Abstract
Previous research indicates that at least some children with specific language impairment (SLI) show a reduced neural response when non-linguistic tones were presented at rapid rates. However, this past research has examined older children, and it is unclear whether such deficits emerge earlier in development. It is also unclear whether atypical refractory effects differ for linguistic versus non-linguistic stimuli or can be explained by deficits in selective auditory attention reported among children with SLI. In the present study, auditory refractory periods were compared in a group of 24 young children with SLI (age 3–8 years) and 24 matched control children. Event-related brain potentials (ERPs) were recorded and compared to 100 ms linguistic and non-linguistic probe stimuli presented at inter-stimulus intervals (ISIs) of 200, 500, or 1000 ms. These probes were superimposed on story narratives when attended and ignored, permitting an experimental manipulation of selective attention within the same paradigm. Across participants, clear refractory effects were observed with this paradigm, evidenced as a reduced amplitude response from 100 to 200 ms at shorter ISIs. Children with SLI showed reduced amplitude ERPs relative to the typically-developing group at only the shortest, 200 ms, ISI and this difference was over the left-hemisphere for linguistic probes and over the right-hemisphere for non-linguistic probes. None of these effects was influenced by the direction of selective attention. Taken together, these findings suggest that deficits in the neural representation of rapidly presented auditory stimuli may be one risk factor for atypical language development.
Keywords: Specific language impairment (SLI), Refractory periods, ERP, Auditory processing
1. Introduction
During the first years of life, most children effortlessly acquire their native language. However, for children with specific language impairment (SLI) the development of oral language does not proceed as expected, despite typical cognitive ability and an absence of frank neurological disorder (Leonard, 1998). It is estimated that SLI affects at least 7% of kindergarten age children (Tomblin et al., 1997), and half of children with SLI will later develop comorbid specific reading disorder (dyslexia) (Bishop and Snowling, 2004; Catts, 1993; Eisenmajer et al., 2005; McArthur and Hogben, 2001). Although linguistic deficits fundamentally characterize both SLI and dyslexia (Bishop and Snowling, 2004; Snowling, 2001; Stanovich, 1988), at least some individuals with these disorders also exhibit non-linguistic deficits in lower-level perceptual skills (e.g., Lovegrove et al., 1980; Talcott et al., 1998; Tallal and Piercy, 1973a) and selective attention (e.g., Hari et al., 1999; Klein and D’Entremont, 1999; Noterdaeme et al., 2000; Spaulding et al., 2008; Sperling et al., 2006; Stevens et al., 2006). However, it is unclear whether a shared neural mechanism underlies both the non-linguistic deficits and the more prominent language difficulties encountered by individuals with language or reading impairment.
One controversial hypothesis proposes that the language difficulties encountered by children with SLI stem from a more basic perceptual deficit in auditory processing. Specifically, it has been proposed that children with SLI have particular difficulty processing auditory information that is presented at rapid rates and/or that is distinguished on the basis of brief auditory cues, forming a non-linguistic perceptual basis for later language difficulties (Tallal, 1980, 2004; Tallal and Piercy, 1973b). Under this auditory deficit hypothesis of SLI, a general deficit in the speed of auditory information processing impairs the perception and processing of some phonemes. For example, the syllables/ba/and/da/are differentiated by cues occurring in the first 40 ms of stimulus onset. A generalized deficit processing rapid auditory information would be expected to impair the effective use of these brief auditory cues, disrupting the ability to form stable representations of these phonemes. Further, as some of the morphemes that are most problematic for children with SLI are also the least perceptually salient (Leonard, 1998), subtle auditory processing deficits might render these forms more difficult to acquire.
Behaviorally, there is considerable evidence that at least some children with SLI exhibit non-linguistic auditory processing deficits. Although the precise nature of these auditory deficits remains an issue of intense debate (for a review, see Rosen, 2003), it is also generally agreed that such non-linguistic deficits do exist, at least among some children with SLI. For example, children with SLI perform more poorly than their typically-developing peers when asked to recall the order of two nonlinguistic auditory stimuli separated by a brief interval and/or presented at rapid rates (Lowe and Campbell, 1965; Tallal and Piercy, 1973a,b). Children with SLI are also less able to separate a brief tone from a similar sound that follows rapidly (Wright et al., 1997). In addition, children with SLI have poorer behavioral frequency discrimination thresholds for auditory tones separated by a 500 ms inter-stimulus interval, and this deficit has been observed for tones of either 25 ms or 250 ms in duration (McArthur and Bishop, 2004, 2005). Further, behavioral measures of non-linguistic rapid auditory processing show positive correlations with concurrent measures of performance on both receptive language assessments (Bavin et al., 2010) and linguistic discrimination tasks (McArthur and Bishop, 2005). Moreover, longitudinal studies indicate that non-linguistic auditory processing ability in infancy predicts later language, but not general intelligence, measures (Benasich and Tallal, 2002; Benasich et al., 2002; Trehub and Henderson, 1996). Taken together, these data suggest that atypical auditory processing is a common correlate of SLI and can precede and even predict the emergence of linguistic deficits.
Neurophysiological measures provide a useful means for further characterizing the nature of auditory processing deficits in SLI. The temporal dynamics of auditory processing are particularly amenable to study using event-related brain potentials (ERPs) and magnetoencephalographic (MEG) responses, which have millisecond precision and can be recorded noninvasively. For example, several ERP and MEG studies document that individuals with SLI show atypical neural responses to both linguistic and non-linguistic auditory stimuli during the first few hundred milliseconds of processing, including delayed latencies, reduced amplitudes, and/or abnormal morphologies of the cortical evoked response (Bishop and McArthur, 2005; Bishop et al., 2007; McArthur and Bishop, 2004, 2005; Pihko et al., 2008). One recent study also reported less robust subcortically-generated phase-locking among children with SLI in response to tone sweep stimuli, with these deficits particularly evident as the sweep rate increased (Basu et al., 2010). These data are suggestive that individuals with SLI exhibit a degraded neural response as the temporal dynamics of auditory stimuli require increasingly rapid rates of processing. Indeed, individuals with SLI tend to show a reduced ability to create a differentiated neural response to two tones presented in rapid succession, including inter-stimulus intervals ranging from 20 to 150 ms (Bishop and McArthur, 2004; Oram Cardy et al., 2005). These data suggest that one aspect of auditory dysfunction in SLI may relate to decreased responsiveness to stimuli presented at rapid rates, with auditory information integrated over a larger than normal time window. Indeed, increased temporal integration windows have been shown in animal models to impair the differentiated response apparent in A1 to consonant–vowel syllables whose identification is dependent on rapid formant transitions (Steinschneider and Fishman, 2011). However, most studies of children with SLI have not analyzed whether atypical neural responses normalize at longer inter-stimulus intervals, which would be predicted by the auditory temporal deficit hypothesis.
The normalization, or recovery, of the neural response to auditory stimuli at longer inter-stimulus intervals provides an index of the auditory recovery cycle. Here, we operationally define auditory recovery cycle as the decrease in the amplitude and/or increase in latency of the evoked response at more rapid rates of stimulus presentation. This decreased responsiveness is believed to reflect the reduced excitability of cortical neurons immediately following an action potential, or the effective processing rate of neurons (Budd et al., 1998; Gastaut et al., 1951). The auditory recovery cycle, as indexed with ERP and MEG responses, may persist for several seconds (Budd et al., 1998; Wang et al., 2004), though discernable effects are observed for ISIs at or under one second in duration (Coch et al., 2005a; Wang et al., 2004). For example, among adults, as the inter-stimulus interval (ISI) between successive auditory stimuli is reduced, the amplitude of the N1 component of the ERP is also reduced (Budd et al., 1998; Coch et al., 2005b; Wang et al., 2004), with some evidence for a delay in N1 latency, as well (Coch et al., 2005b). Developmental studies further indicate changes in recovery cycles during childhood, with younger children showing larger refractory effects, or refractory effects that persist at longer ISIs (Coch et al., 2005b; Rojas et al., 1998). Developmental changes may show laterality effects, as well, with some evidence that developmental changes in ISI effects beyond the age of 8 years are limited to the right hemisphere, at least for non-linguistic tones (Rojas et al., 1998).
Prolonged auditory recovery cycles in SLI may be one source of the atypical ERP responses reported above. Indeed, prolonged recovery cycles may contribute to longer temporal integration windows, such that at rapid rates of presentation stimuli are not faithfully represented at a neural level. However, to date, only a handful of studies have explicitly examined the neurophysiology of responses in SLI as a function of the inter-stimulus interval (ISI) between auditory stimuli. One report used ERPs to examine refractory period effects in individuals with SLI (Neville et al., 1993). In this study, children with SLI who scored poorly on a behavioral test of rapid auditory processing also showed atypical auditory ERP refractory effects. Over right frontal electrode sites, these children with SLI showed reduced amplitude ERPs relative to their typically-developing peers in response to simple tones presented at a brief 200 ms ISI, though not at longer 1000 or 2000 ms ISIs. A more recent study examined auditory brainstem responses to very brief (100 μs) tones presented at ISIs of approximately 14–90 ms (Basu et al., 2010; note that the short ISIs are in line with the brief total duration of auditory brainstem responses). Children with SLI showed delayed latencies and reduced amplitudes of several brainstem waves that generalized across the ISIs tested. Moreover, as the ISI decreased, both groups showed delays in the latency of brainstem responses, though this effect was more pronounced for children with SLI.
While these studies suggest that SLI is associated with depressed recovery cycles at both the cortical and brainstem level, they also leave several questions unanswered. For example, as previous studies of auditory recovery cycles in SLI have used only non-linguistic probes, it is unclear whether or how atypical refractory effects in SLI might vary by stimulus type. One possibility is that linguistic stimuli may display greater refractory cycle vulnerabilities in SLI or that the distribution of differences in cortical evoked potentials may be more broadly distributed over left-hemisphere regions, as opposed to restricted to right hemisphere regions as shown previously (Neville et al., 1993). It is also unclear whether such differences in refractory cycles occur relatively early in development. The children in the two refractory cycle studies described above were relatively old at the time of testing, with all but two children in the studies over the age of six years. However, the auditory processing deficit hypothesis for SLI suggests that atypical refractory effects in SLI should be apparent early in development, when language difficulties are initially emerging. Indeed, such differences might be expected as studies of young children with SLI or infants at-risk for language impairment have reported atypical auditory evoked potentials (Benasich et al., 2006; Choudhury and Benasich, 2011). Moreover, ERP and MEG indices of non-linguistic auditory processing have reported correlations with both concurrent and future language abilities (Benasich et al., 2006; Choudhury and Benasich, 2011; Oram Cardy et al., 2008). However, it remains an open question whether or how auditory refractory cycles differ in younger children with SLI, or between linguistic as compared to non-linguistic stimuli.
A separate issue concerns the nature of the auditory refractory cycle differences. Most critically, it is unclear whether differences in refractory cycles, or, more generally, impaired processing at rapid rates of presentation, can be explained by a separate factor. For example, several studies indicate differences in selective attention among children with SLI (Noterdaeme et al., 2000; Spaulding et al., 2008; Stevens et al., 2006; Ziegler et al., 2005). Furthermore, it has been suggested that attentional differences among individuals with language or reading disorder may account for some differences reported in auditory processing (Hari and Renvall, 2001; Ziegler et al., 2005). Thus, it is unclear whether differences in selective attention might account for some or all of the refractory effect differences previously reported in children with SLI.
Interestingly, the effects of selective attention and the effects of ISI on neural processing show remarkable similarity. When attention is directed selectively to one of two simultaneously presented auditory streams, within 100 ms the neural response is 50–100% larger to stimuli when attended as compared to when unattended (Hansen and Hillyard, 1983; Hillyard et al., 1973). We have developed a child-friendly ERP paradigm that elicits similar effects of selective auditory attention in young children (Coch et al., 2005a; Sanders et al., 2006). In this paradigm, two stories are played simultaneously from speakers located to the left and right of the participant, who is cued to attend selectively to one of the two stories. Despite the crowded acoustic environment, both adults and children show clear ERPs in response to probe stimuli, though the morphology of the response differs in adults and children. Whereas adults display an evoked response that includes an early positivity (P1) followed by a negative component around 100 ms (N1), children show a broad positivity from ~100 to 250 ms in response to probe stimuli. This difference in morphology is consistent with developmental research showing that children’s auditory evoked potentials are dominated by a broad, positive response, particularly in acoustically crowded environments with short inter-stimulus intervals between stimuli (Ponton et al., 2000; Sharma et al., 1997). However, despite these differences in ERP morphology, both adults and children show clear amplification of the neural response within 100 ms, evident as an increased negativity of the N1 in adults and an enhancement of the broad positivity in children (Sanders et al., 2006).
In a comparison of typically-developing (TD) children and children with SLI, we have further observed that whereas TD children as young as three years of age demonstrate ERP effects of selective auditory attention within 100 ms after stimulus presentation, children with SLI do not, despite being willing and able to perform the task (Stevens et al., 2006). Further, this deficit in selective attention is specific to a reduced ability to enhance the neural response to probes presented in the attended channel. However, it is unclear whether or how these attention deficits might interact with the rate of stimulus presentation or account for the refractory cycle differences previously reported in children with SLI.
1.1. Goals and overview of the present study
The present study compared auditory refractory cycles in a group of young (age 3–8 years) children with SLI to typically-developing peers using a unique ERP paradigm. Crucially, the present study examined auditory refractory period effects embedded in a selective attention paradigm, thus permitting an assessment of the role of selective attention deficits in atypical recovery cycles. Additionally, both linguistic and non-linguistic stimuli were presented, to assess possible differences in refractory effects across stimulus types.
Briefly, children were cued to attend to one of two auditory narratives presented simultaneously to the left and right ears, respectively. ERPs were recorded to 100 ms linguistic and non-linguistic probe stimuli superimposed on both narratives, presented at ISIs of 200, 500, or 1000 ms. We have previously reported data on a set of 12 children with SLI and 12 matched controls examining only the effects of the selective attention manipulation using the paradigm (Stevens et al., 2006). Here, we combine the data from this previous study with new data from an additional 24 children (half with SLI) and ask whether children with SLI show atypical refractory effects in this paradigm, as well as whether those effects vary as a function of attention condition (attend/unattend), probe type (linguistic/non-linguistic), and electrode hemisphere (left/right).
2. Results
2.1. Demographic variables
As expected based on selection criteria, the SLI and TD groups differed significantly in CELF receptive language, expressive language, and total language scores, all t(46)>5, P<.001, as shown in Table 1. On average, the SLI group scored in the 6th percentile for receptive language, and the TD group in the 47th percentile. Children with SLI had an average discrepancy of 1.33 standard deviations (20 standard scores) between their receptive language and non-verbal IQ. Aside from these group differences in language ability, the SLI and TD groups did not differ in age, gender, handedness, or non-verbal IQ (all P>.4). The groups also did not differ in socioeconomic status (SES), either as measured using the Hollings-head 4-Factor Index (Hollingshead, 1975) or a measure of maternal education (all P>.6).
Table 1.
Demographic and behavioral characteristics of specifically language impaired (SLI) and typically-developing (TD) groups. Standard deviations in parentheses.
| TD
|
SLI
|
P | |
|---|---|---|---|
| n=24 | n=24 | ||
| Age | 5.4 (1.2) | 5.3 (1.1) | .61 |
| Gender | 9 M, 15 F | 8 M, 16 F | .99 |
| Handedness | 24 R, 0 L | 22 R, 2 L | .99 |
| Socio-economic status a | 33 (12) | 35 (12) | .68 |
| Maternal education b | 4.8 (0.8) | 4.7 (1.0) | .72 |
| Stanford–Binet 5 non-verbal IQ, standard scores | |||
| Non-verbal IQ | 97.8 (6) | 96.4 (7) | .44 |
| Clinical evaluation of language fundamentals, standard scores | |||
| Receptive language | 98.6 (8) 47th %ile |
76.5 (8) 6th %ile |
<.001 |
| Expressive language | 100.5 (8) 53rd %ile |
82.5 (8) 19th %ile |
<.001 |
| Total language | 100.7 (8) 53rd %ile |
80.5 (12)10th %ile | <.001 |
Socio-economic status (SES) estimated using the Hollingshead 4-Factor Index of Social Position. Values range from 8 to 66. Higher scores represent higher SES. Scores of 30–39 represent middle class.
Maternal education coded using the Hollingshead scoring code. Values range from 1 to 7. Higher scores represent higher levels of attained education. Score of 5 represents partial college or specialized training.
2.2. Behavioral data
Following artifact rejection, there were no differences between groups in the number of ERP trials available for analysis in the average for any condition (all P>.25). In response to comprehension questions about the attended story, all children answered at least six of the 12 questions correctly, and there were no differences between groups in the average number of questions correctly answered indicating that both groups were willing and able to complete the task (typically-developing children: M=9.1, SD=1.5; children with SLI: M=8.5, SD=1.6, P=.20).
2.3. Electrophysiological data
Given the focus of the present study on refractory cycle effects, analyses were restricted to a main effect of ISI or group, as well as interactions including ISI as a factor, over a frontal electrode ROI implicated in previous studies of refractory effect differences in SLI and reading disorder (Neville et al., 1993; Sharma et al., 2007).1
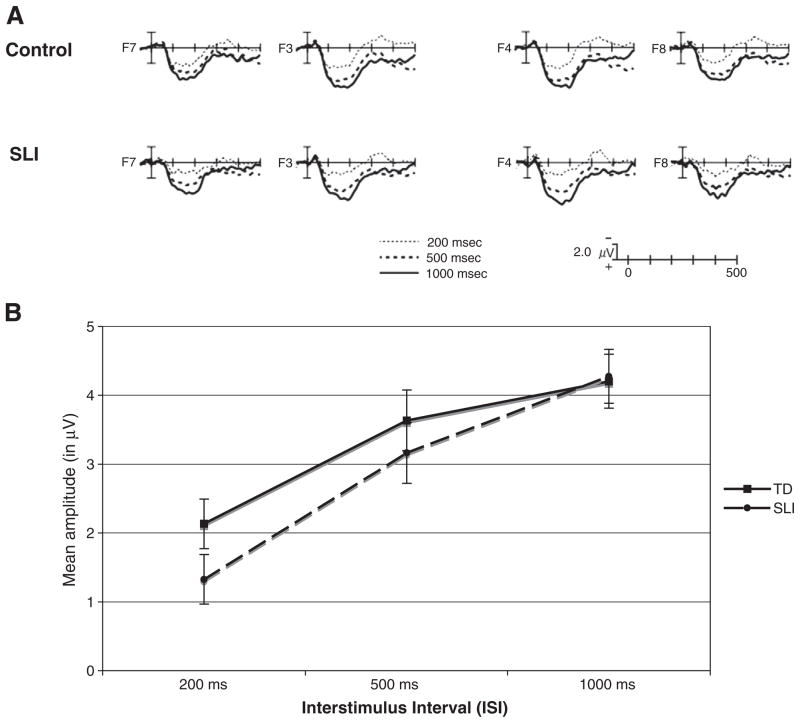
As shown in Fig. 1, both TD and SLI children showed a single, broad positivity in response to probe stimuli, peaking around 150 ms after probe onset. Clear refractory effects were evident on this broad positivity, with overall amplitude increasing at longer inter-stimulus intervals (ISI, F (2, 92) = 27.7, P<.001, partial η2=.38), see Fig. 1. This overall refractory effect did not differ as a function of group, probe type, or hemisphere (ISI×Group, ISI×Probe Type, ISI×Attention, and ISI×Hemi, all P>.3), and neither were there overall amplitude differences between groups (Group, F (1, 46)=1, P=.32).
Fig. 1.
(A) Grand average ERP responses to probe stimuli (collapsed across linguistic and non-linguistic probes) at the three inter-stimulus-intervals over frontal electrode sites, separately for children with SLI and typically-developing children. (B) Mean amplitude from 100 to 200 ms post-stimulus onset at each ISI, collapsed across probe type, separately for SLI and typically-developing groups. Bars represent standard errors. Both groups showed clear refractory effects, evident as a decreased amplitude ERP response with increasing inter-stimulus interval. The overall group×ISI interaction was not significant.
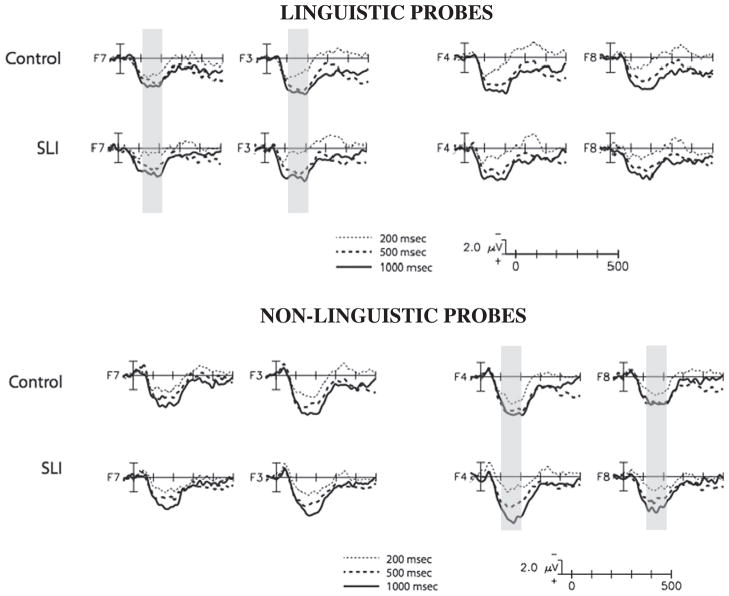
Critical to the main hypothesis of the study, the two groups showed differences in the refractory effects, and these differences interacted with both probe type and hemisphere (Group×ISI×Probe Type×Hemi, F (2, 92)=3.6, P=.032, partial η2=.07). Table 2 presents the mean amplitude and standard deviation as a function of ISI, Probe Type, and electrode hemisphere, separately for each group. As shown in Figs. 2 and 3, the SLI group showed reduced amplitude ERPs relative to the TD group at only the shortest, 200 ms, ISI to both probe types, and this difference was over the left-hemisphere for linguistic probes and over the right-hemisphere for non-linguistic probes. Post-hoc t-tests (one-tailed) comparing the two groups at each ISI separately over the left and right hemisphere ROI confirmed this pattern: linguistic probes after 200 ms ISI (LH: t(46)=−1.92, P<.03, one-tailed; RH: t(46)=−0.43, ns); non-linguistic probes after 200 ms ISI (RH: t(46)=1.66, P=.05, one-tailed; LH: t(46)= 0.65, ns). There were no differences between groups at the longer ISIs in either hemisphere, for either probe type (all other comparisons ns, smallest P=.25).
Table 2.
Mean amplitude in μV from 100 to 200 ms in response to linguistic and non-linguistic probes at each of the three ISIs (200, 500, 1000 ms) in each hemisphere, separately for children in the TD and SLI groups.
| Left hemisphere
|
Right hemisphere
|
||||||
|---|---|---|---|---|---|---|---|
| 200 | 500 | 1000 | 200 | 500 | 1000 | ||
| Linguistic probes | |||||||
| TD | M (SD) | 2.17 (3.12) | 3.50 (2.78) | 4.02 (3.36) | 1.44 (2.43) | 3.22 (3.11) | 4.20 (2.93) |
| SLI | M (SD) | 0.65 (2.33) | 2.90 (3.10) | 3.77 (1.94) | 1.16 (2.15) | 3.08 (3.05) | 4.03 (2.09) |
| Non-linguistic probes | |||||||
| TD | M (SD) | 2.32 (1.91) | 3.68 (3.76) | 4.42 (2.58) | 2.60 (2.30) | 4.15 (3.23) | 4.17 (2.48) |
| SLI | M (SD) | 1.93 (2.15) | 3.19 (2.16) | 4.27 (2.87) | 1.57 (1.99) | 3.49 (1.94) | 5.03 (2.61) |
Fig. 2.
ERP responses to linguistic probes (top row) and non-linguistic probes (bottom row) over frontal electrode sites, separately for children with SLI and typically-developing children. Children with SLI showed a reduced amplitude response to probes at 200 ms ISI that was specific to the left-hemisphere for linguistic probes and the right hemisphere for non-linguistic probes. Regions of significant differences between groups for the 200 ms ISI are shaded in gray.
Fig. 3.

Mean amplitude from 100 to 200 ms ISI, separately for each group over left-and right-hemisphere ROIs. Children with SLI showed a reduced amplitude response to probes at 200 ms ISI that was specific to the left-hemisphere for linguistic probes and the right hemisphere for non-linguistic probes.
There were no overall effects of attention on refractory effects (ISI×Attention, P=.92) nor were the group difference in ISI effects influenced by attention condition (all other interactions with attention and ISI, ns).
3. Discussion
The present study extends previous reports of atypical auditory refractory cycles in SLI in three important ways. First, the data clarify the nature of refractory cycle impairments for linguistic as compared to non-linguistic stimuli. Specifically, children with SLI show a reduction in the amplitude of neural responses relative to their typically-developing peers to both linguistic and non-linguistic stimuli when presented at a brief, 200 ms, ISI. However, these effects are observed over right-hemisphere sites for non-linguistic probes and over left-hemisphere sites for linguistic probes. Second, the present study informs whether deficits in selective auditory attention, previously reported in children with SLI (Stevens et al., 2006), can account for these refractory effect differences. Here, we find no evidence that group differences in refractory effects are influenced by the direction of selective attention, suggesting that deficits in selective attention cannot account for a sluggish refractory cycle. Finally, these results demonstrate that atypical refractory effects are apparent in young children with SLI, at ages closer in temporal proximity to the emergence of linguistic deficits than previously reported. Taken together, these findings suggest that atypical auditory refractory cycles may be one factor contributing to abnormal language development in children with SLI.
3.1. Group differences in refractory effects
A unique ERP paradigm was used in the present study to assess auditory refractory cycles. In this paradigm, probe stimuli were superimposed on two continuous narratives, permitting an experimental manipulation of selective attention within the same paradigm. However, even in this dense acoustic environment, clear ERP refractory effects were observed in both groups of children. Refractory effects were evident as reduced amplitude ERP responses at shorter ISIs, similar to those reported in previous studies in which stimuli were presented in silence (e.g., Budd et al., 1998; Coch et al., 2005b). However, in contrast to previous studies, the underlying auditory evoked potential was a single broad positivity, as compared to the more canonical P1–N1–P2 morphology. These differences in morphology are consistent with developmental studies of changes in the auditory evoked potential with age and/or stimulus complexity (Paetau et al., 1995; Ponton et al., 2000; Sharma et al., 1997). Interestingly, one advantage of the broad positivity elicited was that it returned to baseline approximately 250 ms after stimulus presentation. This permitted an assessment of short 200 ms ISIs, which in some previous work has been problematic due to the overlap of preceding evoked responses on the baseline, preventing analysis of short ISIs (e.g. Coch et al., 2005b). Yet these ISIs are within the range implicated in auditory processing deficits in SLI (Tallal and Piercy, 1973a,b) and thus the neural response to such stimuli is of particular interest.
Using this ERP paradigm, children with SLI were compared directly to a control group of typically-developing children matched for age, gender, socio-economic status, and non-verbal IQ. In comparison to typically-developing peers, children with SLI showed atypical auditory recovery cycles, and this difference varied as a function of both probe type (linguistic vs nonlinguistic) and electrode hemisphere (right vs left). Specifically, children with SLI showed a more attenuated response at the shortest 200 ms ISI, but no group differences were observed for either probe type at the longer 500 or 1000 ms ISI. However, the group difference at the 200 ms ISI was specific to the left hemisphere for linguistic probes and the right hemisphere for non-linguistic probes. It is possible that developmental changes in cerebral lateralization account for the hemisphere-specific effects observed. For example, there is evidence that developmental change in refractory cycle effects for non-linguistic stimuli is more prominent over right-hemisphere regions (Rojas et al., 1998). Future studies are needed to examine whether the refractory effects for linguistic stimuli show the opposite pattern, with a longer developmental time course over left-hemisphere regions. If so, this might suggest that individuals with SLI show differences in later-maturing neural processes, consistent with previous reports suggesting that some atypical neural responses observed in SLI reflect immature patterns of activity (e.g., Bishop and McArthur, 2004).
The hemispheric differences observed further underscore the importance of considering the nature of the stimuli when interpreting the distribution of group differences across electrode sites. For example, Neville et al. (1993) reported attenuated auditory recovery cycles over right frontal electrodes sites in older children with SLI (age 8–10 years), but this previous study used only non-linguistic tones presented in silence. The present study is consistent with these earlier findings and further shows that a similar deficit exists for linguistic stimuli, over left hemisphere frontal regions. Further, whereas Neville and colleagues tested ISIs of 200, 1000, and 2000 ms, the present study tested an additional short-duration ISI (i.e., both 200 and 500 ms). However, as no group differences were apparent at either 500 or 1000 ms, this suggests that differences in the recovery cycles of children with SLI are limited to very short ISIs, at least in the age range and stimulus types examined here.
The probe stimuli in the present study were superimposed over two narratives, with children selectively attending to one of the two narratives. This permitted an assessment of selective auditory attention within the same paradigm, and we have previously reported that in this paradigm, the ERP response to probe stimuli is enhanced in the attended versus unattended channel in adults, as well as children as young as three years of age, within 100 ms of processing (Sanders et al., 2006, results collapsed across the three ISIs). Further, in a separate report based on a subset of 24 children from the present study and examining an ROI focused on our previous studies of selective attention, we have documented that children with SLI do not show these same early attentional enhancements (Stevens et al., 2006). In the present study, we used a larger sample of children and asked whether refractory cycle effects (i.e., the ERP amplitude modulations with varying ISI) were influenced by whether a probe occurred in the attended or unattended channel. There was no evidence to support this hypothesis. When entered as a factor, attention condition did not interact either with ISI overall or in higher-order interactions with group and ISI. Thus, it is unlikely that selective attention difficulties can account for the refractory effects observed here. However, future studies can examine refractory periods within an attended as compared to unattended channel (i.e., in which both stimuli occur within the same channel) to further test the effects of selective attention on refractory cycles.
3.2. Implications for the auditory deficit hypothesis of SLI
According to the auditory deficit hypothesis of SLI, subtle auditory processing deficits in children with SLI may render language learning more difficult, particularly for stimuli presented rapidly and/or that requires sensitivity to subtle spectral and temporal differences. However, this causal link remains controversial (e.g., see Leonard, 1998; Rosen, 2003). The present study further clarifies one neural mechanism whereby the coding of speech input may be compromised in SLI. Specifically, at rapid rates of presentation, the auditory system of some individuals with SLI may be more refractory (i.e., populations of neurons may require more time to recover in preparation to respond to new incoming stimuli), leading to a less robust neural representation of the acoustic signal. Given the continuous nature of the speech stream and the subtle acoustic cues differentiating some speech sounds, language development might be particularly vulnerable in the face of such differences in neural refractory cycles.
If the auditory deficit hypothesis can account for SLI, problems with auditory processing should be observed among infants and young children who later manifest SLI, but prior to the emergence of linguistic deficits. While the children in this study were already lingual and experiencing difficulties, the participants were young relative to those in previous investigations (Basu et al., 2010; Neville et al., 1993). The present data suggest that auditory recovery cycle impairments can occur at least coincident with the emergence of language difficulties, in children as young as three years of age. It is interesting to consider these findings in light of prospective longitudinal studies of infants with or without a family history of language impairment (FH+/FH−) that include ERP measures (Benasich et al., 2006; Choudhury and Benasich, 2011). While these earlier studies have not used a refractory cycle paradigm per se, they have demonstrated several differences in the auditory evoked response among FH+ infants to tone pairs when the gap between tones is either 70 or 300 ms, and the ISI between pairs of tones is ~700 ms. Although the pattern of differences varies depending upon the gap length, differences in the FH+ group including delayed latencies, altered laterality, and an absence of some peaks are consistent with abnormalities in processing rapid auditory stimuli. These studies also report that the latency and amplitude of the response to occasional deviant tones that differ in frequency from the first tone in a pair are predictive of later language skills. As children in these studies begin to be classified specifically for the presence or absence of SLI, it will be interesting for re-analyses of the data sets to examine what aspects of the ERP responses in infancy are predictive of this later classification. Given the differences in morphology of the evoked responses observed across groups in some conditions, it will also be interesting to determine whether those morphological differences – which to date have only been described qualitatively at the group level – have predictive value for the outcomes of individual children.
3.3. Limitations and future directions
The present study included a number of story sets and narrators on which probe stimuli were overlaid. While these factors were balanced across groups, rendering group-level comparisons valid, it limited the feasibility of analyses of individual subject differences. It also introduced a source of noise that would not have been present if all participants were tested with probes overlaid on the same narrators/story sets. Future designs can balance these conditions, even within an individual subject, or use a restricted set of narrators (as in our previous study (Stevens et al., 2006)) to permit these comparisons.
Equating the acoustic characteristics of linguistic and nonlinguistic stimuli is a difficult issue, and the specific stimuli used here should be considered when interpreting results. While the linguistic and non-linguistic probes were matched in many respects (e.g., duration, amplitude, and rise time), the amplitude envelop varied across the two probe types, which may have impacted the neural response to the two probe types, including the trend for a larger amplitude response across all participants to the non-linguistic relative to linguistic probes. Future studies could also match the amplitude envelop of linguistic and non-linguistic stimuli by imposing a similar envelop over the non-linguistic stimulus after the scrambling procedure.
Finally, although the present study was focused on refractory effect differences across groups, it was somewhat surprising not to see overall group differences in the effects of attention on neural processing, as reported previously in a subset of the participants (Stevens et al., 2006). Differences across studies might reflect differences in participant characteristics across studies. Indeed, our initial study of selective auditory attention, which included an analysis based on individual subjects, suggested two possible participant characteristics that could influence results. First, the magnitude of the attention effect showed a modest positive correlation with age (r= +.37), though this relationship was observed only in the TD (r=+.48) and not SLI (r=+.20) groups. This suggests that group differences in selective attention may increase over time, as typically-developing children begin to show larger effects of selective attention on neural processing. Indeed, children in the present study were on average about six months younger than those in the 2006 report, which may have biased the study against finding differences in selective auditory attention. Our previous report also suggested that children with both language and reading difficulties were most likely to show reduced effects of selective attention on neural processing. Unfortunately, it was not possible to compare overall reading scores across studies as only 8 participants with SLI had reading test data available (and these were 8 participants also included in the 2006 report). However, if the current sample had a larger representation of “pure SLI” participants (without concomitant reading disorder), group differences in selective attention would have been less apparent. Further studies exploring the nature of individual differences in both selective attention and refractory cycle effects may help shed light on these differences, and the different profiles of vulnerabilities in the heterogeneous population of children with SLI.
It is important to cast the present findings in a framework of multiple risk and protective factors. That is, SLI is clearly a heterogeneous disorder, and other reports support the existence of additional risk factors for SLI, including deficits in selective auditory attention (Stevens et al., 2006), increased effects of backward masking (Wright et al., 1997), and immature auditory evoked responses (Bishop and McArthur, 2004), among others. Indeed, there is some evidence that different children with SLI exhibit different patterns of vulnerabilities (e.g., Neville et al., 1993), and future research can continue to assess the extent to which these various deficits are shared or overlapping.
3.4. Conclusion
In summary, the present study provides neurophysiological evidence for a low-level auditory processing deficit in children with SLI. When auditory stimuli were presented in rapid succession, children with SLI showed an attenuated neural response to those stimuli relative to typically-developing peers. This deficit extended across linguistic and nonlinguistic stimuli, showed no evidence of being influenced by selective attention, and appeared relatively early in development. This suggests that deficits in the neural representation of rapidly presented stimuli may be one risk factor for atypical language development.
4. Experimental procedures
4.1. Participants
The final sample included 48 children (17 male; mean age 5.4 years, SD 1.2, range 3.5–8.8 years). All children satisfied the following inclusion criteria: (1) normal hearing (20 dB at 500, 1000, and 4000 MHz), (2) normal or corrected-to-normal vision, (3) monolingual, native English speaker, (4) absence of ADHD diagnosis, (5) not taking psychoactive medications, (6) no known neurological disorders, and (7) non-verbal IQ above 80 as assessed using the Stanford–Binet 5 non-verbal composite (Roid, 2003). Half of these children were also included in an earlier study examining selective auditory attention alone, collected in the same experimental paradigm (Stevens et al., 2006).2
Twenty-four children who scored at least one standard deviation below normal (i.e., below the 17th percentile) on the CELF receptive language composite (Semel et al., 1995; Wiig et al., 2004) and whose receptive language score was at least two-thirds of a standard deviation (10 standard scores) below their non-verbal IQ were classified as having specific language impairment (SLI). Fifteen of these children also scored below the 17th percentile on the CELF expressive language composite. One additional child with SLI was tested but excluded from analysis due to poor data quality, indicated by abnormal raw electroencephalogram data and lack of any identifiable components in averaged data.
Twenty-four children who scored above the 22nd percentile on the CELF receptive language composite were classified as typically-developing (TD). The TD controls were selected from a larger database of participants included in our ongoing studies of typically-developing children (Sanders et al., 2006; Stevens et al., 2010) in order to match the SLI group in terms of age, gender, handedness, socio-economic status (SES), non-verbal IQ, and experimental conditions. Groups were compared on demographic and behavioral measures using two-tailed independent samples t-tests or, for nominal variables, Fischer’s Exact Tests.
All study procedures were conducted with the approval of the University of Oregon Institutional Review Board. Parents of children signed a consent form to participate. Verbal assent was obtained from the children. Families were paid for their participation.
4.2. Stimuli and procedures
Auditory probe stimuli were 100 ms sounds presented at inter-stimulus intervals (ISIs) of 200, 500, or 1000 ms. Half of the probe stimuli were linguistic (a CV syllable) and half were non-linguistic (a broad spectrum buzz). The linguistic probe was the syllable/ba/, spoken by a female speaker (different from the story narrators, described below), and then digitized and edited to 100 ms in duration. The non-linguistic probe was created by scrambling 4–6 ms segments of the/ba/stimulus. This resulted in a 100 ms broad-spectrum ‘buzz’ that, while sounding non-linguistic, preserved many of the acoustic properties of the linguistic probe. Both probes had a 12 ms rise time, though the non-linguistic probe had a more variable amplitude envelope resulting from the scrambling procedure used during stimulus creation. Linguistic and non-linguistic probes were intermixed within the recording session. We have previously shown that children in the age range studied here show a similar auditory evoked response in this paradigm to both probe types, characterized by a broad positivity from ~100 to 250 ms (Sanders et al., 2006).
The probe stimuli were superimposed on one of two auditory narratives presented simultaneously from separate speakers located 90° to the left and right of the child, who was cued to attend selectively to one of the two narratives. The three ISIs reflected the time between successive probes, regardless of whether they occurred in the attended or unattended narrative. The narratives provided an age-appropriate task for children and permitted an experimental manipulation of selective attention embedded in the same paradigm (see Stevens et al., 2006 for results of a study comparing TD and LI children based on the attention manipulation). The stories differed in location (left/right speaker), narration voice (male/female), and content (different story series). Small images from the attended story were presented on a central monitor. Fig. 4 presents a schematic representation of the experimental paradigm.
Fig. 4.
Schematic representation of the experimental paradigm. Children were instructed to attend to the story presented from either the left or right speaker. ERPs were recorded to linguistic (ba) and non-linguistic (bz) probe stimuli superimposed on both the attended and ignored narrative, with the ISI between probes 200, 500, or 1000 ms.
The narratives used in the present study were drawn from the following children’s book series: Blue Kangaroo (Clark, 1998, 2000, 2002, 2003), Harry the Dog (Zion and Graham, 1956, 1960, 1965, 1976), Henry (Johnson, 2000, 2002, 2003, 2004), Max and Ruby (Wells, 1991, 1997, 2000, 2002), and/or Munsch for Kids (Munsch and Martchenko, 1988, 1992, 2007; Munsch and Suomalainen, 1996). Stories were read by one of six possible narrators (three male, three female). Across participants, all story series and narrator voices occurred in both the attended and unattended position. The additional story sets were recorded to permit longitudinal assessment of participants in our ongoing intervention studies (Neville et al., 2008, under review), with the same probe stimuli overlaid on all stories.
The majority of children (14 in each group) were tested with the Blue Kangaroo and Harry the Dog stories, as described in our previous research (Sanders et al., 2006). Four participants in each group had probes overlaid on the Max & Ruby and Henry stories, read by a different male and female narrator. The final six participants in each group attended once to each of four different narrators/story sets (including the Munsch series and a new male/female narrator), with each narrator/story set also occurring once in the unattended channel. Thus, in different participants the probe stimuli were overlaid on different narrator voice(s) and/or specific stories, depending upon protocol randomization and whether assessed after the addition of the new story sets. While this introduced a source of variability across participants, the two groups (LI and non-LI) were matched with respect to protocol assignment, rendering group-level comparisons unconfounded with the story or narrator on which probes were overlaid. However, as our ongoing analyses suggest the amplitude of ERP responses to probes can vary depending upon the narrator voice on which probes are overlaid, this rendered individual-subject analysis unwise with these data. Thus, the present analysis is focused on group-level comparisons only.
Each participant attended to a total of four 2.5–3.5 minute stories (two from each speaker location), with a total of 804–1004 total probe stimuli played across the session. The two stories were played at 60 dB SPL (A-weighted), and the probe stimuli were played at 70 dB. Across the session, children heard 67–84 probe stimuli in each of the 12 possible conditions: ISI (200/500/1000)×Probe Type (linguistic/non-linguistic)×Attention Condition (attended/unattended). All children had at least 10 clean trials/condition for calculating averages, and most children many more (on average 45 trials/condition), available for analysis.
An adult experimenter sat next to the child at all times to administer instructions and monitor the child’s behavior. To encourage the child to pay attention, following each story the experimenter asked the child three basic two-alternative comprehension questions about the attended story. Children could also answer ‘I don’t know,’ which was counted as an incorrect response. These questions were not designed as a sensitive assay of children’s language abilities (this was the purpose of the standardized tests), but were instead included to reinforce to the child the goal of paying careful attention to a single story. After answering the three questions, the child heard another story. This procedure was repeated four times until the child had listened to four stories (attending twice to the left speaker and twice to the right speaker) and answered 12 comprehension questions.
Behavioral and ERP assessments took place across separate days of testing at the University of Oregon. Behavioral testing took place during one or two sessions and was supervised by a certified speech language pathologist. On a separate day, children visited the electrophysiology lab for ERP testing, described in detail below. All testing sessions were completed within a 30-day time window.
4.3. Electrophysiological recording
Electroencephalogram (EEG) was recorded from 29 tin electrodes mounted in an elastic cap (Electro-Cap International, Easton, OH). Recording sites included: FP1/2, F7/8, FT7/8, F3/4, FC5/6, C3/4, C5/6, T3/4, CT5/6, P3/4, T5/6, TO1/2, O1/2, Fz, Cz, and Pz, see Fig. 5. Additional electrodes were placed at the outer canthi of each eye and beneath the right eye to monitor blinks and eye movement. On-line, electrodes were referenced to the right mastoid, and off-line electrodes were re-referenced to the average of the left and right mastoid. Electrode impedances were below 10 kΩ for eye electrodes, 5 kΩ for scalp electrodes, and 3 kΩ for mastoid electrodes. EEG was amplified 10,000 times using Grass 7P511 amplifiers (bandpass .01 to 100 Hz) and digitized online (250 Hz sampling rate). To reduce electrical noise in the data, a 60 Hz digital filter was applied offline.
Fig. 5.
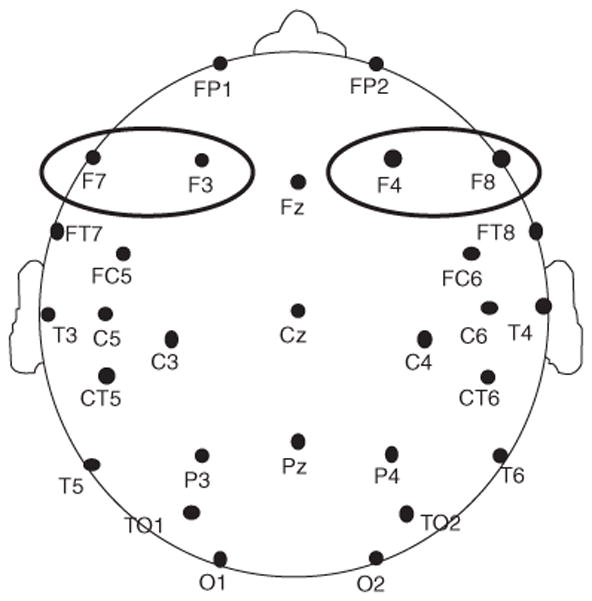
Electrode configuration used in ERP recording. The left and right-hemisphere anterior ROIs are circled: left ROI (F7/F3) and right ROI (F8/F4).
To remove artifacts due to blinks, muscle movement, or eye movement, individual artifact rejection parameters were selected for each participant. Parameters were selected based on inspection of the raw data to identify the smallest change in amplitude observed during a blink (based on shape in electrooculogram [EOG] electrodes and reversal in polarity above and below the eye) or eye movement (based on shape and distribution). Muscle movement was assessed based on channel blocking.
Offline, separate event-related brain potentials (ERPs) were averaged to the probe stimuli at the three ISIs, separately for the two probe types (linguistic/non-linguistic) and attention conditions (attended/unattended channel). ERPs were averaged for each subject at each electrode site over a 500 ms epoch, using the 50 ms pre-stimulus onset as baseline. Mean amplitude was measured from 100 to 200 ms post-stimulus onset at a set of four anterior electrodes (F3/4 and F7/8) in a region-of-interest (ROI) based on previous literature (Neville et al., 1993; Sharma et al., 2007). This region of interest was selected based on previous literature reporting reduced refractory period effects to non-linguistic probe stimuli over right frontal electrodes in older children with SLI at similar ISIs. The 100–200 ms time window was selected based on visual inspection of the individual data. Preliminary examination indicated that the broad positivity elicited in this paradigm did not produce stable latency measures so latency measures were not analyzed.
Mean amplitude within the 100–200 ms window was averaged for the two anterior right-hemisphere electrodes (F8/F4) and left-hemisphere electrodes (F7/3) and submitted to a mixed design ANOVA. The between-subjects factor was group (SLI/TD). Within-subject factors included ISI (200/500/1000), probe type (linguistic/non-linguistic), attention condition (attended/unattended), and hemisphere (right/left). Analyses were restricted to a main effect of ISI or group, as well as interactions including ISI as a factor. The Greenhouse–Geisser correction was applied to all tests involving factors with more than two levels.
Acknowledgments
This research was supported by NIH/NIDCD grant DC00481 to HJN and a Science Collaborative Research Program (SCRP) grant from Willamette University to CS. We are grateful to the members of the child testing team at the Brain Development Laboratory at the University of Oregon involved in collecting the behavioral and ERP data.
Footnotes
Although not reported in the main text, for the interested reader we note that over the frontal ROI there was an overall effect of attention condition (F (1, 46)=12.5, P=.001; Mattended=3.5 μV, Munattended= 2.7 μV), a trend toward a main effect of Probe Type (F (1, 46)=2.85, P<.1; Mlinguistic=2.8 μV, Mnon-linguistic =3.4 μV), and a four-way interaction between Group×Attention×Probe Type×Hemisphere (F (1, 46)= 7.8, P<.01). The pattern of means from the four-way interaction suggested that whereas TD children showed relatively right-lateralized attention effects for non-linguistic stimuli and left-lateralized attention effects for linguistic probes, children with SLI showed the reverse asymmetry for attention effects over this frontal ROI. No other effects in the ANOVA were significant at the P<.1 level.
In the 2006 report, only the main selective auditory attention manipulation was examined given the smaller sample size (data collapsed across the three inter-stimulus intervals). The larger sample size in the present study permitted a focused examination of the separate question of group differences as a function of inter-stimulus interval, as well as whether any recovery cycle differences varied as a function of attention condition or probe type.
References
- Basu M, Krishnan A, Weber-Fox C. Brainstem correlates of temporal auditory processing in children with specific language impairment. Dev Sci. 2010;13:77–91. doi: 10.1111/j.1467-7687.2009.00849.x. [DOI] [PubMed] [Google Scholar]
- Bavin E, Grayden D, Scott K, Stefanakis T. Testing auditory processing skills and their associations with language in 4-to-5-year-olds. Lang Speech. 2010;53:31–47. doi: 10.1177/0023830909349151. [DOI] [PubMed] [Google Scholar]
- Benasich A, Choudhury N, Friedman J, Realpe-Bonilla T, Chojnowska C, Gou Z. The infant as a prelinguistic model for language learning impairments: predicting from event-related potentials to behavior. Neuropsychologia. 2006;44:396–411. doi: 10.1016/j.neuropsychologia.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benasich A, Tallal P. Infant discrimination of rapid auditory cues predicts later language impairment. Behav Brain Res. 2002;136:31–49. doi: 10.1016/s0166-4328(02)00098-0. [DOI] [PubMed] [Google Scholar]
- Benasich A, Thomas J, Choudhury N, Leppánen P. The importance of rapid auditory processing abilities to early language development: evidence from converging methodologies. Dev Psychobiol. 2002;40:278–292. doi: 10.1002/dev.10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D, Hardiman M, Uwer R, Suchodoletz W. Atypical long-latency auditory event-related potentials in a subset of children with specific language impairment. Dev Sci. 2007;10:576–587. doi: 10.1111/j.1467-7687.2007.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D, McArthur G. Immature cortical responses to auditory stimuli in specific language impairment: evidence from ERPs to rapid tone sequences. Dev Sci. 2004;7:F11–F18. doi: 10.1111/j.1467-7687.2004.00356.x. [DOI] [PubMed] [Google Scholar]
- Bishop D, McArthur G. Individual differences in auditory processing in specific language impairment: a follow-up study using event-related potentials and behavioral thresholds. Cortex. 2005;41:327–341. doi: 10.1016/s0010-9452(08)70270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D, Snowling M. Developmental dyslexia and specific language impairment: same or different? Psychol Bull. 2004;130:858–886. doi: 10.1037/0033-2909.130.6.858. [DOI] [PubMed] [Google Scholar]
- Budd T, Barry R, Gordon E, Rennie C, Michie P. Decrement of the N1 auditory event-related potential with stimulus repetition: habituations vs. refractoriness. Int J Psychophysiol. 1998;31:51–68. doi: 10.1016/s0167-8760(98)00040-3. [DOI] [PubMed] [Google Scholar]
- Catts H. The relationship between speech-language impairments and reading disabilities. J Speech Lang Hear Res. 1993;36:948–958. doi: 10.1044/jshr.3605.948. [DOI] [PubMed] [Google Scholar]
- Choudhury N, Benasich A. Maturation of auditory evoked potentials from 6 to 48 months: prediction to 3 and 4 year language and cognitive abilities. Clin Neurophysiol. 2011;122:320–328. doi: 10.1016/j.clinph.2010.05.035. [DOI] [PubMed] [Google Scholar]
- Clark E. I Love You, Blue Kangaroo. Bantom Doubleday Dell Publishing Group, Inc; Italy: 1998. [Google Scholar]
- Clark E. Where Are You, Blue Kangaroo? Random House Children’s Books; Italy: 2000. [Google Scholar]
- Clark E. It Was You, Blue Kangaroo. Random House Children’s Books; Italy: 2002. [Google Scholar]
- Clark E. What Shall We Do, Blue Kangaroo? Random House Children’s Books; Italy: 2003. [Google Scholar]
- Coch D, Sanders L, Neville H. An event-related potential study of selective auditory attention in children and adults. J Cogn Neurosci. 2005a;17:605–622. doi: 10.1162/0898929053467631. [DOI] [PubMed] [Google Scholar]
- Coch D, Skendzel W, Neville H. Auditory and visual refractory period effects in children and adults: an ERP study. Clin Neurophysiol. 2005b;116:2184–2203. doi: 10.1016/j.clinph.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Eisenmajer N, Ross N, Pratt C. Specificity and characteristics of learning disabilities. J Child Psychol Psychiatry. 2005;46:1108–1115. doi: 10.1111/j.1469-7610.2004.00394.x. [DOI] [PubMed] [Google Scholar]
- Gastaut H, Gastaut Y, Roger A, Carriol J, Naquet R. Etude électro-encéphalographique du cycle d’excitabilité cortical. Electroencephalogr Clin Neurophysiol. 1951;3:401–428. doi: 10.1016/0013-4694(51)90029-6. [DOI] [PubMed] [Google Scholar]
- Hansen J, Hillyard S. Selective attention to multidimensional auditory stimuli. J Exp Psychol Hum Percept Perform. 1983;9:1–19. doi: 10.1037//0096-1523.9.1.1. [DOI] [PubMed] [Google Scholar]
- Hari R, Renvall H. Impaired processing of rapid stimulus sequences in dyslexia. Trends Cogn Sci. 2001;5:525–532. doi: 10.1016/s1364-6613(00)01801-5. [DOI] [PubMed] [Google Scholar]
- Hari R, Valta M, Uutela K. Prolonged attentional dwell time in dyslexic adults. Neurosci Lett. 1999;271:202–204. doi: 10.1016/s0304-3940(99)00547-9. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Hink R, Schwent V, Picton T. Electrical signs of selective attention in the human brain. Science. 1973;182:177–180. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. Yale University; New Haven, Connecticut: 1975. Unpublished manuscript. [Google Scholar]
- Johnson DB. Henry Hikes to Fitchburg. Houghton Mifflin Co; New York: 2000. [Google Scholar]
- Johnson DB. Henry Builds a Cabin. Houghton Mifflin Co; New York: 2002. [Google Scholar]
- Johnson DB. Henry Climbs a Mountain. Houghton Mifflin Co; New York: 2003. [Google Scholar]
- Johnson DB. Henry Works. Houghton Mifflin Co; New York: 2004. [Google Scholar]
- Klein R, D’Entremont B. Filtering performance by good and poor readers. In: Everatt J, editor. Attention, Reading, and Dyslexia. Routledge; London: 1999. [Google Scholar]
- Leonard L. Children with Specific Language Impairment. Massachusetts Institute of Technology; Cambridge, MA: 1998. [Google Scholar]
- Lovegrove W, Bowling A, Badcock D, Blackwood M. Specific reading disability: differences in contrast sensitivity as a function of spatial frequency. Science. 1980;210:439–440. doi: 10.1126/science.7433985. [DOI] [PubMed] [Google Scholar]
- Lowe A, Campbell R. Temporal discrimination in aphasoid and normal children. J Speech Hear Res. 1965;8:313–314. doi: 10.1044/jshr.0803.313. [DOI] [PubMed] [Google Scholar]
- McArthur G, Bishop D. Which people with specific language impairment have auditory processing deficits? Cogn Neuropsychol. 2004;21:79–94. doi: 10.1080/02643290342000087. [DOI] [PubMed] [Google Scholar]
- McArthur G, Bishop D. Speech and non-speech processing in people with specific language impairment: a behavioral and electrophysiological study. Brain Lang. 2005;94:260–273. doi: 10.1016/j.bandl.2005.01.002. [DOI] [PubMed] [Google Scholar]
- McArthur G, Hogben J. Auditory backward recognition masking in children with a specific language impairment and children with a specific reading disability. J Acoust Soc Am. 2001;109:1092–1100. doi: 10.1121/1.1338559. [DOI] [PubMed] [Google Scholar]
- Munsch R, Martchenko M. Angela’s Airplane. Turtleback; Eastsound, WA: 1988. [Google Scholar]
- Munsch R, Martchenko M. 50 Below Zero. Turtleback; Eastsound, WA: 1992. [Google Scholar]
- Munsch R, Martchenko M. Thomas’ Snowsuit. Annick Press; Toronto, ON: 2007. [Google Scholar]
- Munsch R, Suomalainen S. Mud Puddle. Perfection Learning; Logan, IA: 1996. [Google Scholar]
- Neville H, Andersson A, Bagdade O, Bell T, Currin J, Fanning J, et al. Effects of music training on brain and cognitive development in under-privileged 3- to 5-year old children: preliminary results. In: Ashbury C, Rich E, editors. Learning, Arts, and the Brain. Dana Press; New York: 2008. pp. 105–116. [Google Scholar]
- Neville H, Coffey S, Holcomb P, Tallal P. The neurobiology of sensory and language processing in language-impaired children. J Cogn Neurosci. 1993;5:235–253. doi: 10.1162/jocn.1993.5.2.235. [DOI] [PubMed] [Google Scholar]
- Neville H, Stevens C, Fanning J, Klein S, Bell T, Pakulak E. Training improves behavior, cognition, and brain functions supporting attention in lower SES children under review. [Google Scholar]
- Noterdaeme M, Amorosa H, Mildenberger K, Sitter S, Minow F. Evaluation of attention problems in children with autism and children with specific language disorder. Eur Child Adolesc Psychol. 2000;10:58–66. doi: 10.1007/s007870170048. [DOI] [PubMed] [Google Scholar]
- Oram Cardy J, Flagg E, Roberts W, Brian J, Roberts T. Magnetoencephalography identifies rapid temporal processing deficit in autism and language impairment. Neuroreport. 2005;16:329–332. doi: 10.1097/00001756-200503150-00005. [DOI] [PubMed] [Google Scholar]
- Oram Cardy J, Flagg E, Roberts W, Roberts T. Auditory evoked fields predict language ability and impairment in children. Int J Psychophysiol. 2008;68:170–175. doi: 10.1016/j.ijpsycho.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Paetau R, Ahonen A, Salonen O, Sams M. Auditory evoked magnetic fields to tones and pseudowords in healthy children and adults. J Clin Neurophysiol. 1995;17:177–185. doi: 10.1097/00004691-199503000-00008. [DOI] [PubMed] [Google Scholar]
- Pihko E, Kujala T, Mickos A, Alku P, Byring R, Korkman M. Language impairment is reflected in auditory evoked fields. Int J Psychophysiol. 2008;68:161–169. doi: 10.1016/j.ijpsycho.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Eggermont JJ, Kwong B, Don M. Maturation of human central auditory system activity: evidence from multi-channel evoked potentials. Clin Neurophysiol. 2000;111:220–236. doi: 10.1016/s1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- Roid GH. Stanford–Binet Intelligence Scales (SB-5) 5. Riverside Publishing; Itasca, IL: 2003. [Google Scholar]
- Rojas D, Walker J, Sheeder J, Teale P, Reite M. Developmental changes in refractoriness of the neuromagnetic M100 in children. Neuroreport. 1998;9:1543–1547. doi: 10.1097/00001756-199805110-00055. [DOI] [PubMed] [Google Scholar]
- Rosen S. Auditory processing in dyslexia and specific language impairment: is there a deficit? What its nature? Does it explain anything? J Phon. 2003;31:509–527. [Google Scholar]
- Sanders L, Stevens C, Coch D, Neville H. Selective auditory attention in 3- to 5-year-old children: an event-related potential study. Neuropsychologia. 2006;44:2126–2138. doi: 10.1016/j.neuropsychologia.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical Evaluation of Language Fundamentals (CELF-3) 3. The Psychological Corporation: Harcourt Brace & Co; San Antonio: 1995. [Google Scholar]
- Sharma AS, Kraus N, McGee T, Nicol T. Developmental changes in P1 and N1 central auditory responses elicited by consonant–vowel syllables. Electroencephalogr Clin Neurophysiol. 1997;104:540–545. doi: 10.1016/s0168-5597(97)00050-6. [DOI] [PubMed] [Google Scholar]
- Sharma M, Purdy S, Newall P, Wheldall K, Beaman R. Refractory effects on auditory-evoked responses in children with reading disorders. Neuroreport. 2007;18:133–136. doi: 10.1097/WNR.0b013e32800fef71. [DOI] [PubMed] [Google Scholar]
- Snowling M. From language to reading and dyslexia. Dyslexia. 2001;7:37–46. doi: 10.1002/dys.185. [DOI] [PubMed] [Google Scholar]
- Spaulding T, Plante E, Vance R. Sustained selective attention skills of preschool children with specific language impairment: evidence for separate attentional capacities. J Speech Lang Hear Res. 2008;51:16–34. doi: 10.1044/1092-4388(2008/002). [DOI] [PubMed] [Google Scholar]
- Sperling AJ, Lu Z-l, Manis F, Seidenberg M. Motion-perception deficits and reading impairment: it’s the noise, not the motion. Psychol Sci. 2006;17:1047–1053. doi: 10.1111/j.1467-9280.2006.01825.x. [DOI] [PubMed] [Google Scholar]
- Stanovich K. Explaining the differences between the dyslexic and the garden-variety poor reader: the phonological-core variable-difference model. J Learn Disabil. 1988;21:590–604. doi: 10.1177/002221948802101003. [DOI] [PubMed] [Google Scholar]
- Steinschneider M, Fishman Y. Enhanced physiologic discriminability of stop consonants with prolonged formant transitions in awake monkeys based on the tonotopic organization of primary auditory cortex. Hear Res. 2011;271:103–114. doi: 10.1016/j.heares.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C, Fanning J, Klein S, Neville H. Development and comparison of two models of attention training. Poster Presented at the Annual Meeting of the Institute of Education Sciences; National Harbor, MD. 2010. [Google Scholar]
- Stevens C, Sanders L, Neville H. Neurophysiological evidence for selective auditory attention deficits in children with specific language impairment. Brain Res. 2006;1111:143–152. doi: 10.1016/j.brainres.2006.06.114. [DOI] [PubMed] [Google Scholar]
- Talcott JB, Hansen MC, Willis-Owen C, McKinnell IW, Richardson AJ, Stein JF. Visual magnocellular impairment in adult developmental dyslexics. Neuro-Ophthamology. 1998;20:187–201. [Google Scholar]
- Tallal P. Auditory temporal perception, phonics, and reading disabilities in children. Brain Lang. 1980;9:182–198. doi: 10.1016/0093-934x(80)90139-x. [DOI] [PubMed] [Google Scholar]
- Tallal P. Improving language and literacy is a matter of time. Nat Rev Neurosci. 2004;5:721–728. doi: 10.1038/nrn1499. [DOI] [PubMed] [Google Scholar]
- Tallal P, Piercy M. Defects of nonverbal auditory perception in children with developmental aphasia. Nature. 1973a;241:468–469. doi: 10.1038/241468a0. [DOI] [PubMed] [Google Scholar]
- Tallal P, Piercy M. Development aphasia: impaired rate of non-verbal processing as a function of sensory modality. Neuropsychologia. 1973b;11:389–398. doi: 10.1016/0028-3932(73)90025-0. [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Records N, Buckwawlter P, Zhang X, Smith E, O’Brien M. Prevalence of specific language impairment in kindergarten children. J Speech Lang Hear Res. 1997;35:832–843. doi: 10.1044/jslhr.4006.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trehub S, Henderson J. Temporal resolution in infancy and subsequent language development. J Speech Hear Res. 1996;39:1315–1320. doi: 10.1044/jshr.3906.1315. [DOI] [PubMed] [Google Scholar]
- Wang M, Liu Z, Zhu Q, Liu J, Wang L, Liu H. Influence of a preceding auditory stimulus on evoked potential of the succeeding stimulus. Chin Sci Bull. 2004;49:1032–1037. [Google Scholar]
- Wells R. Max’s Dragon Shirt. Penguin Group; New York: 1991. [Google Scholar]
- Wells R. Bunny Money. Penguin Group; New York: 1997. [Google Scholar]
- Wells R. Max Cleans Up. Puffin Books; New York: 2000. [Google Scholar]
- Wells R. Ruby’s Beauty Shop. Penguin Group; New York: 2002. [Google Scholar]
- Wiig EH, Secord WA, Semel E. Clinical Evaluation of Language Fundamentals — Preschool (CELF-P:2) 2. The Psychological Corporation: Harcourt Assessment, Inc; 2004. [Google Scholar]
- Wright B, Lombardino L, King W, Puranik C, Leonard C, Merzenich M. Deficits in auditory temporal and spectral resolution in language-impaired children. Nature. 1997;387:176–178. doi: 10.1038/387176a0. [DOI] [PubMed] [Google Scholar]
- Ziegler J, Pech-Georgel C, George F, Alario F, Lorenzi C. Deficits in speech perception predict language learning impairment. Proc Natl Acad Sci. 2005;102:14110–14115. doi: 10.1073/pnas.0504446102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zion E, Graham M. Harry the Dirty Dog. HarperCollins Children’s Books; United States: 1956. [Google Scholar]
- Zion E, Graham M. Harry and the Lady Next Door. HarperCollins Children’s Books; United States: 1960. [Google Scholar]
- Zion E, Graham M. Harry by the Sea. HarperCollins Children’s Books; United States: 1965. [Google Scholar]
- Zion E, Graham M. No Roses for Harry. HarperCollins Children’s Books; United States: 1976. [Google Scholar]