Abstract
Objective
It is unclear how changes in ovarian hormones during the menopausal transition contribute to age-associated arterial stiffening. We sought to evaluate differences in arterial stiffness and the role of oxidative stress across the stages of the menopausal transition in healthy women.
Methods
Arterial stiffness (carotid artery compliance and ultrasound) was measured during immediate infusions of saline (control) and ascorbic acid (experimental model to immediately decrease oxidative stress) in 97 healthy women (22-70 y) classified as premenopausal (n = 24; mean [SD] age, 33 [7] y), early perimenopausal (n = 21; 49 [3] y) or late perimenopausal (n = 21; 50 [4] y), or postmenopausal (n = 31; 57 [5] y).
Results
Basal carotid artery compliance was different among the groups (P < 0.001). Mean [SD] compliance was highest in premenopausal women (1.31 [0.25] mm2/mm Hg × 10−1), with progressive decrements in perimenopausal (early perimenopausal, 0.98 [0.31] mm2/mm Hg × 10−1; late perimenopausal, 0.90 [0.25] mm2/mm Hg × 10−1) and postmenopausal (0.75 [0.24] mm/mm Hg × 10−1) women. Ascorbic acid infusion improved compliance in late perimenopausal (15% [18%] increase, P = 0.001) and postmenopausal (17% [26%] increase, P = 0.002) women but not in early perimenopausal or premenopausal women.
Conclusions
Arterial stiffening worsens across the stages of the menopausal transition in healthy women. This seems to be mediated, in part, by oxidative stress, particularly during the late perimenopausal and postmenopausal periods. It remains uncertain whether this is specifically caused by loss of ovarian function or aging.
Keywords: Oxidative stress, Arterial stiffening, Estrogen, Menopause
Aging is associated with increased stiffness of large elastic arteries in the cardiothoracic circulation.1 The physiological changes that occur with arterial stiffening, including systolic hypertension and left ventricular hypertrophy, make this a major risk factor for cardiovascular disease (CVD), which frequently precedes the development of clinical CVD.2-5 Age-associated arterial stiffening seems to be accelerated in women after menopause, presumably caused by estrogen deficiency.4-7 Estrogen is thought to have protective effects on the vasculature, as evidenced by the low incidence of CVD in women—compared with men—before menopause; this differential rapidly narrows after menopause.8,9 Little is known about how arterial stiffness changes across the stages of the menopausal transition or the mechanisms by which changes in ovarian hormones during the perimenopausal years contribute to age-associated arterial stiffening. Understanding the contributions of age and estrogen deficiency to arterial stiffening thus has important implications for developing strategies for the prevention of CVD in women.
Using a well-described experimental model (systemic infusion of a pharmacological dose of ascorbic acid) in human vascular research to immediately reduce reactive oxygen species (ROS)/oxidative stress, we previously demonstrated that oxidative stress plays a mechanistic role in arterial stiffening in estrogen-deficient postmenopausal women.10 Specifically, we found that infusion of supraphysiological doses of ascorbic acid increased carotid artery compliance in post-menopausal women but not in premenopausal women. In addition, recent data from the Study of Women's Health Across the Nation Heart Study suggest that inflammation, which can induce oxidative stress by increasing ROS production, may contribute to arterial stiffening more strongly among women during the late perimenopausal transition.11 Accordingly, in the present study, we determined differences in arterial stiffness across the stages of the menopausal transition in healthy women and used an intravenous infusion of a pharmacological dose of ascorbic acid (a potent water-soluble antioxidant that scavenges superoxide anions and many other ROS) to experimentally examine whether oxidative stress contributes to arterial stiffening in healthy perimenopausal and postmenopausal women.
Methods
Study population
Ninety-seven healthy women were recruited from the Denver metropolitan area. Premenopausal women were aged 22 to 49 years with regular menstrual cycles (21-35 d) and with no change in cycle length, as confirmed with menstrual cycle calendars and ovulation prediction kits. Perimenopausal women were aged 40 to 55 years with irregular cycles, and postmenopausal women were aged 45 to 70 years with amenorrhea for 12 months or more. Perimenopausal women were classified as early perimenopausal if they had two or more cycles with cycle length changes of 7 days or more, or as late perimenopausal if they had amenorrhea for 2 months or more, as defined by the Stages of Reproductive Aging Workshop.12 Women with a history of hysterectomy or oo-phorectomy were excluded. Participants were sedentary or recreationally active (vigorous exercise for <3 d/wk); had not used oral contraceptives or hormone therapy (HT) for 6 months or more before enrollment; were nonsmokers, normotensive (resting blood pressure <140/90 mm Hg), nondiabetic, and normoglycemic (fasting plasma glucose <110 mg/dL); were not taking medications that influence cardiovascular function (ie, antihypertensive and lipid-lowering medications); and were healthy, as determined by medical history, physical examination, and standard blood chemistry tests. Participants taking vitamin supplements or nonsteroidal anti-inflammatory medications were asked to refrain from using them for 4 weeks or more before vascular testing. The study was approved by the Colorado Multiple Institutional Review Board, and all participants provided a written informed consent form.
Measurements
Participants were studied in supine position after an overnight fast with proper hydration (water only) and without caffeine. Participants maintained their normal dietary patterns, including sodium intake, for 2 days before testing. Because of irregular cycles in perimenopausal women, premenopausal and perimenopausal (when possible) women were tested 7 to 10 days after the onset of menses (ie, midfollicular phase) so that arterial stiffness comparisons between premenopausal and perimenopausal would be representative across the menstrual cycle. Late perimenopausal women were tested regardless of menstrual cycle phase after 2 months of amenorrhea. The study took place at the University of Colorado Clinical Translational Sciences Institute Clinical Translational Research Center.
Carotid artery imaging
Carotid artery compliance was determined by high-resolution ultrasound imaging and measures of arterial pressure, as previously described.13 Briefly, a longitudinal image of the cephalic portion of the carotid artery was acquired approximately 1 to 2 cm distal to the carotid bulb. Carotid images were analyzed for systolic and diastolic diameters and carotid artery intima-media thickness (IMT) using a computerized semiautomated edge-detection software that allows accurate identification and measurements of carotid artery lumen diameter and IMT across a length of the artery (Vascular Analysis Tools v. 5.5; MIA LLC, Coralville, IA). Peripheral artery blood pressures were measured over the brachial artery using a semiautomated device (Dinamap; Johnson & Johnson, New Brunswick, NJ). In a subsample of women (n = 58), carotid artery compliance was also computed using carotid artery blood pressures measured by applanation tonometry arterial waveforms (SPT-301; Millar Instruments, Houston, TX), as previously described.13 Carotid artery IMT was measured during end-diastole, as previously described.14 All images were coded by number, blinded to menopause group and testing condition, and analyzed by the same individual (K.L.M.).
Blood sampling
Fasting plasma concentrations of glucose, insulin, total cholesterol (Roche Diagnostic Systems, Indianapolis, IN), and high-density lipoprotein cholesterol (Diagnostic Chemicals Ltd, Oxford, CT) were determined using enzymatic/colorimetric methods. Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation.15 Serum concentrations of follicle-stimulating hormone (FSH), estradiol, progesterone, and sex hormoneYbinding globulin were measured using chemiluminescence. Estrone was measured using radioimmunoassay, and total testosterone was measured using one-step competitive assay. Dehydroepiandrosterone sulfate (DHEAS) was measured using quantitative electrochemiluminescent immunoassay. Oxidized LDL was measured using an enzyme-linked immunosorbent plate assay (Alpco Diagnostics, Wind-ham, NH). Serum total antioxidant status (TAS), a measure of overall antioxidant defenses, was measured using an enzymatic kit (Randox Laboratories, Oceanside, CA). Glutathione peroxidase, an antioxidant found in the cytoplasm and mitochondria, was measured using the Randox Ransel glutathione peroxidase assay (Randox Laboratories). Serum norepinephrine was measured by high-performance liquid chromatography, with ethylene glycol tetraacetic acid/glutathione added as preservative. Plasma endothelin-1 and interleukin-6 were measured using an enzyme-linked immunoassay. High-sensitivity C-reactive protein (CRP) was measured using an immunoturbidimetric method. All blood samplings occurred on the day of vascular testing. All assays were performed at the University of Colorado Clinical Translational Sciences Institute Clinical Translational Research Center core laboratory.
Ascorbic acid infusion protocol
To investigate the role of oxidative stress as a possible mechanism for increased arterial stiffness, we adopted the experimental model previously used by our laboratory16-18 involving an intravenous infusion of a pharmacological dose of ascorbic acid (vitamin C) to immediately counteract oxidative stress. Ascorbic acid is a potent water-soluble antioxidant that can be immediately infused at rates that attain supraphysiological plasma ascorbic acid concentrations known to reduce the bioavailability of superoxide anions. Carotid artery compliance, blood pressure, and heart rate were measured after a 20-minute normal saline infusion (control) and after a 20-minute systemic ascorbic acid infusion on the same day. The saline infusion always preceded ascorbic acid infusion to avoid any persistent effects of ascorbic acid on the vasculature. The time between saline and ascorbic acid measurements was approximately 60 minutes. The concentration of the ascorbic acid solution prepared by the University of Colorado pharmacy was 0.06 g of ascorbic acid per kilogram of fat-free mass per 100 mL of normal saline. Participants received a 100-mL bolus of ascorbic acid solution at 5 mL/minute followed by a drip infusion at 1.7 mL/minute until testing was completed (total ascorbic acid dose did not exceed 7.5 g). This dose of ascorbic acid has been previously shown to improve carotid artery compliance in estrogen-deficient postmenopausal women. In a subset of women in each group, TAS and CRP were measured during the ascorbic acid infusion to demonstrate increased antioxidant status and correction of inflammation-induced vascular dysfunction,11 respectively.
Statistical analysis
Descriptive statistics were used to examine all data elements. Parameters with skewed distributions were log-transformed and are presented as median (interquartile range). One-way analysis of variance (ANOVA) was used to assess differences in participant characteristics. ANOVA with post hoc tests using Bonferroni correction was used to compare baseline carotid artery compliance, hemodynamics, and carotid artery diameter across menopausal stages. Pearson and Spearman ρ correlation analyses were used to test for the presence of significant linear bivariate relations between variables of interest (ie, potential modulators of vascular function) and carotid artery compliance; analysis of covariance was used to control for variables that were significantly correlated with carotid artery compliance. To determine the effect of the ascorbic acid infusion on arterial stiffness and hemodynamic parameters, we used repeated-measures ANOVA. If significant differences were observed, post hoc analyses were performed using paired t tests with Bonferroni correction to identify significant differences among mean values. Pearson and Spearman ρ correlation analyses were used to test whether the change in carotid artery compliance with ascorbic acid was related to basal oxidative stress and inflammatory markers and clinical characteristics. Because menopausal stage and age were highly correlated (r = 0.84), exploratory analyses of carotid artery compliance during saline and ascorbic acid infusions in perimenopausal and postmenopausal women aged 50 to 59 years were conducted to distinguish the independent effect of menopausal stage. Based on previous studies,10,17 20 participants per group would provide more than 80% power to detect an anticipated change of 0.1 mm2/mm Hg × 10−1 in response to ascorbic acid. Data analysis was performed with IBM SPSS Statistics version 21.0.
Results
Participant characteristics
Among postmenopausal women, the reported age at menopause and time since menopause were 50.7 (4.0) and 6.6 (5.1) years, respectively. Forty-two percent of postmenopausal women were prior HT users with a mean (SD) duration of 2.9 (2.2) years. Seventy-five percent of premenopausal women, 81% of early perimenopausal women, and 91% of late perimenopausal women had used hormonal contraceptives for an average of 7.1 (6.7), 6.7 (5.2), and 8.5 (8.1) years, respectively. Total body fat, trunk fat, total cholesterol, LDL cholesterol, triglycerides, and carotid IMT were elevated across the stages of the menopausal transition, whereas maximal aerobic power VO2max was lower (all P < 0.005; Table 1). Sex hormone levels followed expected patterns; FSH was higher across the stages of menopause, whereas estradiol, progesterone, estrone, testosterone, and DHEAS were lower across the stages of menopause (all P < 0.005). Similar associations—except for body mass index, waist circumference, and fasted glucose concentrations, which were elevated across menopausal stages (P < 0.05; Table S1, Supplemental Digital Content 1, http://links.lww.com/MENO/A72), and testosterone, which was not different—were observed in the cohort of women in whom local carotid blood pressures were measured.
Table 1. Baseline characteristics of participants.
| Variable | Premenopausal (n = 24) | Early perimenopausal (n = 21) | Late perimenopausal (n = 21) | Postmenopausal (n = 31) | P |
|---|---|---|---|---|---|
| Age, y | 33 (7) | 49 (3) | 50 (4) | 57 (5) | <0.001 |
| Body mass, kg | 66.4 (15.0) | 68.8 (10.6) | 66.9 (13.3) | 73.3 (14.1) | 0.09 |
| Height, cm | 166 (6) | 164 (6) | 167 (9) | 163 (7) | 0.31 |
| BMI, kg/m2 | 24.2 (5.6) | 25.5 (3.5) | 24.1 (4.3) | 27.6 (5.2) | 0.13 |
| Total body fat, % | 31 (7) | 34 (6) | 36 (7) | 40 (5) | <0.001 |
| Trunk fat, % | 29 (9) | 33 (6) | 34 (8) | 38 (6) | <0.001 |
| Waist circumference, cm | 77 (8) | 83 (10) | 82 (13) | 88 (13) | 0.07 |
| WHR | 0.77 (0.07) | 0.80 (0.06) | 0.80 (0.06) | 0.83 (0.06) | 0.24 |
| VO2max, mL/kg/min | 33.6 (7.3) | 27.4 (4.5) | 27.5 (5.4) | 23.6 (3.8) | <0.001 |
| LTPA, MET h/wk | 17.3 (11.5) | 18.6 (12.4) | 14.6 (11.8) | 14.6 (17.7) | 0.86 |
| Seated SBP, mm Hg | 109 (8) | 113 (11) | 116 (13) | 119 (14) | 0.06 |
| Seated DBP, mm Hg | 70 (6) | 73 (7) | 72 (8) | 74 (9) | 0.36 |
| Resting HR, beats/min | 67 (12) | 62 (7) | 64 (10) | 64 (7) | 0.39 |
| Total cholesterol, mmol/L | 3.9 (0.8) | 4.2 (0.7) | 4.4 (0.8) | 4.9 (0.8) | <0.001 |
| LDL cholesterol, mmol/L | 2.3 (0.6) | 2.5 (0.7) | 2.6 (0.8) | 3.1 (0.8) | 0.002 |
| HDL cholesterol, mmol/L | 1.1 (0.2) | 1.3 (0.4) | 1.3 (0.2) | 1.2 (0.3) | 0.19 |
| Triglycerides, mmol/La | 0.8 (0.6-0.9) | 0.9 (0.7-1.2) | 0.9 (0.9-1.2) | 1.1 (0.8-1.5) | 0.04 |
| Fasting insulin, pmol/La | 42 (21-57) | 28 (21-56) | 28 (21-28) | 35 (28-83) | 0.48 |
| Fasting glucose, mmol/L | 4.6 (0.6) | 4.7 (0.4) | 4.5 (0.4) | 4.9 (0.6) | 0.10 |
| FSH, IU/L | 5.5 (2.9) | 22.5 (32.0) | 70.8 (35.8) | 84.2 (32.9) | <0.001 |
| Estradiol, pmol/La | 231 (114-338) | 198 (128-503) | 143 (37-433) | 37 (37-48) | <0.001 |
| Progesterone, nmol/La | 1.9 (1.0-2.9) | 1.9 (1.0-3.3) | 1.0 (1.0-1.7) | 1.0 (0.3-1.3) | 0.002 |
| Estrone, pmol/L | 218 (99) | 222 (138) | 190 (100) | 109 (40) | <0.001 |
| SHBG, nmol/L | 60 (28) | 84 (56) | 73 (39) | 60 (31) | 0.36 |
| Testosterone, nmol/La | 1.0 (1.0-1.6) | 0.8 (0.6-1.2) | 0.7 (0.6-0.9) | 0.6 (0.6-1.0) | 0.004 |
| DHEAS, nmol/L | 384 (165) | 222 (116) | 177 (85) | 163 (85) | <0.001 |
| Carotid IMT, mm | 0.509 (0.083) | 0.591 (0.081) | 0.588 (0.070) | 0.651 (0.099) | <0.001 |
Data are presented as mean (SD), unless otherwise stated.
BMI, body mass index; WHR, waist-to-hip ratio; VO2max, maximal aerobic power; LTPA, leisure time physical activity; MET, metabolic equivalent of task; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; LDL, low-density lipoprotein; HDL, high-density lipoprotein; FSH, follicle-stimulating hormone; SHBG, sex hormone–binding globulin; DHEAS, dehydroepiandrosterone sulfate; IMT, intima-media thickness.
Data are presented as median (interquartile range).
Oxidative stress, inflammation, and humoral factors
Basal plasma levels of norepinephrine were elevated across the menopausal transition (Table 2), whereas TAS tended to be reduced and endothelin-1 tended to be elevated. There were no other differences in basal markers of oxidative stress and inflammation between groups. TAS increased and CRP decreased after ascorbic acid infusion, with similar responses observed across groups. In the cohort of women with carotid blood pressures, TAS and norepinephrine were not different, whereas oxidized LDL tended to be elevated across menopausal stages (Table S2, Supplemental Digital Content 2, http://links.lww.com/MENO/A73).
Table 2. Circulating humoral factors at baseline.
| Variable | Premenopausal (n = 24) | Early perimenopausal (n = 21) | Late perimenopausal (n = 21) | Postmenopausal (n = 31) | P |
|---|---|---|---|---|---|
| Oxidized LDL, U/L | 43.7 (17.9) | 44.0 (12.0) | 46.2 (17.3) | 52.7 (18.4) | 0.27 |
| TAS (saline), mmol/L | 1.43 (0.22) | 1.35 (0.14) | 1.30 (0.13) | 1.31 (0.14) | 0.09 |
| TAS (ascorbic acid), mmol/L | 2.82 (0.50) | 2.59 (0.51) | 2.56 (0.37) | 2.57 (1.18) | 0.74 |
| Glutathione peroxidase, U/L | 6,218 (1,845) | 7,259 (1,532) | 6,587 (1,376) | 7,250 (1,736) | 0.16 |
| Norepinephrine, pmol/L | 1,047 (394) | 1,394 (477) | 1,479 (672) | 1,567 (736) | 0.006 |
| Endothelin-1, pg/mLa | 5.4 (4.4-6.0) | 5.6 (5.1-6.7) | 5.7 (5.3-6.6) | 6.2 (5.3-7.0) | 0.06 |
| IL-6, pg/mLa | 0.67 (0.54-1.32) | 0.76 (0.62-1.29) | 0.90 (0.49-1.32) | 0.83 (0.66-1.22) | 0.92 |
| C-reactive protein (saline), mg/dLa | 0.68 (0.32-2.38) | 1.03 (0.70-2.21) | 0.89 (0.47-1.61) | 1.24 (0.67-2.36) | 0.17 |
| C-reactive protein (ascorbic acid), mg/dLa | 0.45 (0.24-0.67) | 0.93 (0.63-2.04) | 0.86 (0.45-1.46) | 1.09 (0.47-1.59) | 0.14 |
Data are presented as mean (SD), unless otherwise stated.
LDL, low-density lipoprotein; TAS, total antioxidant status; IL, interleukin.
Data are presented as median (interquartile range).
Basal arterial stiffness
Basal carotid artery compliance was reduced across the stages of the menopausal transition; similar patterns and rates of decline were observed with carotid compliance computed using brachial and carotid blood pressures (both P < 0.005; Fig. 1). Supine brachial systolic and pulse pressures were elevated across the stages of menopause, but carotid artery diameters and carotid artery blood pressures were not different (Table 3; Table S3, Supplemental Digital Content 3, http://links.lww.com/MENO/A74).
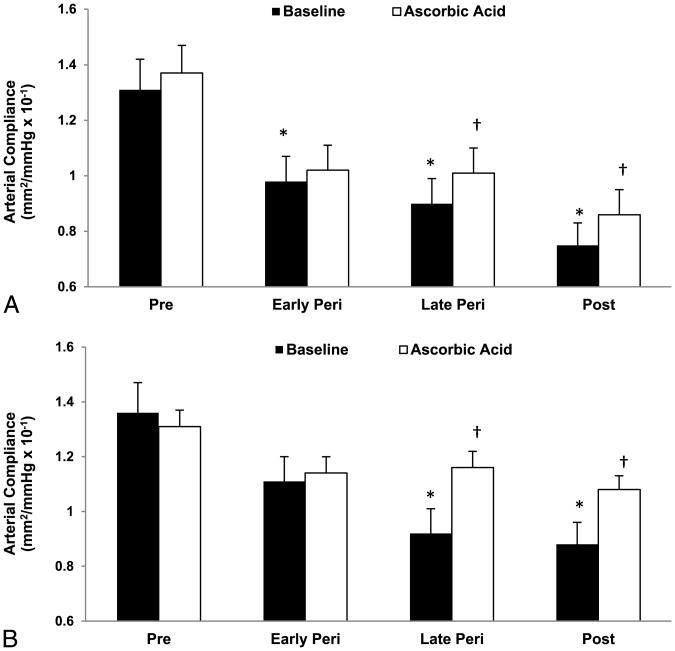
Fig. 1.
Carotid artery compliance at baseline (saline) and after ascorbic acid infusion in premenopausal (Pre), early and late perimenopausal (Peri), and postmenopausal (Post) women in the whole cohort (A) and in a subset with carotid blood pressures (B). *P < 0.05 versus premenopausal women. †P < 0.05 versus saline of the same group.
Table 3. Carotid and hemodynamic parameters with saline (control) and ascorbic acid infusions.
| Variable | Premenopausal (n = 24) | Early perimenopausal (n = 21) | Late perimenopausal (n = 21) | Postmenopausal (n = 31) |
|---|---|---|---|---|
| Carotid diameter, mm | ||||
| Saline | 6.12 (0.46) | 6.06 (0.59) | 5.93 (0.38) | 6.14 (0.41) |
| Ascorbic acid | 6.21 (0.46) | 6.32 (0.60)a | 6.05 (0.41) | 6.28 (0.42) |
| Brachial systolic blood pressure, mm Hg | ||||
| Saline | 105 (11) | 108 (9) | 113 (16) | 122 (15)b,c |
| Ascorbic acid | 105 (11) | 109 (11) | 113 (15) | 120 (17) |
| Brachial pulse pressure, mm Hg | ||||
| Saline | 39 (7) | 39 (6) | 44 (10) | 51 (12)b,c |
| Ascorbic acid | 39 (8) | 41 (5) | 44 (10) | 50 (11) |
Data are presented as mean (SD).
P < 0.05 versus saline.
P < 0.001 versus premenopausal.
P < 0.05 versus early perimenopausal.
Correlations between baseline carotid artery compliance computed with brachial blood pressure and participant characteristics are presented in Table 4. Similar associations were observed with carotid artery compliance computed with carotid blood pressures. After adjustments for all correlates, except for age, the association between menopause stage and carotid artery compliance remained significant (P = 0.035). However, the addition of age as a covariate abolished the association between menopause stage and carotid artery compliance.
Table 4. Correlations of baseline carotid artery compliance and change in compliance with ascorbic acid with participant characteristics.
| Baseline compliance | Change in compliance | |||
|---|---|---|---|---|
|
|
|
|||
| Participant characteristic | Correlation coefficient | P | Correlation coefficient | P |
| Age | −0.72 | <0.001 | 0.12 | 0.23 |
| Time since menopause | −0.23 | 0.21 | 0.34 | 0.07 |
| Body mass | −0.21 | 0.04 | −0.04 | 0.70 |
| BMI | −0.35 | <0.001 | −0.01 | 0.96 |
| Total body fat | −0.50 | <0.001 | 0.23 | 0.02 |
| Trunk fat | −0.49 | <0.001 | 0.23 | 0.02 |
| Waist circumference | −0.29 | 0.007 | 0.04 | 0.70 |
| WHR | −0.20 | 0.07 | 0.06 | 0.56 |
| VO2max | 0.56 | <0.001 | −0.1 | 0.35 |
| LTPA | 0.20 | 0.06 | −0.14 | 0.19 |
| Seated SBP | −0.40 | <0.001 | −0.09 | 0.40 |
| Seated DBP | −0.21 | 0.04 | −0.10 | 0.32 |
| Total cholesterol | −0.40 | <0.001 | 0.02 | 0.84 |
| LDL cholesterol | −0.69 | <0.001 | 0.04 | 0.71 |
| HDL cholesterol | 0.04 | 0.67 | −0.05 | 0.63 |
| Triglycerides | −0.27 | 0.009 | 0.07 | 0.48 |
| Fasting insulin | −0.09 | 0.39 | 0.03 | 0.76 |
| Fasting glucose | −0.13 | 0.21 | 0.02 | 0.82 |
| FSH | −0.44 | <0.001 | 0.16 | 0.13 |
| Estradiol | 0.36 | <0.001 | −0.14 | 0.17 |
| Progesterone | 0.26 | 0.01 | −0.36 | <0.001 |
| Estrone | 0.26 | 0.01 | −0.20 | 0.05 |
| SHBG | 0.06 | 0.54 | −0.08 | 0.45 |
| Testosterone | 0.18 | 0.08 | −0.07 | 0.48 |
| DHEAS | 0.49 | <0.001 | −0.16 | 0.13 |
| Oxidized LDL | −0.30 | 0.003 | −0.13 | 0.19 |
| TAS | 0.10 | 0.35 | −0.14 | 0.17 |
| Glutathione peroxidase | −0.02 | 0.87 | 0.07 | 0.51 |
| Norepinephrine | −0.23 | 0.03 | −0.03 | 0.76 |
| Endothelin-1 | −0.09 | 0.41 | 0.20 | 0.06 |
| IL-6 | −0.21 | 0.04 | 0.17 | 0.11 |
| CRP | −0.32 | 0.001 | 0.02 | 0.84 |
| Carotid IMT | −0.46 | <0.001 | −0.10 | 0.31 |
BMI, body mass index; WHR, waist-to-hip ratio; VO2max, maximal aerobic power; LTPA, leisure time physical activity; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein; FSH, follicle-stimulating hormone; SHBG, sex hormone–binding globulin; DHEAS, dehydroepiandrosterone sulfate; TAS, total antioxidant status; IL, interleukin; CRP, C-reactive protein; IMT, intima-media thickness.
Oxidative stress–mediated arterial stiffening
Ascorbic acid infusion increased carotid artery compliance by 14.7% (17.9%) in late perimenopausal women (P = 0.001) and by 16.8% (26.4%) in postmenopausal women (P = 0.002; Fig. 1A). Carotid artery compliance did not change with the ascorbic acid infusion in early perimenopausal (P = 0.30) or premenopausal (P = 0.09) women. The increases in carotid artery compliance with ascorbic acid in late perimenopausal and postmenopausal women were more robust when computed with local carotid pressures (26.1% [22.0%] and 22.6% [28.0%], respectively; Fig. 1B). Similar results were observed in the subsample of women aged 50 to 59 years, with significant increases in carotid artery compliance computed with carotid artery blood pressures during ascorbic acid infusion in late perimenopausal and postmenopausal women, but not in early perimenopausal women. The increase in carotid artery compliance computed with brachial blood pressure did not reach statistical significance owing to lack of power attributable to sample size (Fig. 2). Brachial blood pressure and heart rate did not change with the ascorbic acid infusion (Table 3). Although carotid artery diameter increased with the ascorbic acid infusion in all groups, the increase was only significant in early perimenopausal women. In women with carotid blood pressures, carotid systolic pressure decreased in the late perimenopausal and postmenopausal groups, and carotid pulse pressure decreased in late perimenopausal women (Table S3, Supplemental Digital Content 3, http://links.lww.com/MENO/A74).
Fig. 2.
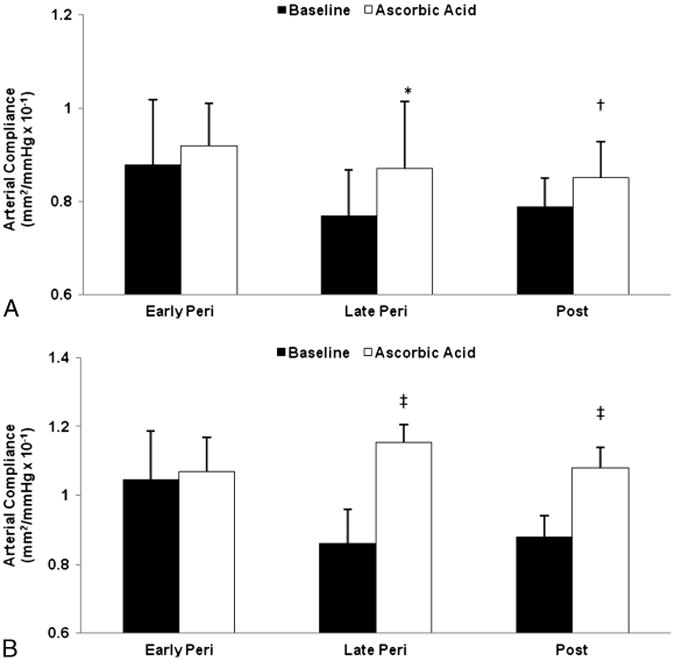
Carotid artery compliance computed with brachial blood pressure (A) and carotid blood pressure (B) at baseline (saline) and after ascorbic acid infusion in early (n = 10; mean [SD] age, 52 [1] y) and late (n = 12; 52 [2] y) perimenopausal (Peri) and postmenopausal (Post) women (n = 21; 55 [2] y) aged 50 to 59 years. *P = 0.04 and †P = 0.08 versus saline of the same group. ‡P ≤ 0.01.
Correlations between the change in carotid artery compliance with ascorbic acid computed with brachial blood pressure and participant characteristics are presented in Table 4. Similar associations were observed with carotid artery compliance computed with carotid blood pressures, except that the change in carotid compliance was correlated with age (r = 0.43), FSH (r = 0.39), oxidized LDL (r = 0.39), DHEAS (r = −0.35), and total cholesterol (r = 0.28; all P < 0.05).
Discussion
The present study provides novel insights into potential mechanisms that may contribute to CVD risk in women. Specifically, we found that stiffening of large elastic arteries was progressively greater across the stages of menopause, beginning in early perimenopause. This process is mediated, at least in part, by oxidative stress; however, oxidative stress seems to contribute to arterial stiffening only in late perimenopausal and postmenopausal women.
Aging, perimenopause, and arterial stiffening
Arterial stiffening is a hallmark of vascular aging.20 In women, vascular aging may be exacerbated by menopause and estrogen deficiency, as indicated by greater arterial stiffening in postmenopausal women compared with age-matched premenopausal women,6 and in postmenopausal non-HT users compared with HT users.3,4 However, changes in arterial stiffness across the menopausal transition have not been well characterized. The present study provides new information on arterial stiffening during the perimenopausal years in healthy women. First, carotid artery compliance computed with brachial blood pressures was reduced by 25%, 31%, and 43% in early perimenopausal, late perimenopausal, and postmenopausal women, respectively, compared with premenopausal women. Similar rates of decline were observed with carotid artery compliance computed with local carotid arterial pressures, except that the rates of decline were similar in late perimenopausal and postmenopausal women (32% and 35%, respectively). Second, the association of menopause stage with reduced carotid artery compliance remained significant after adjusting for adiposity, blood pressure, and blood lipids. To our knowledge, the only other data on arterial stiffening in perimenopausal women are those from the Study of Women's Health Across the Nation Heart Study11 and from O'Neill et al,21 neither of which found elevations in pulse wave velocity across the menopausal transition after adjusting for age. In the present study, adjusting for age abolishes the effect of menopause stage; however, this finding must be interpreted cautiously because of the strong correlation between age and menopause stage (r = 0.84). Indeed, adjusting for menopausal stage also abolishes the effect of age. We cannot rule out that the reduced carotid artery compliance in perimenopausal and postmenopausal women is attributable to age. However, the possibility that the loss of ovarian function mediates changes in vascular function is supported by (1) the associations of reduced carotid artery compliance with higher FSH and lower estradiol levels characteristic of the menopausal transition and (2) the larger rates of decline in carotid artery compliance from premenopausal levels in late perimenopausal women compared with early perimenopausal women of similar age. Because it is impossible to uncouple the tight association between age and menopause in cross-sectional comparisons of women in different stages of menopause, investigations that use an intervention approach to isolate the role of ovarian hormones will be necessary to address this issue.
Oxidative stress and arterial stiffening
Oxidative stress represents an imbalance between the production and the destruction of ROS and is a phenotypic characteristic of aging. Menopause is associated with a greater burden of oxidative stress, as indicated by higher levels of oxidative stress markers and lower levels of endogenous antioxidants in estrogen-deficient postmenopausal women compared with premenopausal women,10,22,23 and reductions in oxidative stress makers in postmenopausal HT users compared with nonusers.24,25 Estrogen is a potent antioxidant that seems to influence the balance of oxidant and antioxidant forces. Estrogen can neutralize excess ROS and inhibit lipid peroxidation via the phenolic hydroxyl group present on the estrogen molecule.26,27 Thus, declines in ovarian hormones during the menopausal transition may tip the oxidant/antioxidant balance toward oxidative stress, with negative consequences for cardiovascular health. In the present study, we found that TAS, a marker of antioxidant status, tended to be reduced across the stages of menopause and that oxidized LDL was inversely correlated with estradiol concentrations (r = 0.31, P = 0.002) and carotid artery compliance. Moreover, immediately countering oxidative stress with a pharmacological dose of the antioxidant ascorbic acid increased carotid artery compliance in late perimenopausal and postmenopausal women, but not in early perimenopausal or premenopausal women. Similar responses were observed in early perimenopausal, late perimenopausal, and postmenopausal women aged 50 to 59 years. These data are consistent with our previous work, which demonstrated a 26% increase in carotid artery compliance in response to ascorbic acid in estrogen-deficient postmenopausal women but no change in premenopausal women.10 The present study extends this work to the stages of menopause and suggests that effects of oxidative stress on arterial stiffness manifest with declines in ovarian function in the late perimenopausal and postmenopausal years.
In this study, the observed improvements in carotid artery compliance with immediate ascorbic acid infusion in late perimenopausal and postmenopausal women were probably attributable to effects on the nitric oxide (NO) modulation of vascular smooth muscle vasodilatory tone, as opposed to structural alterations in the arterial wall. Endothelium-derived NO is a potent modulator of arterial stiffness.28 ROS can decrease NO bioavailability by suppressing NO synthesis and scavenging NO, consequently increasing the tonic state of vascular smooth muscle cell contraction.29 When infused at supraphysiological concentrations, the potent water-soluble antioxidant ascorbic acid scavenges superoxide anion, preventing its interaction with NO.19 Immediate infusions of ascorbic acid have been shown to increase NO bioavailability and endothelial-vasodilatory function in older adults.30 Ascorbic acid could also increase NO bioavailability by counteracting inflammation-induced oxidative stress or vice versa and/or by stabilizing the NO-producing enzyme endothelial NO synthase (eNOS) by recycling its essential co-factor, tetrahydrobiopterin (BH4).31,32 In the present study, plasma CRP was reduced during the ascorbic acid infusion in all groups; previous work has demonstrated decreases in plasma isoprostanes with ascorbic acid infused at similar concentrations.33
The mechanisms by which oxidative stress contributes to arterial stiffening in late perimenopausal and postmenopausal women are speculative. The fact that ascorbic acid only improved carotid artery compliance in these two groups could reflect reduced NO bioavailability and increased vascular smooth muscle cell contraction because of diminished antioxidant defenses with declines in estrogen concentrations during late perimenopause and postmenopause. Our recent work demonstrating more pronounced reductions in endothelium-dependent vasodilatory tone in late perimenopausal women compared with early perimenopausal women, with further reductions in postmenopausal women, supports this idea. In addition, the menopausal transition, particularly late perimenopause, is associated with changes in metabolic risk factors that are known mediators of oxidative stress.35-37 In the present study, measures of adiposity and cholesterol were elevated across the menopausal transition, and exploratory analyses revealed that central adiposity, total cholesterol, and LDL cholesterol were correlated with oxidized LDL concentrations (between r = 0.37 and r = 0.61, P ≤ 0.001) and with the increase in carotid artery compliance with ascorbic acid. Thus, adverse changes in CVD risk factors during the late perimenopausal to postmenopausal periods may represent an independent amplifier of oxidative stress and arterial stiffening in women.
Experimental considerations and limitations
In the present study, ascorbic acid infusion did not restore carotid artery compliance in perimenopausal or postmenopausal women to premenopausal levels. The reasons for this are unclear. One potential explanation is incomplete suppression of ROS. However, plasma ascorbic acid concentrations measured during the saline and ascorbic acid infusions in eight women (two per group) revealed similar concentrations (∼ 18 × basal levels) that have been reported to immediately lower plasma isoprostanes in healthy older men.33 Moreover, TAS concentrations increased twofold with the ascorbic acid infusion. Another potential explanation is involvement of other sources of ROS not scavenged by ascorbic acid. For example, ascorbic acid is a relatively weak scavenger of peroxynitrite,38 a strong oxidant that can damage lipids, DNA, proteins, and mitochondria, leading to cell death. Peroxynitrite can also oxidize BH4, causing eNOS uncoupling and reduced NO. Recent work from our laboratory suggests that BH4 deficiency is mechanistically linked to reduced endothelial vasodilatory tone and arterial stiffening in estrogen-deficient postmenopausal women.39 Although ascorbic acid can stabilize eNOS by recycling oxidized BH4, BH4 reacts with peroxynitrite 6 to 10 times faster than ascorbic acid. Thus, ascorbic acid may not have improved carotid artery compliance in early perimenopausal women or fully restored it in late perimenopausal and postmenopausal women because ascorbic acid did not fully protect BH4 from oxidation by peroxynitrite.
This study has some important limitations. The cross-sectional design precludes any conclusions about causality. Menopause stage was based on self-reported menstrual cycle characteristics; thus, participants may have been misclassified. However, sex hormone concentrations for each group were consistent with previously reported values.40 Furthermore, the updated Stages of Reproductive Aging Workshop + 10 menopausal staging system41 had not been released at the time of this study; therefore, menopause stage was based on older criteria, and we did not collect bleeding/flow patterns or endocrine measures other than FSH to further characterize the women. Participants were primarily white, healthy, nor-motensive, nonsmoker, and sedentary or recreationally active. Whether similar findings would be observed in other populations requires further study. It is important to emphasize that the aim of the present study was to investigate the mechanistic role of oxidative stress in arterial stiffening across the stages of menopause, not to determine the efficacy of ascorbic acid as a potential therapeutic intervention. Clinical trial data demonstrate no effect of vitamin C on CVD outcomes,42 which may reflect the inability of oral vitamin C supplementation to maintain plasma ascorbic acid concentrations at levels required to scavenge ROS.19,43 Indeed, improvements in endothelial function in response to immediate administration of pharmacological levels of ascorbic acid are not necessarily observed with longer-term oral supplementation.43 Finally, local carotid pressures were not available for all participants; however, we were able to demonstrate similar patterns of declines and improvements in carotid artery compliance with ascorbic acid computed with brachial pressures.
Conclusions
Despite major advances in prevention and treatment, CVD continues to be the leading cause of death among women in developed countries. The etiology of CVD in women is particularly complex because many of the age-associated risk factors for CVD occur during the menopausal transition, when women are experiencing profound changes in hormone levels. The current study suggests that the perimenopausal period, characterized by a changing hormonal milieu and menstrual cycle irregularities, is a potentially critical time during which interventions may be most effective. However, whether change in menopause status or chronological age was the primary determinant of changes in arterial stiffening cannot be determined. Future studies should investigate whether pharmacological or nonpharmacological interventions that maintain estrogen levels and/or mitigate oxidative stress can attenuate arterial stiffening and reduce cardiovascular events in women.
Supplementary Material
Acknowledgments
We thank Chelsea Bergman, Lauren Tobin, and Tracy Swibas for their technical assistance.
Funding/support: This work was supported by the National Institutes of Health (awards R01 AG027678 and K01 AG20683), the Colorado Clinical and Translational Sciences Institute (grant UL1 TR000154), the Colorado Nutrition Obesity Research Center (grant P30 DK048520), and the University of Colorado Center for Women's Health Research.
Footnotes
Financial disclosure/conflicts of interest: None reported.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.menopause.org).
References
- 1.Lakatta EG. Cardiovascular aging in health. Clin Geriatr Med. 2000;16:419–444. doi: 10.1016/s0749-0690(05)70021-5. [DOI] [PubMed] [Google Scholar]
- 2.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 3.Moreau KL, Donato AJ, Seals DR, DeSouza CA, Tanaka H. Regular exercise, hormone replacement therapy and the age-related decline in carotid arterial compliance in healthy women. Cardiovasc Res. 2003;57:861–868. doi: 10.1016/s0008-6363(02)00777-0. [DOI] [PubMed] [Google Scholar]
- 4.Nagai Y, Earley CJ, Kemper MK, Bacal CS, Metter EJ. Influence of age and postmenopausal estrogen replacement therapy on carotid arterial stiffness in women. Cardiovasc Res. 1999;41:307–311. doi: 10.1016/s0008-6363(98)00219-3. [DOI] [PubMed] [Google Scholar]
- 5.Smulyan H, Asmar RG, Rudnicki A, London GM, Safar ME. Comparative effects of aging in men and women on the properties of the arterial tree. J Am Coll Cardiol. 2001;37:1374–1380. doi: 10.1016/s0735-1097(01)01166-4. [DOI] [PubMed] [Google Scholar]
- 6.Westendorp IC, Bots ML, Grobbee DE, et al. Menopausal status and distensibility of the common carotid artery. Arterioscler Thromb Vasc Biol. 1999;19:713–717. doi: 10.1161/01.atv.19.3.713. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Miura S, Mori-Abe A, et al. Impact of menopause on the augmentation of arterial stiffness with aging. Gynecol Obstet Invest. 2005;60:162–166. doi: 10.1159/000086570. [DOI] [PubMed] [Google Scholar]
- 8.Messerli FH, Garavaglia GE, Schmieder RE, Sundgaard-Riise K, Nunez BD, Amodeo C. Disparate cardiovascular findings in men and women with essential hypertension. Ann Intern Med. 1987;107:158–161. doi: 10.7326/0003-4819-107-2-158. [DOI] [PubMed] [Google Scholar]
- 9.Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. 1986;111:383–390. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]
- 10.Moreau KL, Gavin KM, Plum AE, Seals DR. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension. 2005;45:1107–1112. doi: 10.1161/01.HYP.0000165678.63373.8c. [DOI] [PubMed] [Google Scholar]
- 11.Woodard GA, Mehta VG, Mackey RH, et al. C-reactive protein is associated with aortic stiffness in a cohort of African American and white women transitioning through menopause. Menopause. 2011;18:1291–1297. doi: 10.1097/gme.0b013e31821f81c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soules MR, Sherman S, Parrott E, et al. Stages of Reproductive Aging Workshop (STRAW) J Womens Health Gender Based Med. 2001;10:843–848. doi: 10.1089/152460901753285732. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 14.Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986;74:1399–1406. doi: 10.1161/01.cir.74.6.1399. [DOI] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 16.Moreau KL, Depaulis AR, Gavin KM, Seals DR. Oxidative stress contributes to chronic leg vasoconstriction in estrogen-deficient postmeno-pausal women. J Appl Physiol. 2007;102:890–895. doi: 10.1152/japplphysiol.00877.2006. [DOI] [PubMed] [Google Scholar]
- 17.Moreau KL, Gavin KM, Plum AE, Seals DR. Oxidative stress explains differences in large elastic artery compliance between sedentary and habitually exercising postmenopausal women. Menopause. 2006;13:951–958. doi: 10.1097/01.gme.0000243575.09065.48. [DOI] [PubMed] [Google Scholar]
- 18.Moreau KL, Gavin KM, Plum AE, Seals DR. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension. 2005;45:1107–1112. doi: 10.1161/01.HYP.0000165678.63373.8c. [DOI] [PubMed] [Google Scholar]
- 19.Jackson TS, Xu A, Vita JA, Keaney JF., Jr Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res. 1998;83:916–922. doi: 10.1161/01.res.83.9.916. [DOI] [PubMed] [Google Scholar]
- 20.Seals DR, Moreau KL, Gates PE, Eskurza I. Modulatory influences on ageing of the vasculature in healthy humans. Exp Gerontol. 2006;41:501–507. doi: 10.1016/j.exger.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 21.O'Neill SM, Liu J, O'Rourke MF, Khoo SK. The menopausal transition does not appear to accelerate age-related increases in arterial stiffness. Climacteric. 2013;16:62–69. doi: 10.3109/13697137.2012.739220. [DOI] [PubMed] [Google Scholar]
- 22.Bednarek-Tupikowska G, Bohdanowicz-Pawlak A, Bidzinska B, Milewicz A, Antonowicz-Juchniewicz J, Andrzejak R. Serum lipid peroxide levels and erythrocyte glutathione peroxidase and superoxide dismutase activity in premenopausal and postmenopausal women. Gynecol Endocrinol. 2001;15:298–303. [PubMed] [Google Scholar]
- 23.Signorelli SS, Neri S, Sciacchitano S, et al. Behaviour of some indicators of oxidative stress in postmenopausal and fertile women. Maturitas. 2006;53:77–82. doi: 10.1016/j.maturitas.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Ozden S, Dildar K, Kadir YH, Gulizar K. The effects of hormone replacement therapy on lipid peroxidation and antioxidant status. Maturitas. 2001;38:165–170. doi: 10.1016/s0378-5122(00)00216-4. [DOI] [PubMed] [Google Scholar]
- 25.Leal M, Diaz J, Serrano E, Abellan J, Carbonell LF. Hormone replacement therapy for oxidative stress in postmenopausal women with hot flushes. Obstet Gynecol. 2000;95:804–809. doi: 10.1016/s0029-7844(00)00822-x. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Lata K, Mukhopadhyay S, Mukherjee TK. Role of estrogen receptors in pro-oxidative and anti-oxidative actions of estrogens: a perspective. Biochim Biophys Acta. 2010;1800:1127–1135. doi: 10.1016/j.bbagen.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Sugioka K, Shimosegawa Y, Nakano M. Estrogens as natural antioxidants of membrane phospholipid peroxidation. FEBS Lett. 1987;210:37–39. doi: 10.1016/0014-5793(87)81293-0. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson IB, Qasem A, McEniery CM, Webb DJ, Avolio AP, Cockcroft JR. Nitric oxide regulates local arterial distensibility in vivo. Circulation. 2002;105:213–217. doi: 10.1161/hc0202.101970. [DOI] [PubMed] [Google Scholar]
- 29.Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res. 1999;43:562–571. doi: 10.1016/s0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 30.Taddei S, Virdis A, Ghiadoni L, et al. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 31.Pleiner J, Mittermayer F, Schaller G, MacAllister RJ, Wolzt M. High doses of vitamin C reverse Escherichia coli endotoxin-induced hy-poreactivity to acetylcholine in the human forearm. Circulation. 2002;106:1460–1464. doi: 10.1161/01.cir.0000030184.70207.ff. [DOI] [PubMed] [Google Scholar]
- 32.Heller R, Unbehaun A, Schellenberg B, Mayer B, Werner-Felmayer G, Werner ER. l-Ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem. 2001;276:40–47. doi: 10.1074/jbc.M004392200. [DOI] [PubMed] [Google Scholar]
- 33.Bell C, Jones PP, Seals DR. Oxidative stress does not modulate metabolic rate or skeletal muscle sympathetic activity with primary aging in adult humans. J Clin Endocrinol Metab. 2003;88:4950–4954. doi: 10.1210/jc.2003-030454. [DOI] [PubMed] [Google Scholar]
- 34.Moreau KL, Hildreth KL, Meditz A, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab. 2012;97:4692–4700. doi: 10.1210/jc.2012-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wildman RP, Colvin AB, Powell LH, et al. Associations of endogenous sex hormones with the vasculature in menopausal women: the Study of Women's Health Across the Nation (SWAN) Menopause. 2008;15:414–421. doi: 10.1097/gme.0b013e318154b6f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matthews KA, Kuller LH, Sutton-Tyrrell K, Chang YF. Changes in cardiovascular risk factors during the perimenopause and postmeno-pause and carotid artery atherosclerosis in healthy women. Stroke. 2001;32:1104–1111. doi: 10.1161/01.str.32.5.1104. [DOI] [PubMed] [Google Scholar]
- 37.Vassalle C, Petrozzi L, Botto N, Andreassi MG, Zucchelli GC. Oxidative stress and its association with coronary artery disease and different ath-erogenic risk factors. J Intern Med. 2004;256:308–315. doi: 10.1111/j.1365-2796.2004.01373.x. [DOI] [PubMed] [Google Scholar]
- 38.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 39.Moreau KL, Meditz A, Deane KD, Kohrt WM. Tetrahydrobiopterin improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Am J Physiol Heart Circ Physiol. 2012;302:H1211–H1218. doi: 10.1152/ajpheart.01065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hale GE, Zhao X, Hughes CL, Burger HG, Robertson DM, Fraser IS. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the Staging of Reproductive Aging Workshop (STRAW) Staging System. J Clin Endocrinol Metab. 2007;92:3060–3067. doi: 10.1210/jc.2007-0066. [DOI] [PubMed] [Google Scholar]
- 41.Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97:1159–1168. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waters DD, Alderman EL, Hsia J, et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: a randomized controlled trial. JAMA. 2002;288:2432–2440. doi: 10.1001/jama.288.19.2432. [DOI] [PubMed] [Google Scholar]
- 43.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.