in both women and men. Premenopausal women are more insulin sensitive with associated improved glucose tolerance and are more resistant to develop IR compared to men.41 In particular, estrogens increase hepatic insulin sensitivity by decreasing gluconeogenesis and glycogenolysis, and increasing insulin release in islets of Langerhans. Estrogen also prevents β-cell apoptosis, reduces proinflammatory signaling, and improves insulin action.68 The effects of estrogen signaling through ERs on insulin production have been studied in vivo using aromatase KO mice, which lack the enzyme responsible for conversion of androgens to estrogens.69 Our data further confirmed the role for ERα in modulating IR. Global ERαKO in mice resulted in elevation of IR and inflammation in skeletal muscle along with impaired glucose tolerance.6 Conversely, ERα agonist with the selective ligand propylpyrazoletriyl in ovariectomized wild-type rats increased insulin-stimulated glucose uptake in skeletal muscle through activating AKT and AMPK signaling pathways.70 Therefore, postmenopausal women with lower endogenous estrogen levels may contribute to the sex differences-related IR, T2DM, and CVD in CRS.
4.3. Estrogen regulates lipogenesis and lipolysis
Adiposity is a growing global epidemic that increases the risk of diabetes, CVD, and CRS. Epidemiological studies show postmenopausal women have low estrogen levels, experience a general increase in weight, and redistribute adipose tissue, which lead to increased abdominal fat deposition.71 The increased abdominal fat in postmenopausal women tends to be visceral and not subcutaneous fat.38 Although estrogen increases the risk for the formation of cholesterol gallstones by promoting hepatic secretion of biliary cholesterol, estrogen decreases plasma LDL, and increases plasma HDL.72 Estrogen significantly enhances the activity of enzyme 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, which is the rate-limiting enzyme in hepatic cholesterol biosynthesis under high dietary cholesterol loads, suggesting that there could be an increased delivery of cholesterol to bile from de novo synthesis in the liver.41 A recent study showed that ERα and ERβ are expressed in both subcutaneous and visceral fat tissues, and premenopausal women have a less atherogenic lipid profile than men due to higher HDL and lower TG levels, which are closely associated with central fat accumulation.8 Further, studies found that ERα predominantly regulates adipose homeostasis via growth and proliferation of adipocytes.38 With the euglycemic-hyperinsulinemic clamp, a defective suppression of hepatic glucose output has been demonstrated in ERαKO mice, along with increased expression of genes involved in lipid biosynthesis and decreased expression of genes involved in lipid transport.6 Thus, estrogen and its receptor may play important roles in adipose tissue biology.
4.4. Estrogen regulates inflammatory responses
The incidence of CRS and T2DM are shown to rise remarkably in women following menopause. It is becoming increasingly evident that chronic activation of proinflammatory pathways may be responsible for obesity-induced IR and T2DM. The proinflammatory cytokines, such as TNFα, Interleukin 6 (IL6), and c-reactive protein (CRP) are elevated in patients with IR and T2DM.41 Suppression of proinflammatory responses represents a promising strategy to combat obesity and IR in CRS. Therefore, it might be expected that estrogenic treatments would modulate inflammatory responses. Currently, the most consistent reports of estrogenic modulation of inflammation in vascular tissue involve activation of cytokine pathways in inflammation. Estrogen may decrease the expression of CRP in vascular injury and increases protein S-nitrosylation in human umbilical vein ECs directly via ERα and indirectly by eNOS upregulation, and in turn prevents Ang II-induced upregulation of intercellular cell adhesion molecule-1.8 Many of the intracellular signaling cascades affected by activating toll-like receptors and interleukin molecules are cross-linked with ERs.73 Activation of nuclear factor kappa B (NF-κB) is known to mediate a variety of chronic inflammatory diseases in CRS. Estrogen has been shown to inhibit NF-κB signaling in an ER-dependent manner through both ERα and ERβ.74 However, the complexity of hormonal effects on inflammation is depended on a variety of factors, including the amount and composition of estrogens, the type of immune stimulus, the types of cells becoming inflamed, and the presence of hormone receptors.37,74 Therefore, the impact and the mechanisms linked to sex hormone therapy in inflammation remain to be fully elucidated.
5. CONCLUSIONS
The CRS consists of a constellation of metabolic, cardiac, and renal abnormalities. As discussed throughout this review, CRS is closely related to mitochondria dysfunction and diminished estrogen signaling in the liver, adipose tissues, skeletal muscles, and vascular tissues. Although conventional estrogen therapy might beneficially affect CRS, it is noted that some side effects such as breast cancer and thromboembolism have been found in patient treatment with estrogen. Future investigation should further identify the potential interactions and cross-talk among ERα, ERβ, and GPR 30 in the regulation of body weight, IR, and CVD in both females and males. Meanwhile, a well-controlled and -designed clinical trial is needed to elucidate whether estrogen therapy could be effective in patients with CRS. This may help to define novel pharmacological targets, and thus we may be able to select cogent and knowledge CRS therapies.
Acknowledgments
The authors would like to thank Brenda Hunter for her editorial assistance. This research was supported by NIH (R01 HL73101, R01 HL107910) and the Veterans Affairs Merit System (0018) for J. R. S.
Footnotes
The authors have no conflict of interest associated with this manuscript.
References
- 1.Sowers JR, Whaley-Connell A, Hayden MR. The role of overweight and obesity in the cardiorenal syndrome. Cardiorenal Med. 2011;1:5–12. doi: 10.1159/000322822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ford ES, Li C, Zhao G. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. J Diabetes. 2010;2:180–193. doi: 10.1111/j.1753-0407.2010.00078.x. [DOI] [PubMed] [Google Scholar]
- 3.Jindal A, Brietzke S, Sowers JR. Obesity and the cardiorenal metabolic syndrome: therapeutic modalities and their efficacy in improving cardiovascular and renal risk factors. Cardiorenal Med. 2012;2:314–327. doi: 10.1159/000343803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallagher EJ, Leroith D, Karnieli E. Insulin resistance in obesity as the underlying cause for the metabolic syndrome. Mt Sinai J Med. 2010;77:511–523. doi: 10.1002/msj.20212. [DOI] [PubMed] [Google Scholar]
- 5.Jindal A, Whaley-Connell A, Sowers JR. Obesity and heart failure as a mediator of the cerebrorenal interaction. Contrib Nephrol. 2013;179:15–23. doi: 10.1159/000346718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manrique C, DeMarco VG, Aroor AR, et al. Obesity and insulin resistance induce early development of diastolic dysfunction in young female mice fed a Western diet. Endocrinology. 2013;154:3632–3642. doi: 10.1210/en.2013-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaspard U. Hyperinsulinaemia, a key factor of the metabolic syndrome in postmeno-pausal women. Maturitas. 2009;62:362–365. doi: 10.1016/j.maturitas.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 8.Masood DE, Roach EC, Beauregard KG, Khalil RA. Impact of sex hormone metabolism on the vascular effects of menopausal hormone therapy in cardiovascular disease. Curr Drug Metab. 2010;11:693–714. doi: 10.2174/138920010794233477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao L, Laude K, Cai H. Mitochondrial pathophysiology, reactive oxygen species, and cardiovascular diseases. Vet Clin North Am Small Anim Pract. 2008;38:137–155. doi: 10.1016/j.cvsm.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nisoli E, Clementi E, Carruba MO, Moncada S. Defective mitochondrial biogenesis: a hallmark of the high cardiovascular risk in the metabolic syndrome? Circ Res. 2007;100:795–806. doi: 10.1161/01.RES.0000259591.97107.6c. [DOI] [PubMed] [Google Scholar]
- 11.Mandavia CH, Aroor AR, Demarco VG, Sowers JR. Molecular and metabolic mechanisms of cardiac dysfunction in diabetes. Life Sci. 2013;92:601–608. doi: 10.1016/j.lfs.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Sowers JR, Ren J. Pathophysiological insights into cardiovascular health in metabolic syndrome. Exp Diabetes Res. 2012;2012:320534. doi: 10.1155/2012/320534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pieczenik SR, Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol. 2007;83:84–92. doi: 10.1016/j.yexmp.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102:401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicolson GL. Metabolic syndrome and mitochondrial function: molecular replacement and antioxidant supplements to prevent membrane peroxidation and restore mitochondrial function. J Cell Biochem. 2007;100:1352–1369. doi: 10.1002/jcb.21247. [DOI] [PubMed] [Google Scholar]
- 16.Whaley-Connell A, McCullough PA, Sowers JR. The role of oxidative stress in the metabolic syndrome. Rev Cardiovasc Med. 2011;12:21–29. doi: 10.3909/ricm0555. [DOI] [PubMed] [Google Scholar]
- 17.Demarco VG, Whaley-Connell AT, Sowers JR, Habibi J, Dellsperger KC. Contribution of oxidative stress to pulmonary arterial hypertension. World J Cardiol. 2010;2:316–324. doi: 10.4330/wjc.v2.i10.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal. 2010;12:537–577. doi: 10.1089/ars.2009.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sowers JR, Bakris GL, Black HR, Giles TD. The cardiometabolic syndrome and calcium channel blocker combination drugs. J Cardiometab Syndr. 2007;2:207–212. doi: 10.1111/j.1559-4564.2007.06656.x. [DOI] [PubMed] [Google Scholar]
- 20.Hayden MR, Tyagi SC, Kolb L, Sowers JR, Khanna R. Vascular ossification-calcification in metabolic syndrome, type 2 diabetes mellitus, chronic kidney disease, and calciphylaxis-calcific uremic arteriolopathy: the emerging role of sodium thiosulfate. Cardiovasc Diabetol. 2005;4:4. doi: 10.1186/1475-2840-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunter TE, Buntinas L, Sparagna G, Eliseev R, Gunter K. Mitochondrial calcium transport: mechanisms and functions. Cell Calcium. 2000;28:285–296. doi: 10.1054/ceca.2000.0168. [DOI] [PubMed] [Google Scholar]
- 22.Turner N, Heilbronn LK. Is mitochondrial dysfunction a cause of insulin resistance? Trends Endocrinol Metab. 2008;19:324–330. doi: 10.1016/j.tem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Sack MN. Type 2 diabetes, mitochondrial biology and the heart. J Mol Cell Cardiol. 2009;46:842–849. doi: 10.1016/j.yjmcc.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharef SW, Al-Senaidi K, Joshi SN. Successful treatment of cardiomyopathy due to very long-chain acyl-CoA dehydrogenase deficiency: first case report from Oman with literature review. Oman Med J. 2013;28:354–356. doi: 10.5001/omj.2013.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whaley-Connell A, Sowers JR. Indices of obesity and cardiometabolic risk. Hypertension. 2011;58:991–993. doi: 10.1161/HYPERTENSIONAHA.111.180406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Shen W, Zhao B, et al. Targeting mitochondrial biogenesis for preventing and treating insulin resistance in diabetes and obesity: hope from natural mitochondrial nutrients. Adv Drug Deliv Rev. 2009;61:1343–1352. doi: 10.1016/j.addr.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 27.de Cavanagh EM, Inserra F, Ferder L. Angiotensin II blockade: a strategy to slow ageing by protecting mitochondria? Cardiovasc Res. 2011;89:31–40. doi: 10.1093/cvr/cvq285. [DOI] [PubMed] [Google Scholar]
- 28.Sowers JR. Diabetes mellitus and vascular disease. Hypertension. 2013;61:943–947. doi: 10.1161/HYPERTENSIONAHA.111.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50:567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiff M, Loublier S, Coulibaly A, Bénit P, de Baulny HO, Rustin P. Mitochondria and diabetes mellitus: untangling a conflictive relationship? J Inherit Metab Dis. 2009;32:684–698. doi: 10.1007/s10545-009-1263-0. [DOI] [PubMed] [Google Scholar]
- 31.Jindal A, Whaley-Connell A, Brietzke S, Sowers JR. Therapy of obese patients with cardiovascular disease. Curr Opin Pharmacol. 2013;13:200–204. doi: 10.1016/j.coph.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aroor AR, Mandavia C, Ren J, Sowers JR, Pulakat L. Mitochondria and oxidative stress in the cardiorenal metabolic syndrome. Cardiorenal Med. 2012;2:87–109. doi: 10.1159/000335675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gleyzer N, Scarpulla RC. PGC-1-related coactivator (PRC), a sensor of metabolic stress, orchestrates a redox-sensitive program of inflammatory gene expression. J Biol Chem. 2011;286:39715–39725. doi: 10.1074/jbc.M111.291575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shao D, Liu Y, Liu X, et al. PGC-1 beta-regulated mitochondrial biogenesis and function in myotubes is mediated by NRF-1 and ERR alpha. Mitochondrion. 2010;10:516–527. doi: 10.1016/j.mito.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Venditti P, Bari A, Di Stefano L, et al. Involvement of PGC-1, NRF-1, and NRF-2 in metabolic response by rat liver to hormonal and environmental signals. Mol Cell Endocrinol. 2009;305:22–29. doi: 10.1016/j.mce.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Yu Y, Zhang XH, Ebersole B, Ribnicky D, Wang ZQ. Bitter melon extract attenuating hepatic steatosis may be mediated by FGF21 and AMPK/Sirt1 signaling in mice. Sci Rep. 2013;3:3142. doi: 10.1038/srep03142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60:210–241. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rettberg JR, Yao J, Brinton RD. Estrogen: a master regulator of bioenergetic systems in the brain and body. Front Neuroendocrinol. 2014;35:8–30. doi: 10.1016/j.yfrne.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Handgraaf S, Riant E, Fabre A, et al. Prevention of obesity and insulin resistance by estrogens requires ERα activation function-2 (ERαAF-2), whereas ERαAF-1 is dispensable. Diabetes. 2013;62:4098–4108. doi: 10.2337/db13-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Velarde MC. Pleiotropic actions of estrogen: a mitochondrial matter. Physiol Genomics. 2013;45:106–109. doi: 10.1152/physiolgenomics.00155.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faulds MH, Zhao C, Dahlman-Wright K, Gustafsson JÅ. The diversity of sex steroid action: regulation of metabolism by estrogen signaling. J Endocrinol. 2012;212:3–12. doi: 10.1530/JOE-11-0044. [DOI] [PubMed] [Google Scholar]
- 42.Guo J, Duckles SP, Weiss JH, Li X, Krause DN. 17β-Estradiol prevents cell death and mitochondrial dysfunction by an estrogen receptor-dependent mechanism in astrocytes after oxygen-glucose deprivation/reperfusion. Free Radic Biol Med. 2012;52:2151–2160. doi: 10.1016/j.freeradbiomed.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johannsen DL, Ravussin E. The role of mitochondria in health and disease. Curr Opin Pharmacol. 2009;9:780–786. doi: 10.1016/j.coph.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vasconsuelo A, Milanesi L, Boland R. Actions of 17β-estradiol and testosterone in the mitochondria and their implications in aging. Ageing Res Rev. 2013;12:907–917. doi: 10.1016/j.arr.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Klinge CM. Estrogenic control of mitochondrial function and biogenesis. J Cell Biochem. 2008;105:1342–1351. doi: 10.1002/jcb.21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simoes DC, Psarra AM, Mauad T, et al. Glucocorticoid and estrogen receptors are reduced in mitochondria of lung epithelial cells in asthma. PLoS One. 2012;7:e39183. doi: 10.1371/journal.pone.0039183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xue B, Johnson AK, Hay M. Sex differences in angiotensin II- and aldosterone-induced hypertension: the central protective effects of estrogen. Am J Physiol Regul Integr Comp Physiol. 2013;305:R459–R463. doi: 10.1152/ajpregu.00222.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bender SB, McGraw AP, Jaffe IZ, Sowers JR. Mineralocorticoid receptor-mediated vascular insulin resistance: an early contributor to diabetes-related vascular disease? Diabetes. 2013;62:313–319. doi: 10.2337/db12-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simpkins JW, Yi KD, Yang SH, Dykens JA. Mitochondrial mechanisms of estrogen neuroprotection. Biochim Biophys Acta. 1800;2010:1113–1120. doi: 10.1016/j.bbagen.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31:529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhatt MP, Lim YC, Kim YM, Ha KS. C-peptide activates AMPKα and prevents ROS-mediated mitochondrial fission and endothelial apoptosis in diabetes. Diabetes. 2013;62:3851–3862. doi: 10.2337/db13-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martins-Maciel ER, Campos LB, Salgueiro-Pagadigorria CL, Bracht A, Ishii-Iwamoto EL. Raloxifene affects fatty acid oxidation in livers from ovariectomized rats by acting as a pro-oxidant agent. Toxicol Lett. 2013;217:82–89. doi: 10.1016/j.toxlet.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 53.O’Lone R, Knorr K, Jaffe IZ, et al. Estrogen receptors alpha and beta mediate distinct pathways of vascular gene expression, including genes involved in mitochondrial electron transport and generation of reactive oxygen species. Mol Endocrinol. 2007;21:1281–1296. doi: 10.1210/me.2006-0497. [DOI] [PubMed] [Google Scholar]
- 54.Lee SA, Kim EY, Jeon WK, et al. The inhibitory effect of raloxifene on lipopolysaccharide-induced nitric oxide production in RAW264.7 cells is mediated through a ROS/p38 MAPK/CREB pathway to the up-regulation of heme oxygenase-1 independent of estrogen receptor. Biochimie. 2011;93:168–174. doi: 10.1016/j.biochi.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 55.Jaubert AM, Mehebik-Mojaat N, Lacasa D, Sabourault D, Giudicelli Y, Ribière C. Nongenomic estrogen effects on nitric oxide synthase activity in rat adipocytes. Endocrinology. 2007;148:2444–2452. doi: 10.1210/en.2006-1329. [DOI] [PubMed] [Google Scholar]
- 56.Dey NB, Lincoln TM. Possible involvement of cyclic-GMP-dependent protein kinase on matrix metalloproteinase-2 expression in rat aortic smooth muscle cells. Mol Cell Biochem. 2012;368:27–35. doi: 10.1007/s11010-012-1339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deenadayalu V, Puttabyatappa Y, Liu AT, Stallone JN, White RE. Testosterone-induced relaxation of coronary arteries: activation of BKCa channels via the cGMP-dependent protein kinase. Am J Physiol Heart Circ Physiol. 2012;302:H115–H123. doi: 10.1152/ajpheart.00046.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neugarten J, Golestaneh L. Gender and the prevalence and progression of renal disease. Adv Chronic Kidney Dis. 2013;20:390–395. doi: 10.1053/j.ackd.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 59.Shirwany NA, Zou MH. Arterial stiffness: a brief review. Acta Pharmacol Sin. 2010;31:1267–1276. doi: 10.1038/aps.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dollery CM, McEwan JR, Henney AM. Matrix metalloproteinases and cardiovascular disease. Circ Res. 1995;77:863–868. doi: 10.1161/01.res.77.5.863. [DOI] [PubMed] [Google Scholar]
- 61.Matic M, Bryzgalova G, Gao H, et al. Estrogen signalling and the metabolic syndrome: targeting the hepatic estrogen receptor alpha action. PLoS One. 2013;8:e57458. doi: 10.1371/journal.pone.0057458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deng JY, Hsieh PS, Huang JP, Lu LS, Hung LM. Activation of estrogen receptor is crucial for resveratrol-stimulating muscular glucose uptake via both insulin-dependent and -independent pathways. Diabetes. 2008;57:1814–1823. doi: 10.2337/db07-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khalil RA. Potential approaches to enhance the effects of estrogen on senescent blood vessels and postmenopausal cardiovascular disease. Cardiovasc Hematol Agents Med Chem. 2010;8:29–46. doi: 10.2174/187152510790796156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krom YD, Pires NM, Jukema JW, et al. Inhibition of neointima formation by local delivery of estrogen receptor alpha and beta specific agonists. Cardiovasc Res. 2007;73:217–226. doi: 10.1016/j.cardiores.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 65.Aroor AR, McKarns S, Demarco VG, Jia G, Sowers JR. Maladaptive immune and inflammatory pathways lead to cardiovascular insulin resistance. Metabolism. 2013;62:1543–1552. doi: 10.1016/j.metabol.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barros RP, Machado UF, Warner M, Gustafsson JA. Muscle GLUT4 regulation by estrogen receptors ERbeta and ERalpha. Proc Natl Acad Sci U S A. 2006;103:1605–1608. doi: 10.1073/pnas.0510391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Mahony F, Razandi M, Pedram A, Harvey BJ, Levin ER. Estrogen modulates metabolic pathway adaptation to available glucose in breast cancer cells. Mol Endocrinol. 2012;26:2058–2070. doi: 10.1210/me.2012-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meyer MR, Clegg DJ, Prossnitz ER, Barton M. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol (Oxf) 2011;203:259–269. doi: 10.1111/j.1748-1716.2010.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones ME, Thorburn AW, Britt KL, et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci U S A. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khor VK, Dhir R, Yin X, Ahima RS, Song WC. Estrogen sulfotransferase regulates body fat and glucose homeostasis in female mice. Am J Physiol Endocrinol Metab. 2010;299:E657–E664. doi: 10.1152/ajpendo.00707.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pansini F, Cervellati C, Guariento A, et al. Oxidative stress, body fat composition, and endocrine status in pre- and postmenopausal women. Menopause. 2008;15:112–118. doi: 10.1097/gme.0b013e318068b285. [DOI] [PubMed] [Google Scholar]
- 72.Stubbins RE, Najjar K, Holcomb VB, Hong J, Núñez NP. Oestrogen alters adipocyte biology and protects female mice from adipocyte inflammation and insulin resistance. Diabetes Obes Metab. 2012;14:58–66. doi: 10.1111/j.1463-1326.2011.01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang SY, Xin H, Sun J, Li R, Zhang XM, Zhao D. Estrogen receptor β agonist diarylpropionitrile inhibits lipopolysaccharide-induced regulated on activation normal T cell expressed and secreted (RANTES) production in macrophages by repressing nuclear factor κB activation. Fertil Steril. 2013;100:234–240. doi: 10.1016/j.fertnstert.2013.02.052. [DOI] [PubMed] [Google Scholar]
- 74.Wang L, Qiu XM, Hao Q, Li DJ. Anti-inflammatory effects of a Chinese herbal medicine in atherosclerosis via estrogen receptor β mediating nitric oxide production and NF-κB suppression in endothelial cells. Cell Death Dis. 2013;4:e551. doi: 10.1038/cddis.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]