Abstract
Targeted Reinnervation is a surgical technique developed to increase the number of myoelectric input sites available to control an upper-limb prosthesis. Because signals from the nerves related to specific movements are used to control those missing degrees-of-freedom, the control of a prosthesis using this procedure is more physiologically appropriate compared to conventional control. This procedure has successfully been performed on three people with a shoulder disarticulation level amputation and three people with a transhumeral level amputation. Performance on timed tests, including the box-and-blocks test and clothespin test, has increased two to six times. Options for new control strategies are discussed.
Index Terms: Arm Prosthesis, Artificial Limbs, Bionic, Electromyography
I. Introduction
For high-level upper-limb amputees, there are traditionally very few sources of control inputs for externally powered prostheses and many degrees-of-freedom of the arm are lost. Only three powered joints are currently available as a replacement: elbow, wrist rotation and hand open/close. For control of these motors, options for the shoulder disarticulation level include switches actuated with residual shoulder movement or myoelectric control using muscles of the chest and back. Although the transhumeral amputee also has residual biceps and triceps, there are still many more degrees-of-freedom lost than there are available control inputs. With current devices, if myoelectric control is used as an input, typically an agonist-antagonist pair is used for control of the two directions of movement of a motor. Often co-contraction or switches will be implemented to alternate the use of these two myoelectric signals to control multiple motors. Body-powered systems use a cable controlled by a strap across the shoulder blades to effect movement. Body-power and myoelectric control can be combined to increase the independent number of degrees-of-freedom. Since there are few muscle pairs available for higher level amputees, usually only one, or at most two, degrees-of-freedom can be controlled at a time, which is slow and cumbersome [1],[2]. Furthermore, the standard inputs used are not physiologically related to the degree-of-freedom being actuated (i.e. contraction of back muscles used to control of hand open), thus the control is not intuitive and operating these devices can be challenging. This limitation in control inputs also restricts the development of more advanced prosthetic devices. Since it is so difficult to control two or three degrees-of-freedom, there is little motivation to develop more. Improvements in the quantity and quality of control inputs will enhance the usability of both commercially available systems and more complex systems with an increased number of powered degrees-of-freedom.
Targeted muscle reinnervation (TMR) has been developed to increase the number of physiologically appropriate control inputs available for use with a prosthetic device. With TMR residual motor nerves are transferred to remaining muscles. The target muscles are denervated in the surgery so that the transferred nerves can then reinnervate these muscles, which serve as biological amplifiers of the residual nerve signals [3]. Electromyographic signals (EMG) can then be recorded from these reinnervated muscles and used to control the prosthesis. For example, a muscle signal using the median nerve transfer site is used to control closing of the prosthetic hand. Since the median nerve previously innervated the muscles associated with closing the hand, this is a physiologically appropriate control.
Prior to performing targeted muscle reinnervation on human subjects, it was necessary to show that reinnervation was possible and that independent signals could be recorded from the target muscles. Animal experimentation showed that muscle recovery improved when muscle was hyper-reinnervated with a large number of motor neurons. Thus, good recovery could be expected when transferring large residual arm nerves to relatively small target muscles [4]. In order to determine if independent surface electromyographic signals (EMG) could be recorded from discrete target muscles, finite element models of EMG propagation through the muscle and soft tissue were constructed [5]–[7]. In order to have myoelectric signals of adequate size, it was found that target muscles must be a minimum of 3 to 5cm across, 1cm thick, and as much soft tissue and fat as possible be removed between the muscle and skin.
Targeted reinnervation surgery has been performed on people with shoulder disarticulation amputations and transhumeral amputations. Early results have been reported as case studies and demonstrated encouraging improvements. In this paper we present the functional results of TMR for six subjects who have been using their new prostheses for up to five years. These results are based on standard myoelectric signal processing (velocity control proportional to amplitude of the EMG signal). The potential for using advanced signal processing methods is also discussed.
II. Methods
Six subjects have undergone targeted reinnervation surgery with internal review board approval and informed consent. During surgical planning and implementation, it is necessary to have independent muscle segments with their own vascular supply and innervation to be target transfer sites. At the transhumeral level, the goal has been to transfer the median and distal radial nerves to target muscle segments creating hand opening and hand closing signals. Target muscles have included the medial biceps for the median nerve and the lateral triceps or brachialis muscles for the distal radial nerve. The procedure has been successfully performed on three people with transhumeral amputations (see [8] for surgical detail). At the shoulder disarticulation level, all four brachial plexus nerves that descend to the missing arm can be transferred to target muscle segments. TMR has been successful on two shoulder disarticulation level amputees and a person with a humeral neck transhumeral amputation who is functionally treated as a shoulder disarticulation amputee. In these patients, a combination of segmented pectoralis major, pectoralis minor, serratus anterior and latissimus dorsi muscles were used (see [9],[10] for surgical detail).
Following the nerve transfer surgery, the subjects have typically returned to wearing their original prosthesis, with slight modifications sometimes necessary to fit and control, while the nerves reinnervate the target muscles. This reinnervation has typically taken four to six months before the subjects can be fit with a prosthesis that utilizes the TMR sites. For people with left shoulder disarticulation amputations, the amplitude of the electrocardiogram (ECG) has significantly overwhelmed some of the new TMR myoelectric signals. It has been possible to eliminate approximately 80% of the ECG with only a 20% reduction of the EMG using second order high pass filters with a cut-off at 60 Hz built into the surface electrodes [11]. This adequately reduces ECG interference although a greater improvement in the signal-to-noise ratio is desired.
All six TMR subjects were fit with a Boston Digital arm, Otto Bock electric wrist rotator and an electric terminal device (hook or hand, depending on subject preference). In all but one case, the subject used the same components pre- and post-surgically. Some users did not use an electric wrist rotator pre-surgery, but all subjects used one post-surgery. Table 1 shows a complete list of inputs used, by individuals who have had TMR, to control their externally powered prostheses. Prior to surgery, all subjects were fit with externally powered device in a clinical prosthetics department. Occupational therapy was performed by the research group to ensure that the device functioned and the subjects were able to operate the devices. Using this conventionally controlled prosthesis baseline functional testing was performed including a modified Block and Boxes test [12], a clothes pin relocation test [13], and the AMPS (Assessment of Motor and Process Skills) testing [14]–[16]. Approximately three to six months after the TMR surgery prosthetic fitting was begun. The prosthetist optimized electrode location, contact, gain and thresholds through clinical experimentation—similar to conventional myoelectric control fitting. Occupational therapy was initiated to train the subjects in the use of the new control system. Therapy first focused on control each joint individually then in combination. Since the targeted reinnervation control was more intuitive to the subjects, less training was needed with the new control compared to the pre-TMR conventional myoelectric control. For some out of town subjects, therapy was extended to the local community. After concluding that the control was optimized and the subjects could use the device effectively at home, functional testing was repeated at one-, three- and six-month intervals.
Table 1.
Summary of successfully TMR subjects, including level of amputation, surgical sites used as inputs and any other prosthetic controls implemented in the post-TMR prosthetic fitting.
| Subject Identifier | Level of Amputation | Elbow Flexion | Elbow Extension | Hand Close | Hand Open | Wrist Rotation |
|---|---|---|---|---|---|---|
| SD-A | Shoulder Disartic. | Musculocutaneous N. to P. Major, Clav. Head | Radial N. to P. Major, inf. Sternal head | Median N. to upper sternal head | Median N. to upper sternal head* | Toggle between Hand Open/Close control using socket-mounted FSR |
| SD-B | Shoulder Disartic.** | Musculocutaneous N. to P. Major, Clav. Head | Native remnant triceps | Median N. to P. Major, sternal head and P. minor | Distal Radial N. to Thoracodorsal N (Serratus Anterior) | Two-site control with two socket-mounted FSRs |
| SD-C | Shoulder Disartic. | Musculocutaneous N. to P. Major, Clav. Head | Native remnant triceps/post. Deltoid | Ulnar N. to remnant P. Major sternal head segment | Radial N to P. Minor | Single-site control with one socket-mounted FSR |
| TH-A | Trans-humeral | Native Biceps, long head | Native Triceps | Median N. to Biceps, short head | Distal Radial N. to Brachialis | Single-site control with harness linear potentiometer |
| TH-B | Trans-humeral | Native Biceps, long head | Native Triceps | Median N. to Biceps, short head | Distal Radial N. to Brachialis | Single-site control with harness linear potentiometer |
| TH-C | Trans-humeral | Native Biceps, long head | Native Triceps, long and medial head | Median N. to Biceps, short head | Distal Radial N. to Triceps, lateral head | Single-site control with harness linear potentiometer |
Unexpected result: subject indicates opening hand with a movement of thumb abduction, a median nerve function
Humeral neck level amputation clinically fit as a shoulder disarticulation level amputee
III. Results
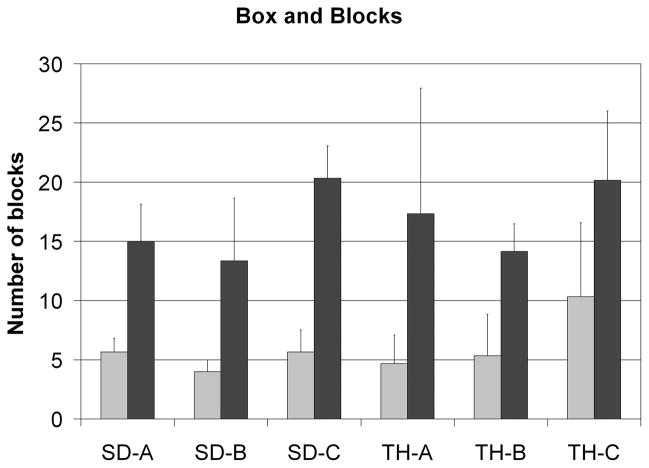
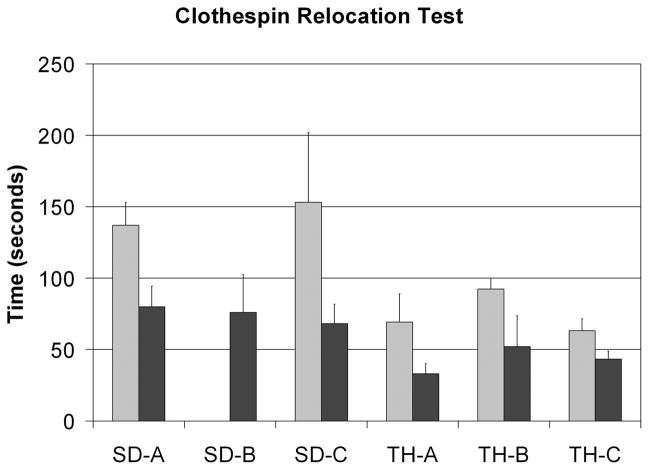
Multiple tasks were executed to judge performance of the pre-surgical prosthesis compared to the control system used after TMR [8]–[10],[13],[17]. The Box and Blocks test of gross manual dexterity required the subject to move one-inch blocks from one compartment to another, modified to allow 120s instead of 60s, and performed standing instead of sitting (Fig. 1a) [12]. All subjects demonstrated marked improvement; the increased ranged from 95% to 271% with an average increase of 198% (Fig. 2). For the Clothespin Relocation Task, adapted from the Roylan Graded Pinch Exerciser [13], the time was recorded as the patient moved three clothespins from a horizontal to a vertical bar (Fig. 1b). Again, all subjects demonstrated improvement ranging from 31% to 55% with an average difference of 45% reduction in time with the TMR controlled device compared to the pre-surgical control (Fig. 3). These tasks were chosen, in part, because speed of performance could be improved if elbow and hand could be actuated in an easier and simultaneous way. Also, the clothespin test requires the addition of the wrist rotation and thus all three degrees-of-freedom in the prosthesis.
Fig. 1.
Timed tasks used to evaluate function pre-surgery and post-TMR: a) box and blocks, b) clothespin relocation task
Fig. 2.
Results for all subjects for the Box and Blocks test. The left bar is the pre-surgical fitting and the right bar is the post-TMR fitting. All subjects had an increase in the number of blocks moved in 120s.
Fig. 3.
Results for all subjects for the Clothespin relocation test. The left bar is the pre-surgical fitting and the right bar is the post-TMR fitting. All subjects reduced the amount of time required to move the three clothespins.
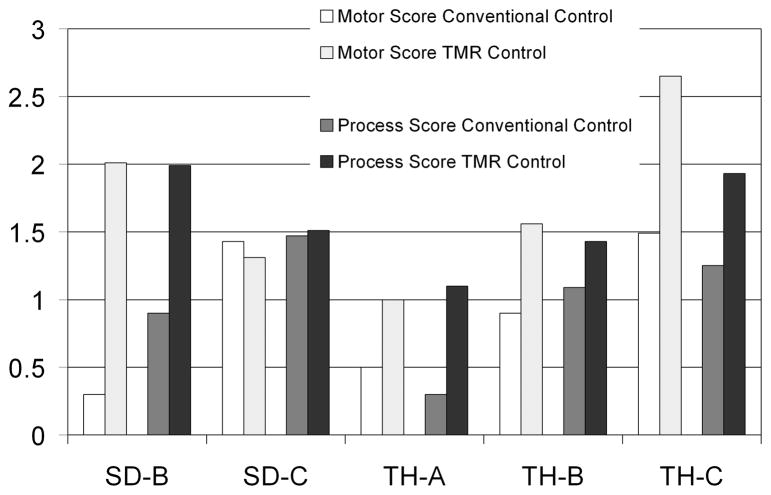
All subjects except for SD-A, a bilateral shoulder disarticulation amputee, have also performed AMPS (Assessment of Motor and Process Skills) testing [14]–[16], a scored evaluation of activities of daily living chosen from a list by the patient. For those that have completed the AMPS testing, results (pre-surgical and 3 months after fitting with TMR-controlled device, except 1 month post-surgical for TH-C) are presented in Fig. 4.
Fig. 4.
Results for four subjects for the AMPS (Assessment of Motor and Process Skills) testing. Both motor and process scores are presented. SD-A did not perform the AMPS testing since these tasks were not possible with bilateral shoulder disarticulation prostheses. A change of .5 logits is considered to be significant. The cutoff for Motor skills is 2 and for Process skills is 1. Measures below the cutoffs indicate that there were problems that affected the quality or effectiveness of task performance.
All subjects reported that the prosthesis was easier to operate using the TMR control compared to conventional control. All subject appreciated the fact that they were able to simultaneously operate the hand and elbow without having to switch control between these two functions. This allowed faster and more seamless positioning and operation of the hand/hook for various functions. They reported many tasks were easier to perform with the new control mechanism. These tasks included cooking, cleaning, housework, yard work, and home maintenance.
IV. DISCUSSION AND FUTURE WORK
Targeted muscle reinnervation has demonstrated both improvements in function both objectively and subjectively. The objective testing shows marked improvement in the speed and quality of movement in each subject. Subjectively, the subjects report their prostheses are much easier to use, they can perform more tasks and they wore their devices more often with TMR control than they did with conventional control.
Current tests may not adequately measure improvement in control or prosthetic design due to ceiling or floor effects. For example, in the AMPS testing, most users are scored above the cutoff indicating impairment. Generally they quickly ceiling out in the process scores, while motor scores vary but consistently improve, although the significance of improvement (.5 logits) is not consistent. Also, the AMPS test is not diagnosis specific (not designed for amputees but for general arm impairment). In order to test improvement with new controls and components as they become available, we have begun to explore additional and better functional evaluation tools. In addition to AMPS, Clothespins and Box and Blocks, we have also begun performing the UNB test of prosthetic function [18], miscellaneous bimanual activities of daily living (timed) which include more complex movements, and tasks that might generally be performed using two hands in an attempt to measure the degree of successful integration of the prosthesis into a functional activity.
Another promising technology that could benefit TMR is implantable myoelectric electrode systems (IMES) [19],[20]. These intramuscular EMG systems that telemeter signals to the prosthesis could alleviate the problems of surface EMG electrodes, stabilize the EMG control information making operation more robust and allow easier donning of advanced prosthetic arm systems.
During the surgery, the nerves that controlled the majority of arm movements (distal to amputation site) were transferred to target muscle segments. However, only four signals are currently being used for the conventional fitting of a TMR-controlled prosthesis. The goal in the future is that more of the information contained in the transferred nerves be used to control additional degrees-of-freedom in a physiologically based manner. For example, the median and distal radial nerves have only been used to operate terminal device opening and closing. Yet these nerves control many complex functions in the human arm including all wrist movement and individual digit movement. Thus the myoelectric signals from the muscle segments reinnervated by these nerves should contain control data for multiple degree of freedom wrists and multi-function hands with independently moving fingers and thumbs. It is possible, with more advanced control algorithms such as pattern recognition, to utilize more information from the targeted reinnervation muscle [21]. These algorithms are the basis of systems that can be trained to recognize and classify repeatable patterns of EMG signals, each corresponding to a specific arm or hand motion. Early work has tested systems of up to 16 classes (movements) using over 100 monopolar EMG inputs. Data trials were collected and used to train a classifier and independent data trials were used to test the classifier. The bilateral shoulder disarticulation level subject achieved higher than 90% accuracies in pattern recognition testing [22]. The goal is to reduce this number of inputs to a more reasonable number (8–16 electrode pairs), while still maintaining similar accuracies. A further discussion of this topic can be found in the companion paper in this issue [23].
An important limitation of current myoelectric prostheses is that they provide very little sensory feedback to close the control loop in operation of an artificial limb. Operation relies primarily on visual feedback, carefully watching the terminal device as it grasps objects rather than any specific knowledge of the grip force. Targeted sensory reinnervation (TSR) [9],[10] has shown potential to provide this important sensory feedback for amputees. The goal of targeted sensory reinnervation (TSR), done at the same time as TMR, is to denervate skin in an area over the nerve transfers so that the hand afferents will reinnervate this skin. When this reinnervated skin is stimulated, the subject perceives it as a sensation in the missing limb. Unlike phantom limb sensation, which is a cortical phenomenon, this sensation is occurring through normal sensory pathways and has been termed ‘transfer sensation.’ TSR provides a potential portal to the sensory system of the amputee and has the potential to greatly change myoelectric prostheses. A key element is that perception of touch on the palmar side of the hand would correlate to function of the prosthetic hand grasping an object. Another important aspect is that the modality is appropriate—the subject feels touch, pressure, heat or cold.. Much research needs to be done before TSR could possibly provide clinically useful feedback to an amputee. Transfer sensation must be characterized, prosthetic terminal devices need to be developed that sense interaction forces with objects and pragmatic devices must be made that can deliver appropriate stimulation to the reinnervated skin. Touch, vibration and thermal information could be relayed from parts of the prosthetic hand to the corresponding part of the transfer sensation [24]. This would allow the amputee to feel tactile input in a physiologically and anatomically appropriate way.
V. CONCLUSIONS
The targeted reinnervation technique makes possible the creation of new EMG control signals for the operation of complex prosthetic systems. With relatively little training, TMR patients showed an ability to control a prosthesis using the additional control signals added through the nerve transfers. These advancements have increased the incentive to develop more advanced artificial arms that will allow people with high level amputations, especially bilateral amputees, to improve their functional abilities and independence.
Acknowledgments
This work was supported by the NIH National Institute of Child and Human Development (Grant numbers R01 HD044798, R01 HD043137-01, R01 HD044798 and NO1-HD-5-3402), the Defense Advanced Research Projects Agency and the Searle Family Foundation.
Biographies

Laura A. Miller (M’94–SM’07) received her BS in BME from Tulane University, New Orleans, LA, in 1992, a MS (1994) and PhD (2003) in BME and a Prosthetics Certificate (1999) from Northwestern University, IL.
She works as a Research Prosthetist for the Neural Engineering Center for Artificial Limbs at the Rehabilitation Institute of Chicago, Chicago, IL and is an Assistant Professor in PM&R at Northwestern University. Her research interests include fitting and evaluation of new advanced prosthetic devices.
Dr. Miller is a member of the International Society of Prosthetics and Orthotics and the American Academy of Orthotists and Prosthetists.

Kathy A. Stubblefield received a BS degree in Occupational Therapy in 1977.
She works as a Research Therapist for the Neural Engineering Center for Artificial Limbs at the Rehabilitation Institute of Chicago, Chicago, IL. Her experience and expertise is in the treatment of patients with stroke, spinal cord injury and amputations. She has participated in research, training and testing of targeted muscle reinnervation patients using conventional and experimental prosthetic components and control schemes.
Ms. Stubblefield is a member of the American Occupational Therapy Association.

Robert D. Lipschutz received his Bachelor of Science Degree in Mechanical Engineering from Drexel University in 1988 and received his certificate in prosthetics and orthotics from New York University’s Post Graduate Medical School in 1990. He then completed his prosthetics training at the Shriner’s Hospital for Crippled Children in Springfield, MA and continued his clinical endeavors at Newington Children’s Hospital in Newington, CT. He currently works as a Research Prosthetist for the Neural Engineering Center for Artificial Limbs at the Rehabilitation Institute of Chicago in Chicago, IL. His current research interests include fitting and evaluation of new advanced prosthetic devices.

Blair A. Lock received a BS in 2003 and MS in 2005 both in EE from the University of New Brunswick, Fredericton, Canada. He received a diploma in Technology Management and Entrepreneurship in 2003 from UNB.
He works as a Research Engineer for the Neural Engineering Center for Artificial Limbs at the Rehabilitation Institute of Chicago, Chicago, IL. His research interests include pattern recognition for improved control of powered prostheses and virtual reality in rehabilitation.
Mr. Lock is a registered Member in Training with the Association of Professional Engineers and Geoscientists of New Brunswick (APEGNB), in Canada.

Todd A. Kuiken, (M’99-SM’ 07) completed his BS in BME at Duke University, Durham, North Carolina in 1983 and PhD in BME (1989) and MD (1990) from Northwestern University, Chicago, IL. He completed his residency in PM&R at the Rehabilitation Institute of Chicago and Northwestern University Medical School, Chicago, Illinois in 1995.
He is the Director of the Neural Engineering Center for Artificial Limbs and Director of Amputee Services at the RIC. He is an Associate Professor in the Depts. of PM&R and BME of Northwestern University. He is also the Associate Dean for Academic Affairs, Feinberg School of Medicine.
Contributor Information
Laura A. Miller, Email: lamiller@northwestern.edu, Neural Engineering Center for Artificial Limbs, Rehabilitation Institute of Chicago, Chicago, Illinois and the Dept. of PM&R at Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA phone: 312-238-2080; fax 312-238-2081.
Kathy A. Stubblefield, Email: k-stubblefield@northwestern.edu, Neural Engineering Center for Artificial Limbs, Rehabilitation Institute of Chicago, Chicago, Illinois, USA
Robert D. Lipschutz, Email: rlipschutz@ric.org, Neural Engineering Center for Artificial Limbs, Rehabilitation Institute of Chicago, Chicago, Illinois, USA
Blair A. Lock, Email: b-lock@northwestern.edu, Neural Engineering Center for Artificial Limbs, Rehabilitation Institute of Chicago, Chicago, Illinois, USA
Todd A. Kuiken, Neural Engineering Center for Artificial Limbs, Rehabilitation Institute of Chicago, Chicago, Illinois and the Dept. of PM&R at Feinberg School of Medicine, Northwestern University, Chicago, Illinois and the Biomedical Engineering Dept. of Northwestern University, Evanston, Illinois, USA.
References
- 1.Williams TW. Control of powered upper extremity prostheses. In: Meier RH, Atkins DJ, editors. Functional Restoration of Adults and Children with Upper Extremity Amputation. New York, NY: Demos Medical Publishing; 2004. pp. 207–224. [Google Scholar]
- 2.Childress DS, Weir RF. Control of limb prostheses. In: Smith DG, Michael JW, Bowker JH, editors. Atlas of Amputations and Limb Deficiencies. 3. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2004. pp. 173–196. [Google Scholar]
- 3.Hoffer JA, Loeb GE. Implantable electrical and mechanical interfaces with nerve and muscle. Ann Biomed Eng. 1980;8:351–360. doi: 10.1007/BF02363438. [DOI] [PubMed] [Google Scholar]
- 4.Kuiken TA, Childress DS, Rymer WZ. The hyper-reinnervation of rat skeletal-muscle. Brain Res. 1995;676:113–123. doi: 10.1016/0006-8993(95)00102-v. [DOI] [PubMed] [Google Scholar]
- 5.Lowery MM, Stoykov NS, Kuiken TA. Independence of myoelectric control signals examined using a surface EMG model. IEEE Trans Biomed Eng. 2003;50:789–93. doi: 10.1109/TBME.2003.812152. [DOI] [PubMed] [Google Scholar]
- 6.Kuiken TA, Lowery MM, Stoykov NS. The effect of subcutaneous fat on myoelectric signal amplitude and cross-talk. Prosthet Orthot Int. 2003;27:48–54. doi: 10.3109/03093640309167976. [DOI] [PubMed] [Google Scholar]
- 7.Kuiken TA, Stoykov NS, Popovic M, Lowery M, Taflove A. Finite element modeling of electromagnetic signal propagation in a phantom arm. IEEE Trans Neural Syst Rehabil Eng. 2001;9:346–354. doi: 10.1109/7333.1000114. [DOI] [PubMed] [Google Scholar]
- 8.O’Shaughnessy KD, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield KA, Kuiken TA. Targeted reinnervation to improve prosthesis control in transhumeral amputees: a report of three cases. J Bone Joint Surg Am. doi: 10.2106/JBJS.G.00268. to be published. [DOI] [PubMed] [Google Scholar]
- 9.Kuiken TA, Miller LA, Lipschutz RD, Lock BA, Stubblefield K, Marasco PD, Zhou P, Dumanian GA. Targeted reinnervation for enhanced prosthetic arm function in a woman with a proximal amputation: a case study. Lancet. 2007;369:371–80. doi: 10.1016/S0140-6736(07)60193-7. [DOI] [PubMed] [Google Scholar]
- 10.Kuiken TA, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield KA. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet Orthot Int. 2004;28:245–253. doi: 10.3109/03093640409167756. [DOI] [PubMed] [Google Scholar]
- 11.Zhou P, Lock BA, Kuiken TA. Real time ECG artifact removal for myoelectric prosthesis control. Physiol Meas. 2007;28:397–413. doi: 10.1088/0967-3334/28/4/006. [DOI] [PubMed] [Google Scholar]
- 12.Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther. 1985;39:386–91. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- 13.Lipschutz RD, Kuiken TA, Miller LA, Dumanian GA, Stubblefield KA. Shoulder disarticulation externally powered prosthetic fitting following targeted muscle reinnervation for improved myoelectric control. J Prosthet Orthot. 2006;18:28–34. doi: 10.3109/03093640409167756. [DOI] [PubMed] [Google Scholar]
- 14.Fisher AG. The assessment of IADL motor skills: An application of many-faceted Rasch analysis. Am J Occup Ther. 1993;47:319–329. doi: 10.5014/ajot.47.4.319. [DOI] [PubMed] [Google Scholar]
- 15.Fisher AG. Development of a functional assessment that adjusts ability measures for task simplicity and rater leniency. In: Wilson M, editor. Objective measurement: Theory into practice. Vol. 2. Norwood, NJ: Ablex; 1994. pp. 145–175. [Google Scholar]
- 16.Fisher AG. AMPS Assessment of Motor and Process Skills. 5. Fort Collins, CO: Three Star Press; 2003. [Google Scholar]
- 17.Hijjawi JB, Kuiken TA, Lipschutz RD, Miller LA, Stubblefield KA, Dumanian GA. Improved myoelectric prosthesis control accomplished using multiple nerve transfers. Plast Reconstr Surg. 2006;118:1573–1578. doi: 10.1097/01.prs.0000242487.62487.fb. [DOI] [PubMed] [Google Scholar]
- 18.Sanderson ER, Scott RN. UNB test of prosthetics function, a test for unilateral upper extremity amputees, Ages 2–13. University of New Brunswick, Bio-engineering Institute; 1985. [Google Scholar]
- 19.Lowery MM, Weir RF, Kuiken TA. Simulation of intramuscular EMG signals detected using implantable myoelectric sensors (IMES) IEEE Trans Biomed Eng. 2006;53:1926–33. doi: 10.1109/TBME.2006.881774. [DOI] [PubMed] [Google Scholar]
- 20.Loeb GE, Peck RA, Moore WH, Hood K. BION(tm) system for distributed neural prosthetic interfaces. Med Eng Phys. 2001;23:9–18. doi: 10.1016/s1350-4533(01)00011-x. [DOI] [PubMed] [Google Scholar]
- 21.Englehart K, Hudgins B. A robust, real-time control scheme for multifunction myoelectric control. IEEE Trans Biomed Eng. 2003;50:848–54. doi: 10.1109/TBME.2003.813539. [DOI] [PubMed] [Google Scholar]
- 22.Zhou P, Lowery MM, Englehart KB, Huang H, Li G, Hargrove L, Dewald JP, Kuiken TA. Decoding a new neural-machine interface for control of artificial limbs. J Neurophysiol. 2007;29:29. doi: 10.1152/jn.00178.2007. [DOI] [PubMed] [Google Scholar]
- 23.Huang H, Zhou P, Li G, Kuiken T. An analysis of EMG electrode configuration for targeted muscle reinnervation based neural machine interface. IEEE Trans Neural Syst Rehabil Eng. doi: 10.1109/TNSRE.2007.910282. to be published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim K, Colgate JE, Santos-Munne JJ, Maklin A. A miniature tactor design for upper extremity prosthesis. presented at Frontiers in the Convergence of Bioscience and Information Technologies; 2007; Jeju Island, Korea. [Google Scholar]