Abstract
Introduction:
The rarity of thymomas and lack of multi-institutional studies have hampered therapeutic progress for decades. To overcome this, the members of the International Thymic Malignancy Interest Group created a worldwide retrospective database. This database was analyzed regarding the demographic and geographic distribution of thymomas and the impact of different variables on survival and recurrence.
Methods:
This study analyzed 4221 thymomas diagnosed between 1983 and 2012 with World Health Organization histotype information from the International Thymic Malignancy Interest Group database. Associations to survival and recurrence were studied by univariate and multivariate analyses.
Results:
Type B2 thymoma is the most common (28%) and type A the least common (12%) histotypes. They are significantly more frequent in Europe and the United States than Asia. Type A and AB occur at significantly higher age than other thymomas (64 and 57 years, respectively). There are no differences in gender distribution. Stage is lower in type A (90% in stages I–II) and AB than B1 to B3 thymomas (38% of type B3 in stage III). In univariate analysis, recurrence is significantly less frequent among stage I/II tumors, in type A and AB (recurrence rates, 1–2%) than B1 to B3 thymomas (2–7%). Multivariate analysis reveals an impact of age, stage, and resection status on survival and recurrence, whereas for histology there is only a significant impact on recurrence.
Conclusion:
New findings are (1) geographic differences such as a lower incidence of type A and B2 thymoma in Asia; and (2) impact of stage and histology, the latter partially limited to early stage disease, on recurrence.
Keywords: Thymoma, International Thymic Malignancy Interest Group, World Health Organization histotype, Prognosis, Epidemiology
Thymomas and thymic carcinomas (TCs) are epithelial tumors of the thymus. They are the most frequent tumors of the anterior mediastinum in adults but account for less than 1% of all human neoplasms. Thymomas comprise a spectrum of unique tumors of low to moderate malignant potential that are subdivided according to the histological classification of the World Health Organization (WHO) into type A, AB, B1, B3, and B3 and rare other subtypes. By contrast, TCs resemble similarly termed carcinomas outside the mediastinum and usually follow an aggressive clinical course.1
The prognosis of thymomas and TCs seems to be mainly dependent on tumor stage and resection status, while the relevance of WHO defined histological subtype as an independent prognostic marker among thymomas is still controversial: some studies and meta-analyses found a more favorable outcome of patients with type A, AB, and B1 compared with B2 and B3 thymoma subtypes,2–6 whereas others did not.7,8 These controversies are partly due to the rarity of these histologically complex tumors, and possibly, difficulties with interobserver reproducibility involving some subtypes of the WHO classification as suggested by discrepancies between relatively small published case series from different parts of the world.9 To overcome inherent problems of previous small and mostly single-center series, and to identify true epidemiological and clinicopathological differences between thymoma patients across the globe, the International Thymic Malignancy Interest Group (ITMIG)10 has compiled a worldwide retrospective database of thymomas and TCs from small to large volume clinical and pathology centers in Asia, in the United States, and in Europe.11 Furthermore, ITMIG launched a worldwide, prospective, Internet-accessible clinicopathological database in 2012 (https://ccehubg.org/itmig).12
An overview of the ITMIG retrospective database has been published elsewhere,11 which includes original data from 4918 thymomas. The present article focuses on the thymoma cases in this database—specifically on those diagnosed between 1983 and 2012 which included data on the WHO subtype and sufficient survival and recurrence data to be germane to an analysis of the impact of the WHO histotype of thymoma. An analysis of outcomes relative to WHO histotypes was beyond the scope of the retrospective database overview.12
This report addresses the following issues: (1) Are there demographic and clinical differences between the different histological thymoma subtypes? (2) Is the WHO histological classification consistently applied across different geographic regions, countries, and centers? (3) Which variables (WHO histotype, stage, resection status) affect survival? and (4) Does the WHO histotype have independent impact on prognosis?
MATERIALS AND METHODS
Of the 4918 thymomas in the ITMIG retrospective database,11 389 lacked histotype information and 308 were excluded due to diagnosis before 1983, leaving 4221 cases (Supplementary Table S1, SDC 1, http://links.lww.com/JTO/A732). An additional 328 lacked sufficient epidemiological data and 26 were excluded due to being of tumor subtypes other than A to B3 (21 micronodular thymoma, 4 metaplastic thymoma, 1 other). The overall survival (OS) analysis involved 2891 and the recurrence analysis 2408 patients after deleting cases with missing data (resection status, intervention date, follow-up date, etc.). Clinical centers were dichotomized by geographic region into low- and high-volume centers, high-volume centers being defined as the top contributors who submitted over 50% of all cases of a particular continent. This study was approved by the Yale Institutional Review Board (HIC # 1307012419).
Thymomas were classified according to WHO criteria13 by local surgical pathologists. Tumors other than type A, B1, B2, B3, and AB thymomas were excluded. There was no central review of slides for the purpose of this study.
Both the Masaoka et al.14 and the Masaoka-Koga15,16 stage classification systems were used by submitting centers. Because the Thymic domain of the Staging and Prognostic Factors Committee found no difference in outcomes between these systems14–16 or between stages I and II,12,17 the Masaoka and Masaoka-Koga systems and stages I and II were combined in the analysis in this article.
The ITMIG statistical core (XY, YD) performed all analyses with SAS 9.3 (SAS Institute Inc., Cary, NC) and R. The primary outcome measures OS and cumulative incidence of recurrence (CIR) were analyzed using the Kaplan–Meier method and the log-rank test. Prognostic factors that were significantly associated with survival in univariate analysis (p < 0.05) were included in a Cox proportional hazards model for multivariate analysis. CIR was assessed using competing risk analysis, with death included as the competing event. The effect of clinical factors on recurrence was assessed using Gray’s test. All p values from pairwise comparisons were adjusted by using Bonferroni method due to multiple comparison problems. Statistical significance was set at p less than 0.05 and all tests were two-tailed.
RESULTS
Patient Characteristics and Frequencies
Characteristics of the 4221 thymoma cases are summarized in Supplementary Table S1 (SDC 1, http://links.lww.com/JTO/A732). There is no gender predilection (49% male and 51% female patients). The largest number of cases came from centers in Europe; center volume ranged from 461 to 2 patients. Demographic and clinical details specifically for each WHO histotype are shown in Table 1. There is no significant gender predilection among the five WHO types. Type A and AB patients are significantly older than B1-3 patients (median, 60 versus 52, p < 0.0001). Myasthenia gravis (MG) is more frequent in type B1-3 thymomas (35–49%) than in type A and AB (25–26%). There is a significant association between WHO type and stage (p < 0.0001, Fig. 1).
Table 1.
Clinical Characteristics of Each WHO Histotype
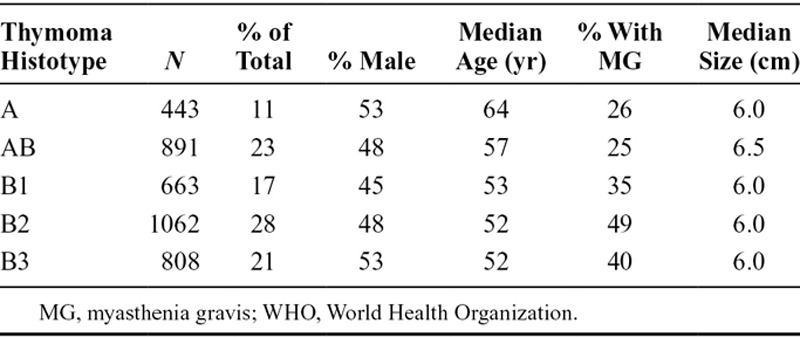
FIGURE 1.
Stage distribution of thymoma histotypes. Stage classification as reported by the centers using either the Masaoka or Masoaka-Koga classification systems. WHO, World Health Organization.
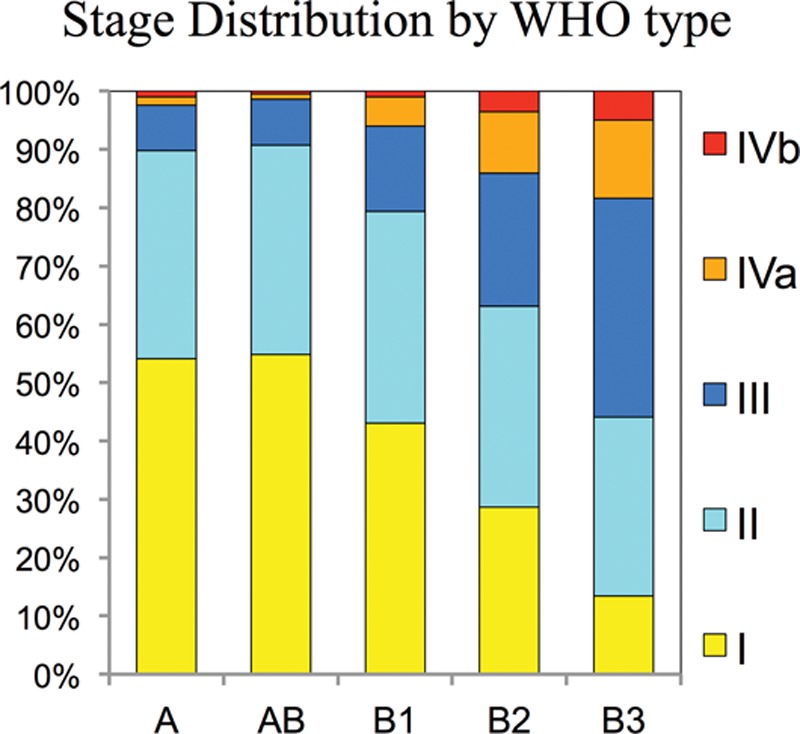
There is remarkable variability in the proportion of WHO histotypes reported from individual centers: In Figure 2A, the centers are ordered by center volume to assess whether this is associated with center experience; no consistent trend is apparent. The results suggested a difference according to geographic regions; to assess this further, we grouped the centers according to the region (Fig. 2B). The frequency of type A thymoma is similar in Europe (15%) and the United States (14%), but significantly lower in Asia (6%, adjusted p < 0.0001). Type AB thymoma is more frequent in Asia (27%) than Europe (23%) and the United States (18%, adjusted p = 0.0002). Type B2 thymoma is similar in Europe (31%) and the United States (32%), but significantly lower in Asia (20%, adjusted p < 0.0001). Type B3 thymoma is more frequent in Asia (32%) than Europe (15%) and the United States (16%, adjusted p < 0.0001). The frequencies of type B1 thymoma (16–20%) are not significantly different between geographic regions.
FIGURE 2.
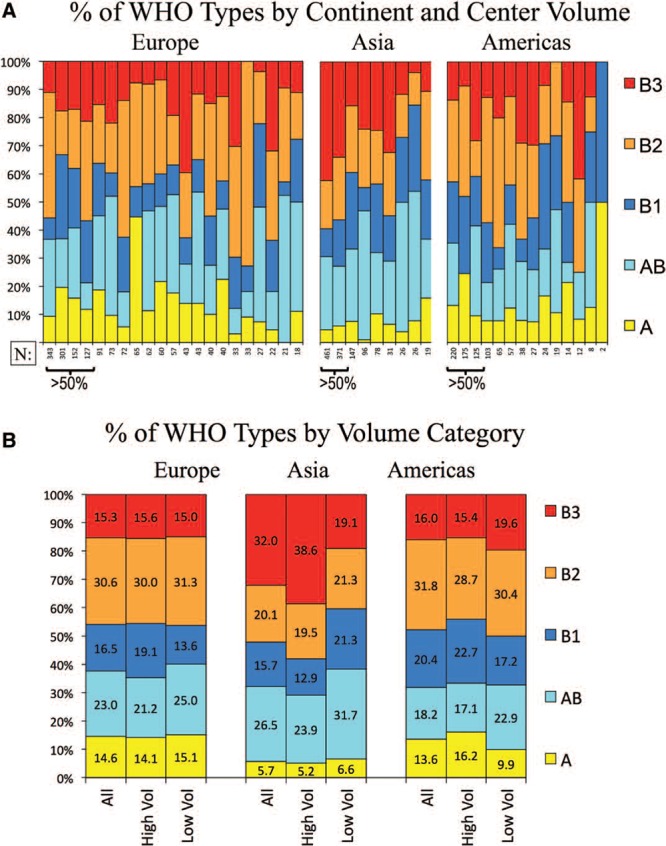
Relative frequency of thymoma histotypes by center volume and geographic region. A, Frequency of thymoma subtypes, ordered by size of center and geographic region. B, The frequency of thymoma subtypes by region and by high- and low-volume centers. “High volume centers” are those contributing more than 50% of the total cases per region. WHO, World Health Organization.
Survival and Recurrence
The OS probability is significantly different among the different WHO types (Supplementary Figure S1A, SDC 2, http://links.lww.com/JTO/A733, R0 resected patients, p = 0.0165). However, there are no significant differences except that OS was significantly lower for B3 versus B1 (adjusted p = 0.043). CIR among R0 resected patients, all stages, is shown in Supplementary Figure S1B (SDC 2, http://links.lww.com/JTO/A733). The 5-year recurrence rate by histotype is as follows: type A 4% (95% confidence interval [CI], 1–9%); type AB 2% (CI, 1–4%); type B1 8% (CI, 5–13%); type B2 13% (CI, 9–17%); and type B3 14% (CI, 9–17%). For CIR, the differences are statistically significant with the exceptions that A and AB are almost identical, B2 and B3 are almost identical, and the difference between B3 versus B1 does not quite reach significance (adjusted p = 0.066). Of note, the OS of type A is relatively poor, whereas the recurrence rate for this histotype is quite low.
Association Between WHO Type and Other Prognostic Factors
The cohort under study in this article was examined for the effect of other known prognostic factors.18 Stage had a significant impact on OS among patients with thymoma (R0 resection, stages I + II versus III versus IVa versus IVb, p < 0.0001; Supplementary Figure S2A, SDC 3, http://links.lww.com/JTO/A734). All differences are significant except that IVa is similar to IVb. There are also significant differences in CIR by stage (all thymoma histotypes, R0 resection, stages I + II versus III versus IVa versus IVb, p < 0.0001; Supplementary Figure S2B, SDC 3, http://links.lww.com/JTO/A734). Resection status also proved to be a significant prognostic factor among thymoma patients (Supplementary Figure S3, SDC 4, http://links.lww.com/JTO/A735).
The effect of WHO subtype on recurrence within each stage group in R0 resected patients is shown in Figure 3A–C, to eliminate the effect of stage and resection status (corresponding data for survival are shown in Supplementary Figure S4, SDC 5, http://links.lww.com/JTO/A736). Thymoma histotype was a prognostic factor in stages I to II (1473 cases, R0); the CIR was significantly lower for type A, AB, and B2 versus B1 and B3 thymomas (5-year recurrence rates: type A 2%, [CI, 0.3–6%]; type AB 1% [0.4–3%]; B2 2% [0.8–4%] versus type B1 6% [3–10%]; and type B3 7% [3–12%]). By contrast, CIR was not significantly different between thymoma histotypes within stages III (277 cases, R0) and IVa (89 cases, R0).
FIGURE 3.
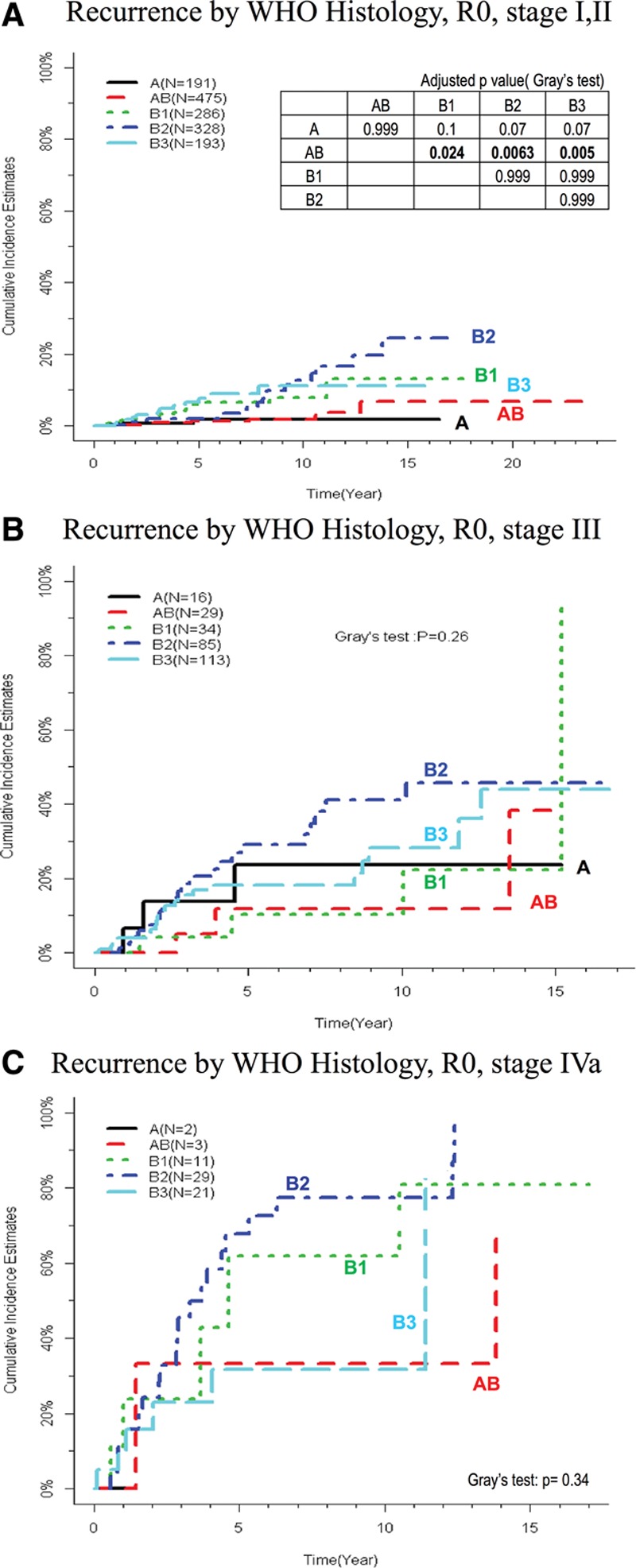
Recurrence by thymoma histotype for specific stage groups. Cumulative incidence of recurrence by World Health organization (WHO) type (R0 patients) for (A) stages I + II, (B) stage III, and (C) stage IVa. Curves for stage IVb are not shown due to low case numbers. The table inserted in (A) shows pairwise comparisons for stages I + II with adjusted p values, asterisk denotes p ≤ 0.05. Data for stages III and IVa are not shown because all results are nonsignificant.
Multivariate Analysis
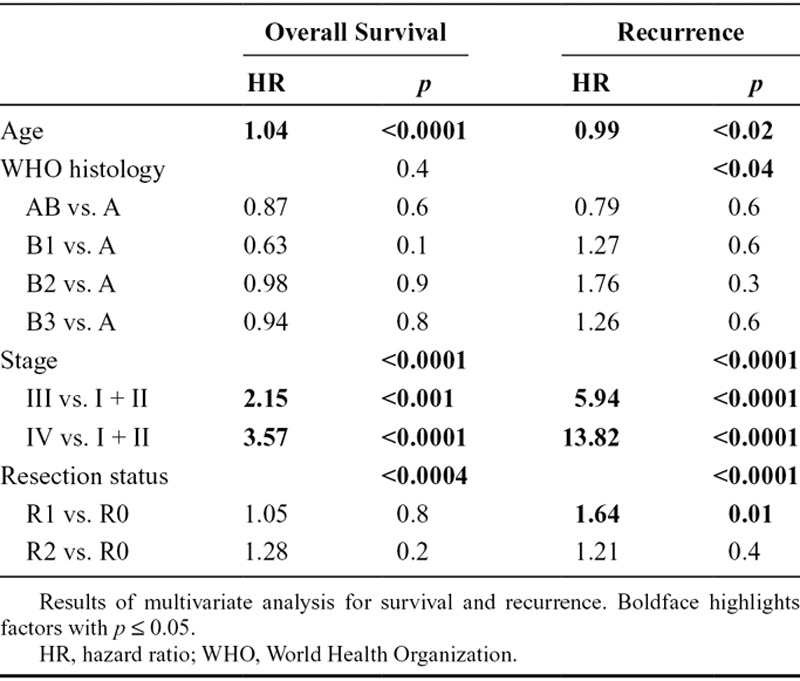
A multivariate analysis (including age, stage resection status, and WHO histotype) was conducted to ascertain the independent prognostic value of WHO subtype (Table 2). Age, stage, and resection status were all significantly associated with both OS and CIR by multivariate analysis. After adjusting for age, stage, and resection status, WHO histology was not significantly associated with survival (p = 0.4); however, there was an association with recurrence (p < 0.04).
Table 2.
Multivariate Analysis for Survival and Recurrence
DISCUSSION
This is the first analysis of the impact of WHO histology, stage, and resection status on survival and recurrence in a large worldwide collection of patients. Our observation that type B2 and AB are most common and type A the least common thymoma subtype is in agreement with findings in most previous studies.9,11,19,20 Furthermore, we confirmed associations of histotype with stage,7,21,22 age,2,7,20,21,23, and MG2,20,23 and a lack of association with gender9 from previous smaller studies.
Demographic and Clinical Characteristics of Thymoma Subtypes
We observed significant variability in the frequency distribution of histotypes at individual centers, which is also consistent with previous observations.9,23,24 These differences could be related to differences in institutional referral patterns regarding age, MG, or earlier detection, given observed associations of these factors with thymoma histotype. The variability in histotype frequency does not seem to be a simple result of sampling error in a rare disease, given that the variability seems to be present among both higher and lower volume programs (Fig. 2). The observed variability in both high- and low-volume centers also argues that this is not due to pathologist inexperience; presumably, the case volume at high-volume institutions would provide adequate exposure. It is more likely that the variability reflects a lack of consistency in histotype classification. However, this study cannot directly evaluate this issue because central pathology review was not possible. ITMIG has proposed refinements to criteria for histotype classification in an effort to improve reproducibility.25,26
Variability in Frequency of Thymoma Subtypes
This is the first study investigating the distribution of histotypes across geographic regions in a large worldwide collection of patients. We observed a significantly lower frequency of type A thymoma in Asia and the higher frequency of B2 thymomas in Europe. This seems to be a fairly consistent finding across centers within a region, further arguing against this observation being due to chance alone. This could represent regional differences in histologic classification and interpretation; however, the phenomenon is observed even in high-volume centers, most of which have pathologists who received training in other geographic regions. It is possible that the regional differences represent epidemiologic differences as has been seen in a variety of cancers. Investigating the histotype distribution among migrant populations might help to distinguish genetic from environmental factors in disease etiology.27 At this point, the suggestion of a possible epidemiologic difference can only be viewed as speculative, requiring development of more reproducible diagnostic criteria for histological subtyping26,28 and additional studies, perhaps including central pathology review.29
Impact of Thymoma Subtype on Outcomes
The discrepancy between the low recurrence rate for type A thymomas and the relatively lower survival rate is likely explained by the significantly higher median age for type A than for AB, B1, B2, and B3 thymomas (i.e., a greater proportion of thymoma-unrelated deaths). This underscores that recurrence may be a better marker of the biologic potential of thymoma.18 It also highlights the benefit of assessing both survival and recurrence together as in this study, in contrast to most previous studies that either focused on either survival2,21,23,24 or recurrence.6,30–32
This study confirms the strong prognostic value of stage, shown in many other smaller studies.7,20–22,32–38 We also confirmed the prognostic impact of resection status. The substantially larger size of the present study permitted a more robust multivariate analysis which demonstrated that both of these factors have independent prognostic significance.
The recurrence rate is strongly correlated with the histotype by univariate analysis, as has been shown in other smaller studies.2,20,22,35–39 However, it must be recognized that there is a strong association between stage and the histotype (Fig. 1). Indeed, in multivariate analysis there is no independent prognostic impact of histotype on survival. An effect of histotype remains with respect to recurrence though. This effect seems to be limited to stages I + II and is only significant for type AB versus B1, B2, or B3 thymoma. The lack of significant differences for type A versus the B subtypes, however, is likely due to the smaller number of type A patients because otherwise type A and AB seem to have the same results. Nevertheless, the fact that the independent prognostic value of histotype on recurrence in the large ITMIG database is limited to very specific patients (stages I + II, AB versus B subtypes) underscores that stage has a more profound prognostic impact.
CONCLUSION
The ITMIG retrospective database provides an unprecedented opportunity to investigate the impact of thymoma histotypes in a large worldwide cohort. This has shown that the average age is higher in type A (and AB), MG is most commonly seen in the B subtypes (especially B2), and there is an increasingly higher proportion of higher stage disease moving from A/AB to B1, B2, and especially B3. Some variability exists in the proportion of histotypes at individual centers. There seems to be a lower proportion of type A thymoma in Asia and more type B2 in Europe for unclear reasons. Although WHO histotype is associated with differences in survival and recurrence, a multivariate analysis shows that age, stage, and resection status have the greatest independent prognostic impact that WHO histotype is an independent prognostic factor in recurrence but not survival. The effect on recurrence is seen in stages I + II thymoma and for type AB versus B1, B2, and B3.
Acknowledgments
Dr. Andrew G. Nicholson was supported by the National Institute of Health Research Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London. The remaining authors have no conflict of interest to disclose.
Footnotes
Disclosure: The authors declare no conflict of interest.
REFERENCES
- 1.Travis W, Brambilla E, Müller-Hermelink H, et al., editors. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press; 2004. [Google Scholar]
- 2.Okumura M. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma. Cancer. 2002;94:581–583. doi: 10.1002/cncr.10226. [DOI] [PubMed] [Google Scholar]
- 3.Park MS, Chung KY, Kim KD, et al. Prognosis of thymic epithelial tumors according to the new World Health Organization histologic classification. Ann Thorac Surg. 2004;78:992–997; discussion 997. doi: 10.1016/j.athoracsur.2004.03.097. [DOI] [PubMed] [Google Scholar]
- 4.Rea F, Marulli G, Girardi R, et al. Long-term survival and prognostic factors in thymic epithelial tumours. Eur J Cardiothorac Surg. 2004;26:412–418. doi: 10.1016/j.ejcts.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 5.Rieker RJ, Hoegel J, Morresi-Hauf A, et al. Histologic classification of thymic epithelial tumors: comparison of established classification schemes. Int J Cancer. 2002;98:900–906. doi: 10.1002/ijc.10255. [DOI] [PubMed] [Google Scholar]
- 6.Wright CD, Wain JC, Wong DR, et al. Predictors of recurrence in thymic tumors: importance of invasion, World Health Organization histology, and size. J Thorac Cardiovasc Surg. 2005;130:1413–1421. doi: 10.1016/j.jtcvs.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 7.Kim DJ, Yang WI, Choi SS, Kim KD, Chung KY. Prognostic and clinical relevance of the World Health Organization schema for the classification of thymic epithelial tumors: a clinicopathologic study of 108 patients and literature review. Chest. 2005;127:755–761. doi: 10.1378/chest.127.3.755. [DOI] [PubMed] [Google Scholar]
- 8.Kondo K, Yoshizawa K, Tsuyuguchi M. WHO histologic classification is a prognostic indicator in thymoma. Ann Thorac Surg 2004;77:1183-1188. [DOI] [PubMed]
- 9.Detterbeck FC. Clinical value of the WHO classification system of thymoma. Ann Thorac Surg. 2006;81:2328–2334. doi: 10.1016/j.athoracsur.2005.11.067. [DOI] [PubMed] [Google Scholar]
- 10.Detterbeck F. International thymic malignancies interest group: a way forward. J Thorac Oncol. 2010;5(10 Suppl 4):S365–S370. doi: 10.1097/JTO.0b013e3181f22d01. [DOI] [PubMed] [Google Scholar]
- 11.Huang J, Ahmad U, Antonicelli A, et al. Development of the International Thymic Malignancy Interest Group International Database: an unprecedented resource for the Study of a Rare Group of Tumors. J Thorac Oncol. 2014 doi: 10.1097/JTO.0000000000000269. [DOI] [PubMed] [Google Scholar]
- 12.Detterbeck FC, Asamura H, Crowley J, et al. Staging and Prognostic Factors Committee; Members of the Advisory Boards; Participating Institutions of the Thymic Domain. The IASLC/ITMIG thymic malignancies staging project: development of a stage classification for thymic malignancies. J Thorac Oncol. 2013;8:1467–1473. doi: 10.1097/JTO.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 13.Rosai J, Sobin L. Histological Typing of Tumours of the Thymus. Springer; 1999. [Google Scholar]
- 14.Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer. 1981;48:2485–2492. doi: 10.1002/1097-0142(19811201)48:11<2485::aid-cncr2820481123>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 15.Koga K, Matsuno Y, Noguchi M, et al. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int. 1994;44:359–367. doi: 10.1111/j.1440-1827.1994.tb02936.x. [DOI] [PubMed] [Google Scholar]
- 16.Girard N, Travis W, Oku- M. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. J Thorac Oncol. 2011;6:1710–1716. doi: 10.1097/JTO.0b013e31821e8cff. [DOI] [PubMed] [Google Scholar]
- 17.Detterbeck FC, Zeeshan A. Thymoma: current diagnosis and treatment. Chin Med J (Engl) 2013;126:2186–2191. [PubMed] [Google Scholar]
- 18.Detterbeck F, Youssef S, Ruffini E, Okumura M. A review of prognostic factors in thymic malignancies. J Thorac Oncol. 2011;6(7 Suppl 3):S1698–S1704. doi: 10.1097/JTO.0b013e31821e7b12. [DOI] [PubMed] [Google Scholar]
- 19.Chalabreysse L, Roy P, Cordier JF, Loire R, Gamondes JP, Thivolet-Bejui F. Correlation of the WHO schema for the classification of thymic epithelial neoplasms with prognosis: a retrospective study of 90 tumors. Am J Surg Pathol. 2002;26:1605–1611. doi: 10.1097/00000478-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Ströbel P, Bauer A, Puppe B, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol. 2004;22:1501–1509. doi: 10.1200/JCO.2004.10.113. [DOI] [PubMed] [Google Scholar]
- 21.Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg. 2003;76:878–884; discussion 884–875. doi: 10.1016/s0003-4975(03)00555-1. [DOI] [PubMed] [Google Scholar]
- 22.Shinohara S, Hanagiri T, So T, et al. Results of surgical resection for patients with thymoma according to World Health Organization histology and Masaoka staging. Asian J Surg. 2012;35:144–148. doi: 10.1016/j.asjsur.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa K, Asamura H, Matsuno Y, et al. Thymoma: a clinicopathologic study based on the new World Health Organization classification. J Thorac Cardiovasc Surg. 2003;126:1134–1140. doi: 10.1016/s0022-5223(03)00798-0. [DOI] [PubMed] [Google Scholar]
- 24.Chen G, Marx A, Chen WH, et al. New WHO histologic classification predicts prognosis of thymic epithelial tumors: a clinicopathologic study of 200 thymoma cases from China. Cancer. 2002;95:420–429. doi: 10.1002/cncr.10665. [DOI] [PubMed] [Google Scholar]
- 25.Mansfield AS, Roden AC, Peikert T, et al. B7-H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis. J Thorac Oncol. 2014;9:1036–1040. doi: 10.1097/JTO.0000000000000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marx A, Ströbel P, Badve SS, et al. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Oncol. 2014;9:596–611. doi: 10.1097/JTO.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 27.Sankaranarayanan R. Commentary: cancer incidence among Asian Indians in India and abroad. Int J Epidemiol. 2008;37:160–161. doi: 10.1093/ije/dym249. [DOI] [PubMed] [Google Scholar]
- 28.Ströbel P, Hartmann E, Rosenwald A, et al. Corticomedullary differentiation and maturational arrest in thymomas. Histopathology. 2014;64:557–566. doi: 10.1111/his.12279. [DOI] [PubMed] [Google Scholar]
- 29.Hadoux J, Girard N, Besse B. Thymic epithelial neoplasms: updates on diagnosis, staging, biology and management in France. Bull Cancer. 2012;99:1045–1055. doi: 10.1684/bdc.2012.1659. [DOI] [PubMed] [Google Scholar]
- 30.Blumberg D, Port JL, Weksler B, et al. Thymoma: a multivariate analysis of factors predicting survival. Ann Thorac Surg. 1995;60:908–913; discussion 914. doi: 10.1016/0003-4975(95)00669-c. [DOI] [PubMed] [Google Scholar]
- 31.Cowen D, Richaud P, Mornex F, et al. Thymoma: results of a multicentric retrospective series of 149 non-metastatic irradiated patients and review of the literature. FNCLCC trialists. Fédération Nationale des Centres de Lutte Contre le Cancer. Radiother Oncol. 1995;34:9–16. doi: 10.1016/0167-8140(94)01493-m. [DOI] [PubMed] [Google Scholar]
- 32.Huang J, Detterbeck FC, Wang Z, Loehrer PJ., Sr Standard outcome measures for thymic malignancies. J Thorac Oncol. 2011;6(7 Suppl 3):S1691–S1697. doi: 10.1097/JTO.0b013e3182254ac1. [DOI] [PubMed] [Google Scholar]
- 33.Dawson A, Ibrahim NB, Gibbs AR. Observer variation in the histopathological classification of thymoma: correlation with prognosis. J Clin Pathol. 1994;47:519–523. doi: 10.1136/jcp.47.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luebke T, Beckurts KT, Wickenhauser C, Schneider P, Hoelscher AH, Moenig SP. Masaoka staging is of prognostic relevance in type B3 / C thymomas. Anticancer Res. 2004;24:4113–4119. [PubMed] [Google Scholar]
- 35.Margaritora S, Cesario A, Cusumano G, et al. Thirty-five-year follow-up analysis of clinical and pathologic outcomes of thymoma surgery. Ann Thorac Surg. 2010;89:245–252; discussion 252. doi: 10.1016/j.athoracsur.2009.08.074. [DOI] [PubMed] [Google Scholar]
- 36.Ríos A, Torres J, Galindo PJ, et al. Prognostic factors in thymic epithelial neoplasms. Eur J Cardiothorac Surg. 2002;21:307–313. doi: 10.1016/s1010-7940(01)01091-0. [DOI] [PubMed] [Google Scholar]
- 37.Ruffini E, Detterbeck F, Van Raemdonck D, et al. Tumours of the thymus: a cohort study of prognostic factors from the European Society of Thoracic Surgeons database. Eur J Cardiothorac Surg. 2014:1–8. doi: 10.1093/ejcts/ezt649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruffini E, Filosso PL, Mossetti C, et al. Thymoma: inter-relationships among World Health Organization histology, Masaoka staging and myasthenia gravis and their independent prognostic significance: a single-centre experience. Eur J Cardiothorac Surg. 2011;40:146–153. doi: 10.1016/j.ejcts.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 39.Rena O, Papalia E, Maggi G, et al. World Health Organization histologic classification: an independent prognostic factor in resected thymomas. Lung Cancer. 2005;50:59–66. doi: 10.1016/j.lungcan.2005.05.009. [DOI] [PubMed] [Google Scholar]


