Abstract
Iron deficiency (ID) in rodents leads to decreased ventral midbrain (VMB) iron concentrations and to changes in the dopamine (DA) system that mimic many of the dopaminergic changes seen in RLS patient where low substantia nigra iron is a known pathology of the disease. The ID-rodent model, therefore, has been used to explore the effects that low VMB iron can have on striatal DA dynamics with the hopes of better understanding the nature of iron-dopamine interaction in Restless Legs Syndrome (RLS). Using a post-weaning, diet-induced, ID condition in rats, the No-Net-Flux microdialysis technique was used to examine the effect of ID on striatal DA dynamics and it reversibility with acute infusion of physiological concentrations of iron into the VMB. This study replicated prior findings by showing that the ID condition is associated with increased extracellular striatal DA, reduced striatal DA uptake, and blunted DA-2-receptor-agonist feedback enhancement of striatal DA uptake. Despite the increase in extracellular striatal DA, intracellular striatal DA, as determined in tissue homogenates, was decrease in the ID rat. The study’s key finding was that an infusion of physiological concentrations of iron into the VMB reversed the ID-induced increase in extracellular striatal DA and the ID-induced decrease in intracellular striatal DA but had no effect on the ID-induced changes in DA uptake or on the blunted DA-uptake response to quinpirole. In summary, the ID-rodent model provides highly reproducible changes in striatal DA dynamics that remarkably parallel dopaminergic changes seen in RLS patients. Some but not all of these ID-induced changes in striatal DA dynamics were reversible with physiological increases in VMB iron. The small changes in VMB iron induced by iron infusion likely represent biologically relevant changes in the non-transferrin-bound labile iron pool and may mimic circadian-dependent changes that have been found in VBM extracellular iron.
Keywords: restless legs syndrome, iron deficiency, ventral midbrain, striatum, microdialysis, dopamine
1. Introduction
Restless Legs Syndrome (RLS) is a neurological disease with well defined pathology (Earley, 2003). Brain autopsy, MRI and ultrasound studies have demonstrated reduced iron stores in substantia nigra (Allen, et al., 2001; Connor, et al., 2003; Godau, et al., 2007; Rizzo, et al., 2013; Schmidauer, et al., 2005). Given the sensitivity of the RLS symptoms to levodopa and to dopamine agonists (Earley, 2003) and the fact that dopamine neurons in the substantia nigra have low iron levels (Connor, et al., 2003), RLS studies have also focused on the dopaminergic (DAergic) system and its relation to low substantia nigra iron. Both autopsy assessment and PET imaging have revealed changes in the striatal DAergic system (Connor, et al., 2009; Earley, et al., 2011; Earley, et al., 2013). Therefore to better understand the nature of iron-dopamine interaction in RLS, investigations have focused on understanding the effects of low substantia nigra iron on striatal DAergic dynamics.
Using a post-weaning, diet-induced iron deficiency (ID) condition in rodents to reduce VMB (i.e., substantia nigra) iron, studies have demonstrated that ID increases extracellular striatal dopamine (DA) (Beard, et al., 1994), decreases striatal dopamine-2 receptors (D2R) density (Erikson, et al., 2001) and diminishes in vitro dopamine transporter (DAT) density and function but not DA release (Erikson, et al., 2000). Similar to the findings in the ID rodent, RLS striatal dopaminergic pathology has shown diminished D2R receptor (Connor, et al., 2009), decreased membrane-bound DAT (Earley, et al., 2011) and increased synaptic DA levels (Earley, et al., 2013). Thus the post-weaning, diet-induced ID condition appears to provide a biological model to understanding the iron-dopamine connection in RLS.
Studies using the diet-induced, ID condition in rodents have demonstrated a strong association between VMB iron concentrations, gene expression of monoamine transporters, and regional monoamine levels (Erikson, et al., 2000; Erikson, et al., 2001). MRI studies in RLS have shown not only lower iron concentrations in the substantia nigra but also that iron concentrations correlated negatively with RLS symptoms severity (Lee, et al., 2007). This finding suggests that small variations in substantia nigra iron concentrations may be pertinent to, at least, disease severity. It has been shown in mice that small but significant changes in extracellular VMB iron occurs in a circadian manner (Unger, et al., 2013). The physiological significance of these small circadian variations in VMB iron is unknown because studies using low physiological concentrations of iron have not been done. Understanding how small physiological changes in extracellular iron, similar to those found with circadian variation, impact DAergic function may be pertinent to our understanding of the role of iron dynamics in influencing DA dynamics in RLS, especially given the distinct circadian nature of RLS symptoms. The primary goals of the current study were to validate and extend previous findings on changes in the striatal DAergic system under ID conditions and to examine the effects of perfusing physiological concentrations of iron directly into the VMB on these ID-induced changes in striatal DA dynamics.
2. Materials and Methods
2.1 Animals
Male weanling (postnatal day 21) Sprague-Dawley rats (Harlan, Indianapolis IN) were randomly divided into a control group fed an iron-sufficient diet (80 µg iron/g diet) and an ID group fed a low iron diet (4 µg iron/g diet). The 4 µg iron/g diet maintained for 5-6 weeks has been shown in prior studies to produce a decrease in VMB iron in Sprague-Dawley rats (Bianco, et al., 2008; Unger, et al., 2008). Control and ID diets were prepared in our laboratory as described previously (Reeves, et al., 1993). The ID diet contained all components of the control diet with the exception of ferric citrate. Rats consumed control or ID diets for 5 weeks, and were provided food and deionized distilled water ad libitum throughout the study. All rats were housed 2 per cage in a temperature (21 ±2°C) and humidity (40%) controlled room maintained on a 12:12 hr light/dark cycle (lights on at 0600) until surgeries were performed. Post-surgery, rats were housed 1 per cage and continued on a 12:12 hr light/dark cycle throughout the microdialysis experiments. Experimental protocols were in accordance with the NIH Animal Care guidelines and were approved by the Pennsylvania State Institutional Animal Care and Use Committee.
2.2 Surgery
Rats were anesthetized with 4% isoflurane, and the top of the head was shaved and sterilized with providone/iodine and 70% ethanol. After placement on a stereotaxic frame (Stoeltig, Wood Dale, Il), the skull was exposed and bregma identified. A hole was drilled through the skull, and a CMA 11 guide cannula was implanted into the striatum of all control and ID rats as previously described (Bianco, et al., 2008). The coordinates used were: A −0.2 mm, L −2.8 mm, and V −5.3 mm. In a subset of rats from each diet group, a second guide cannula for iron infusions was implanted in the substantia nigra following the same protocol. The coordinates used for substantia nigra were: A 2.0 mm, L −6.5mm, V −5.7 mm. All rats received a sham surgery in the region of iron infusion in the contra-lateral side of the brain.
After 3–5 day recovery time, rats were lightly anesthetized with 25.0/2.0/0.5 mg/kg ketamine/xylazine/acepromazine. A CMA 11 (2 mm) probe was then inserted into the guide cannula implanted in the striatum of all rats. In rats with a guide cannula implanted in substantia nigra, an infusion cannula (2 mm) was inserted into the guide cannula. The rats were allowed to recover overnight with a slow perfusion of artificial cerebrospinal fluid (aCSF; 1.3 µL/min) into the microdialysis probe (Bianco, et al., 2008).
2.3 NNF/Quinpirole NNF
The No-Net-Flux (NNF) microdialysis technique provides quantitative real-time in vivo determination of extracellular neurotransmitter concentrations by utilizing the principle of diffusion, which takes into account both diffusion and catabolism (Mefford, 1981; Refshauge, et al., 1974). In this study, two primary measures of extracellular DA are determined from NNF technique: extraction fraction and the extracellular DA concentration (Bungay, et al., 2003; Chefer, et al., 2006). The extraction fraction has been shown to reflect primarily DAT-dependent neurotransmitter uptake (Smith and Justice, 1994) and has been used as an estimate of DAT function (Bianco, et al., 2008). For greater details of the NNF technique and its application, see our prior publication (Bianco, et al., 2008) but in brief, artificial CSF samples containing 100, 50, 20, and 10 nM DA were infused into the brain via the microdialysis probe as described previously (Bianco, et al., 2008 ; Chefer, et al., 2006). Each concentration of DA was perfused for 75 min, with a random ordering for each rat. The perfusate was collected continuously into a loading loop on the HPLC and injected in 15 min intervals. After the last concentration of DA for NNF was perfused, a potent DA-2/3 receptor agonist, quinpirole (5 µM), was added to the perfusate. NNF was performed after equilibration (1.5 hr) using 100, 50, 20, and 10 nM DA + 5 µM quinpirole in artificial CSF. This equilibration time for quinpirole was chosen based on previous studies, which demonstrated that the maximal effect of quinpirole occurs 1-2 hr after perfusion is initiated (Mao, et al., 1996; See, et al., 1991). Quinpirole through its agonist effects on DA-2/3 receptors and subsequent feedback to pre-synaptic mechanism will decrease DA release and increase membrane-bound DAT and extraction of DA from the extracellular space (Bolan, et al., 2007; Cooper, et al., 2008; Robinson, 2002). Used within the context of the NNF techniques, it provides an assessment of the integrity of the feedback-loop mechanism that can modify pre-synaptic functions like DAT density and DA release.
2.4 Iron Infusion
Ferrous sulfate (FeSO4, 1 µM) was infused as described previously at a rate used in previous studies (Prikhojan, et al., 2002; Santiago, et al., 2000) of 1.3 µL/min for 20 min resulting in the delivery of 26 picomole (pmol) of iron into the extracellular space. This concentration of extracellular iron is within the physiological range seen with the circadian peak-to-trough differences in extracellular VMB iron (Unger, et al., 2013). In a previous study, infusion of 10-100 µM FeCl3 into the striatum did not cause lipid peroxidation or affect neuronal health (Santiago, et al., 2000). The infusion of iron into the substantia nigra was started at 2400 hr and into the striatum at 0600 hr, with the NNF/Quinpirole NNF experiment being performed starting at 0600 hr. In the contra-lateral brain region, sterile saline was perfused under the same conditions. As has been previously described (Bianco, et al., 2008; Connor, et al., 1992), verification of cannula placement and distance of iron diffusion was determined with cytology and iron histochemistry, respectively. The distance of iron diffusion was on average about 350 µm from the infusion site.
2.5 High Performance Liquid Chromatography
Dialysate samples (10 µl) were injected every 15 min onto an ESA MD-150 narrow-bore HPLC column 150×2 mm (ESA Inc., Chelmsford, MA) for separation followed by detection by an ESA 5014B microdialysis cell (+300 mV; ESA Coulochem III, ESA, Inc., Chelmsford, MA). A guard cell (ESA 5020) placed in line before the injection loop was set at a potential of +350 mV. The mobile phase consisted of 75 mM sodium phosphate monobasic (EMD Chemical, Gibbstown, NJ), 1.7 mM 1-octanesulfonic acid (EMD Chemical), 25 µM EDTA (Acros, Morris Plains, NJ), 10% acetonitrile (EMD), and 0.01% triethylamine (Sigma Aldrich, St. Louis, MO) in a volume of 1L (pH 3.0). The neurotransmitter and metabolite peak areas were integrated using EZ Chrom Elite software (Scientific Software Inc, Pleasanton, CA) and quantified against known standards of dopamine (DA) (ESA Inc., Chelmsford, MA), DA (ESA Inc.), 3,4-dihydroxyphenylacetic acid (DOPAC; Sigma Aldrich), and homovanillic acid (HVA; Sigma Aldrich).
2.6 Tissue collection and hematology
Brains were removed from the skull and dissected for striatum and VMB on ice. Brain regions were stored at −80°C until prepared for iron or monoamine analysis. Blood was collected at sacrifice into heparinized microcapillary tubes and used for the determination of hemoglobin (Hgb) and hematocrit (Hct). The Hgb was determined photometrically using cyanomethemoglobin standard solution as described by the manufacturer (Sigma Aldrich, St. Louis MO) and the Hct was calculated after centrifugation of blood samples (9000×g, RT, 10 min).
2.7 Tissue preparation
The striatum (caudate-putamen) and VMB were homogenized in cold phosphate buffered saline (PBS; 9.1 mmol/L Na2HPO4, 1.7 mmol/L NaH2PO4, 150 mmol/L NaCl, pH 7.4) with protease inhibitors (Roche, Mannheim Germany). The resulting homogenates were aliquoted for iron analysis by atomic absorption spectroscopy and catecholamine analysis by HPLC (Bianco, et al., 2008; Unger, et al., 2007).
2.8 Lipid Peroxidation
Lipid peroxidation was determined in brain region homogenates by thiobarbituric acid reactive substances (TBARS) assay as described by (Ohkawa, et al., 1979). TBARS levels in samples were measured at an absorbance of 532 nm and calculated using malondialdehyde standards.
2.9 Statistical Analysis
The first hypothesis to be tested in this study was that ID-diet, which produces low VMB iron, will result in altered striatal dopaminergic dynamics. The dietary condition (normal vs iron-deficient) was the independent variable and primary dependent variables were the measures of striatal dopaminergic dynamics: extracellular DA, DA extraction from the extracellular space and the response of these two factors to quinpirole. Total striatal concentration of DA and its metabolites were used as a secondary measure of striatal dopaminergic dynamic. Our second hypothesis was that small but physiological increases in VMB extracellular iron can reverse the changes in striatal DAergic function seen with diet-induced, low brain iron. Infusion of iron into the striatum was exploratory. Data are presented as means ±SEM. One-sided, t-test (Microsoft, Excel) was used to test the effect of ID diet on decreasing Hgb, Hct, VMB iron and striatal iron. ANOVA was used to test the effects of VMB iron infusion and diet condition. Diet effects were also tested by one-way ANOVA for iron treatment and brain region. A two-way ANOVA with Tukey’s post-hoc test was used to analyze the microdialysis data (SYSTAT, Systat Software INC, Richmond CA).
3. Results
The ID diet, as expected, resulted in significantly reduced hemoglobin (Control vs ID: 144 ±3 g/l vs 63 ±6 g/l, p < 0.001), hematocrit (Control vs ID: 43.0 ±0.01% vs 22.4 ±0.02%, p < 0.001) and total iron concentration in VMB (Control vs ID: 24.9 ±2.5 µg iron/g tissue vs 16.0 ±1.2 µg iron/g tissue, p < 0.01) and striatum (Control vs ID: 24.3 ±3.6 µg iron/g tissue vs 18.6 ±1.4 µg iron/g tissue, p < 0.05). Data are presented as means ±sem with N=12-13 rats/group in Table 1.
Table 1.
Hemoglobin (Hgb), hematocrit (Hct) and brain iron levels from control and iron deficient (ID) rats.
| Hgb (g/l) |
Hct (%) |
VMB Iron (µg/g) |
Striatum Iron (µg/g) |
|
|---|---|---|---|---|
| Control | 144 ± 3 | 43.0 ± 0.01 | 24.9 ± 2.5 | 24.3 ± 3.6 |
| ID | 63 ± 6c | 22.4 ± 0.02c | 16.0 ± 1.2b | 18.6 ± 1.4a |
Data are presented as means ±SEM.
p<0.05,
p<0.01,
p<0.001 relative to control diet fed rats. N=12 – 13 rats/group.
3.1 Effects of diet and VMB iron infusion on striatal dopaminergic dynamics
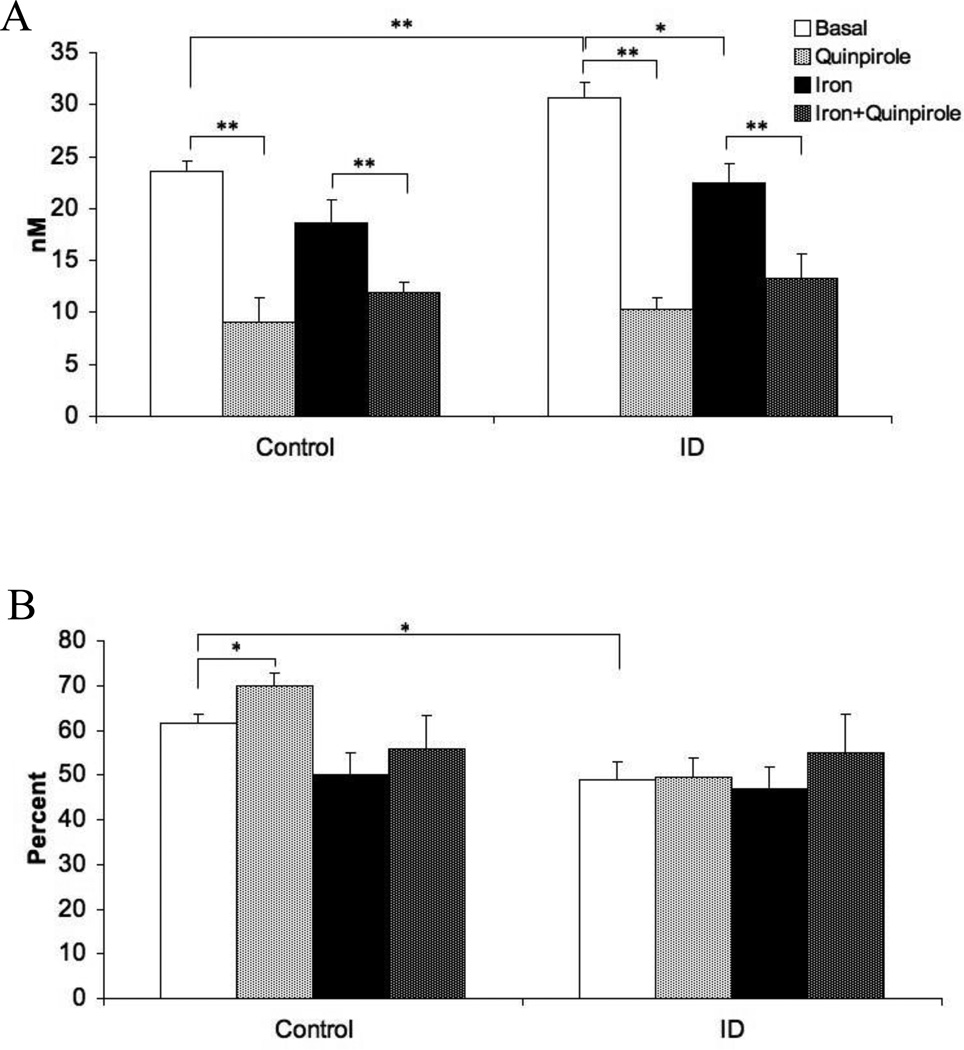
For extracellular dopamine levels, there were significant main effects for diet (F=15.1, p < 0.05) and for iron treatment (F=21.8, p < 0.01). The ID group had elevated extracellular striatal DA compared to controls (Figure 1A: Basal-Control vs Basal-ID, p < 0.01) and iron infusion in the VMB reduced extracellular DA in both diet groups (Figure 1A: Basal vs Iron, p < 0.05). The addition of quinpirole to the perfusate, as a test of the feedback-loop dynamics, produced the predicted drop in extracellular dopamine independent of diet (Figure 1A: F=71.8, p < 0.01). The interactions between diet and VMB iron infusion and between diet and quinpirole challenge were not significant (F= 1.21, p=0.5; F= 1.98, p=0.09, respectively), indicating that both diet groups had similar responses to iron infusion and to the quinpirole challenge (Figure 1A).
Figure 1.
(A) Extracellular dopamine concentration in the striatum before and after quinpirole perfusion into the striatum and before and after iron infusion into the midbrain. (B) Extraction fraction of extracellular DA in the striatum before and after quinpirole perfusion into the striatum and before and after iron infusion into the midbrain. Measurements were collected by in vivo microdialysis in control and iron deficient (ID) rats. Data are presented as means ± sem of 6-7 rats per group. Significance is denoted as *p < 0.05, **p < 0.01
For extraction fraction, the main effect for diet (F= 24.32, p < 0.01), as well as the interaction between diet and VMB iron infusion (F=12.74, p < 0.05), were significant. Iron deficiency caused a reduction in extraction fraction relative to controls (Figure 1B, Basal-Control vs Basal-ID, p < 0.05). The VMB iron infusion had, however, no overall significant effect on the extraction fraction (F=7.01, p=0.08). Although the main effect of the quinpirole challenge was not significant (F=5.36, p=0.09), there was a strong diet-by-quinpirole interaction (F=27.83, p < 0.01). The primary effect in this interaction appears to be the increase in extraction fraction in control (Figure 1B: Basal vs Quinpirole, p < 0.05) but not ID rats (Figure 1B: Iron-ID and Iron-Quinpirole-ID) in response to quinpirole perfusion.
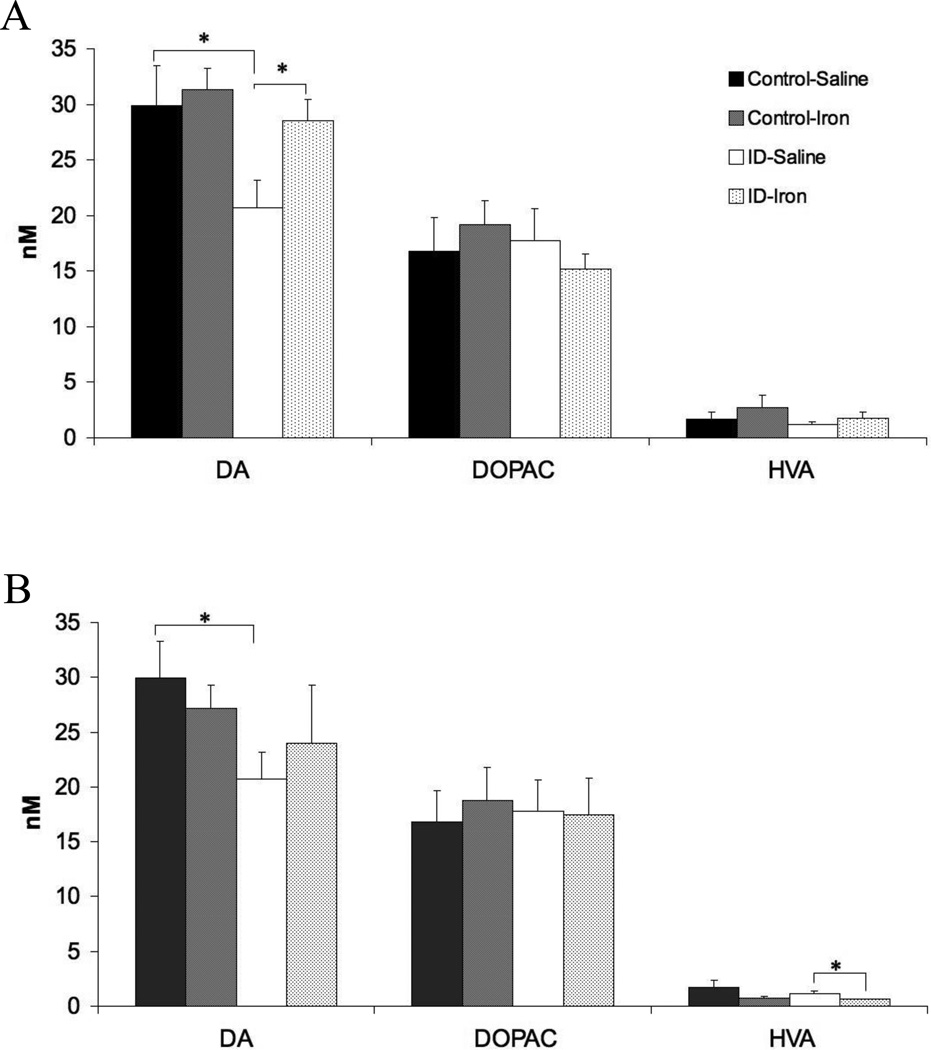
Tissue concentrations of DA, DOPAC, and HVA were measured in homogenates derived from brain tissue 24 hours after striatal DA dynamics were assessed. The intracellular striatal concentration of DA was decreased in the ID group (Figure 2: Control-Saline vs ID-Saline, p < 0.05) while there were no differences seen between the Control and ID groups for striatal DOPAC and HVA. Iron infusion into the VMB 30 hours before assessing striatal DA and its metabolites resulted in a reversal of ID-induce decrease in intracellular striatal DA (Figure 2). Thus small physiological increases in VMB extracellular iron can have sustain effects on striatal DA dynamics. This long-term change in striatal intracellular DA appears to differ from the VMB DA dynamics, as VMB iron infusion in ID animals did not change intracellular DA or its metabolites in the VMB (data not shown).
Figure 2.
Dopamine (DA), DOPAC, and HVA concentrations in the striatum (A) 30 hours after VMB iron infusion or (B) 24 hours after striatal iron infusion. Tissue levels were determined in tissue homogenates and analyzed via HPLC. Data are presented as means ± sem of 6-7rats per group. Significance is denoted as *p < 0.05, **p < 0.01
3.2 Effects of diet and striatal iron infusion on striatal dopaminergic dynamics
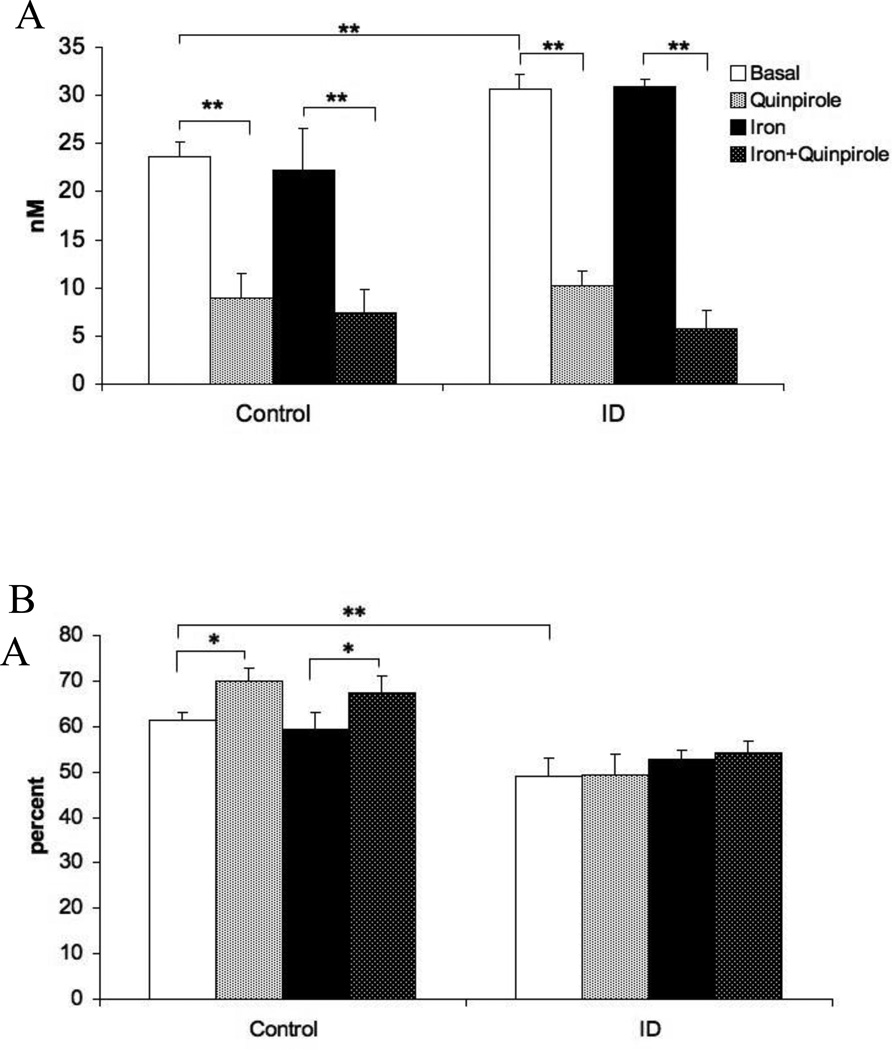
Analysis of extracellular dopamine levels revealed a significant main effect of diet (Figure 3A: F=34.4, p < 0.05). The main effect of iron infusion in the striatum was not significant (Figure 3A: Basal vs Iron, F=0.18, p=0.84), nor was the interaction between diet and iron infusion (F=0.41, p=0.76). The main effect of the quinpirole challenge was also significant (Figure 3A: F=75.7, p < 0.001) showing that quinpirole did reduce striatal extracellular dopamine, however, both control and ID rats responded similarly (F=2.95, p=0.07).
Figure 3.
(A) Extracellular dopamine concentration in the striatum before and after quinpirole perfusion into the striatum and before and after iron infusion into the striatum. (B) Extraction fraction of extracellular DA in the striatum before and after quinpirole perfusion into the striatum and before and after iron infusion into the striatum. The basal and quinpirole conditions for Control and ID groups displayed in this figure are the same as in figure 1. Measurements were collected by in vivo microdialysis in control and iron deficient (ID) rats. Data are presented as means ± sem of 6-7 rats per group. Significance is denoted as *p < 0.05, **p < 0.01
For extraction fraction, there was a significant main effect of diet (F=42.2, p < 0.01), supporting the diminished DA-extraction capability found in the ID group (Figure 3B). Striatal iron infusion had no effect on striatal extraction fraction irrespective of diet (Figure 3B; F=0.44, p=0.72). The diet-by-iron-infusion interaction was also not significant (F=3.64, p=0.31). The main effect of quinpirole treatment was significant (F=6.11, p < 0.05), but the interaction of quinpirole and diet was not significant (F=3.63, p=0.10).
Iron infusion into the striatum of the ID group did not significantly alter intracellular striatal, DOPAC but did reduce HVA compared to the vehicle-infused striatum (Figure 4: ID-saline vs ID-Iron, p < 0.05). No changes in striatal DA, HVA, or DOPAC were seen in the Control group with striatal iron infusion (Figure 4). Thus, iron infusion into the striatum did not reverse any of the four effects seen with ID on the dopamine dynamics in the striatum. Striatal lipid peroxidation levels, obtained 24 hr after the infusion, did not differ between vehicle- and iron-infused striatum (Data not shown).
4. Discussion
The ID-rodent model has been used extensively to explore the relation between brain iron status and dopamine system (Beard and Connor, 2003). ID diet also produces peripheral organ (e.g., liver and spleen) iron deficiency and may also produce an anemia in some animals (Yin, et al., 2012), thus raising concerns as to the relevance of peripheral ID effects on the ID effects found in brain. Studies utilizing a broad range of BXD RI mice strains under normal and ID condition have not identified any significant relation between peripheral iron status and VMB iron status (Jellen, et al., 2012; Yin, et al., 2012). In cell culture models, where the issue of anemia or altered peripheral metabolism are not operational, changes in DAergic system with diminished iron concentrations were similar to DAergic system changes seen in ID animal (Connor, et al., 2009; Unger, et al., 2008; Wiesinger, et al., 2007). Equally pertinent to the argument that the dietary-ID rodent, in which anemia is a physiological covariant, has relevance to understanding RLS pathology, is the fact the ID anemia in human populations is associated with a 6-fold increase in the prevalence of RLS (Allen, et al., 2013). More importantly, however, is the comparative nature of the findings: how similar are the findings in ID-rodent model to what has been found in RLS. Prior studies have demonstrated that the ID condition leads to a reduction VMB iron, an increase in extracellular striatal DA (Beard, et al., 1994), a decreases in striatal dopamine-2 receptors (D2R) density (Erikson, et al., 2001) and a diminishes in vitro dopamine transporter (DAT) density and function (Erikson, et al., 2000). Similar to the findings in the ID rodent, RLS show low iron in the substantia nigra, diminished D2R receptor (Connor, et al., 2009), decreased membrane-bound DAT (Earley, et al., 2011) and increased synaptic DA levels (Earley, et al., 2013). Thus the post-weaning, diet-induced ID condition in rodents appears to provide a biological model to understanding the iron-dopamine connection in RLS.
The study by Bianco et al (Bianco, et al., 2008) further advanced our understanding of the effects of ID-diet on striatal DAergic dynamic by utilizing the NNF techniques as was used in the current study. Bianco found that ID rats compared to control rats had elevated extracellular striatal DA, diminished ability to extract DA from the extracellular space, a lack of a quinpirole-induced change in extracellular DA extraction, but a preserved capacity to reduce DA release into the synapse under quinpirole challenge. These are the same results found in the current study indicating the highly reproducible nature of these ID-induced effects. Although not new findings, they do form the background for the more unique findings in this study, which is the consequence of iron infusion on these outcome variables. Therefore it is important to understand the physiological relevance of these replicated findings.
ID condition was associated with decrease in uptake of extracellular DA and an absence of quinpirole-induced increase in DA extraction. The extraction fraction as calculated using the NNF technique has been shown to reflect primarily DAT-dependent neurotransmitter uptake (Smith and Justice, 1994). Thus the decrease in DA extraction seen in the ID rats is in keeping with the know effects of low iron on decreasing membrane bound DAT (Erikson, et al., 2000; Wiesinger, et al., 2007). Studies have also demonstrated a strong correlation between striatal iron levels and densities of striatal DAT, as well as, a strong association between ventral midbrain iron concentrations, gene expression of DAT, and regional monoamine levels (Bianco, et al., 2008; Erikson, et al., 2000). ID condition was also associates with an increase in extracellular DA. It has been proposed that the increase in extracellular DA is related to the decrease in DAT function (Bianco, et al., 2008; Erikson, et al., 2001). One would have expected, however, that a DAT-dependent change in extracellular DA would be in the long term countered by receptor signaling and feedback to change the synthesis or release of DA (Cooper, et al., 2008), which as the quinpirole challenge indicted, appears to be intact. It is possible, however, that chronic agonist challenge, compared to the acute quinpirole-effects seen in this study, might reveal more dynamic ID effects on DA-receptor-release signal transduction pathway. Also in this study, we only challenged the dopamine-2/3 receptors when other DA receptors may be affected by ID conditions (Erikson, et al., 2000).
Even though extracellular DA is increased, the intracellular DA concentrations appear to be decreased relative to rats on a normal iron diet. The DA metabolites, HVA and DOPAC, were however unchanged. Without knowing the extracellular concentrations or the turnover rate of these metabolic, it is difficult to know what the relevance of these finding are to changes seen in intracellular DA. A study has evaluated changes in extracellular DA, HVA and DOPAC and found diminished concentrations of all three in ID rats after an injection of levodopa (Bianco, et al., 2009). At best that prior study supports the concept of altered pre-synaptic DA metabolism under ID conditions but gives minimal insight into why intracellular DA is decreased but HVA and DOPAC are unchanged. One potential cause for the low intracellular DA concentrations would be diminished vesicular DA storage. Systemic injection of amphetamine, commonly used to assess DA vesicular stores, resulted in a marked increase in extracellular DA, rather than the predicted decrease (Bianco, et al., 2009). The overall implication of the current and prior studies is that ID rodents have alter striatal DA metabolism and most likely at the level of pre-synaptic control of DA storage, release and re-uptake to create what has been referred to as a “hyper-dopaminergic” state as evinced by the chronic elevation extracellular DA (Bianco, et al., 2009). Importantly, a similar presynaptic “hyperdopaminergic” state with increased intrasynaptic DA and decrease in striatal membrane-bound DAT have been proposed as part of the pathology in RLS (Earley, et al., 2011; Earley, et al., 2013).
The most significant and novel aspect of this study is that small physiological increases in VMB iron can affect some of the changes in striatal DAergic dynamics associated with lower brain iron. The timing and dosing of the VMB iron infusion were based upon our primary interest in the circadian changes in VMB iron and its potential relation to striatal DA dynamic as part of our developing the ID-rodent model for RLS. As RLS symptoms have a distinct circadian presentation (worse at night and diminished in the morning) (Trenkwalder, et al., 1995) and as extracellular VMB iron also shows a distinct circadian variation with a nadir around 2400 hrs (Unger, et al., 2013), the VMB iron infusion was given at 2400 hrs and NNF measures were performed in the striatum 6 hours after the VMB infusion. Our study showed that a 26 pmol concentration change in VMB iron can reduce extracellular DA to levels compatible with control and increase intracellular DA even 30 hours after the infusion. It did not, however, have an effect on any of ID-induced changes seen in extracellular DA extraction (i.e, DAT function). As the reversal of the ID-induced increase in extracellular DA was not associated with a concomitant increase in DA extraction, the findings suggest that subacute changes in VMB iron appear to have an effect on striatal DA release. Striatal iron infusion, on the other hand, had no effect on any of the four, ID-induced changes in DAergic dynamics. For the striatal-iron effect, iron was infused into the striatum and the NNF measures of striatal DA were done soon afterwards. The timing was based on prior studies that had used similar acute intervals in assessing the effects of iron infusion on striatal DA. These studies did find in striatal DA function but also used substantially higher (> 1000 fold), non-physiological concentrations (Ben-Shachar and Youdim, 1991; Prikhojan, et al., 2002), which would be predicted to lead to lipid peroxidation and cell death (Santiago, et al., 2000). There was no indication that the iron concentrations used in the current study lead to an elevations in lipid peroxidation (TBARS levels). As VMB infusion-to-assessment interval was different than the striatal infusion-to-assessment interval, one cannot directly compare the two results. It is quite possible that a striatal infusion-to-assessment interval of 6 hours would have produced a similar effect to that seen with VMB infusion.
So what is the biological relevance of small picomole concentrations of extracellular iron? Most of the ferrous sulfate used in this study would have likely not been bound to transferrin, as only the ferric (Fe3+) form of iron can bind to transferrin. Most of the ferrous sulfate would likely have dissociated into ferrous ascorbate (i.e., non-transferrin bound iron (NTBI)) given the very high concentrations of ascorbate found in the extrasynaptic space (Rice, 2000). Although a substantial amount of research has focused on the iron-transferrin complex and its biology (Moos, 2002), less attention has been spent on understanding biological importance of NTBI in brain. That NTBI has biological relevance that can be deduced from the fact that astrocytes and neurons have transporters that utilize only NTBI. The most common of the NTBI transporters is the divalent metal transporter-1 (DMT-1), which can transport only the ferrous (Fe2+) form of iron (Conrad and Umbreit, 2000). Ferrous iron via an interaction with ascorbate and nitric oxide has been shown to modulate DA release (Rocchitta, et al., 2005) and NMDA-receptor-dependent Ca++ flux (Nakamichi, et al., 2002). The up regulation of hippocampal DMT-1 with learning, indirectly implicates the role for NTBI in this process (Haeger, et al., 2010). Using a dialysis probe in the VMB, which excludes proteins the size of transferrin, extracellular NTBI has been shown to have significant circadian fluctuations in a range compatible with the 26 pmol concentration used in this study (Unger, et al., 2013). Translating the data from ID-rodent models as a whole, a possible biological basis for the distinct circadian-dependent nature of RLS symptoms (Earley, 2003) may be small circadian changes in substantia nigra iron concentrations that then drive changes in striatal DA dynamics and thus changes in RLS symptoms.
Acutely increasing VMB iron reversed the ID-induced increase in extracellular DA but did not reverse the ID effects on the basal or quinpirole-induced-change in DA uptake. Similarly, quinpirole reduced extracellular DA to an almost identical concentration in both Control and ID even though basal levels were higher in the ID group. Quinpirole had no effect, however, on DA uptake in the ID group. Thus the ID-induced increase in extracellular DA seems the most responsive to acute changes as both the quinpirole-challenge and increase in VMB iron negated the ID-effect. The ID-induced effect on DA extraction from the extracellular, on the other hand, was unaffected by either quinpirole-challenge or increasing VMB iron. These results with the ID-rodent model raise the issue that the initial benefits commonly seen with levodopa or the DA-2/3 receptor agonist in RLS patient, may only partially correct the underlying DAergic abnormally and leaving unchecked, the ID-induced DAT dysfunction. The consequences of this select effect may in the long term, however, undermine the initial benefits. The ID-rodent model has, to date, accurately identified alternations in the DAergic system similar to those identified RLS. Assuming the ID-rodent model’s translational component holds true then this divergent effect of DA agonist on ID-induced changes in striatal DA dynamic may explain why acute DA agonist can dramatically improve RLS symptoms but with chronic use, the DA agonist actually causes the symptoms to worsen, referred to as “augmentation” (Allen and Earley, 1996; Garcia-Borreguero, 2004).
Conclusion
The ID-rodent model provides highly reproducible changes in the striatal DA dynamics that remarkably parallel DAergic changes seen in RLS patient. Some but not all of these ID-induced changes in striatal DA dynamic were reversible with physiological increases in VMB iron. The small changes in VMB iron induced by iron infusion likely represent biologically relevant changes in the non-transferrin-bound labile iron pool and may mimic circadian-dependent changes that have been found in VBM extracellular iron. Future work with this model needs to evaluate the consequents of chronic DA2/3 receptor agonist on the four, ID-induced changes in striatal DA dynamics to potentially understand why DA-agonist-induced RLS symptoms augmentation occurs. Furthermore, future work should evaluate drugs with different DA-receptor classification or news drugs from different classes that might negate all of the ID-induced changes in striatal DA dynamics.
Highlights.
Iron deficiency (ID) in rats results in altered striatal dopamine (DA) dynamics.
ID effects included altered DA uptake dynamics but not altered release mechanisms.
Infusion of iron into the ventral midbrain reversed some of the ID effects
ID effects where similar to striatal DA changes seen in Restless Legs Syndrome
Acknowledgement
This research was supported by a grant from National Institutes of Health PO1-AG21190.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allen RP, Auerbach S, Bahrain H, Auerbach M, Earley CJ. The prevalence and impact of restless legs syndrome on patients with iron deficiency anemia. Am J Hematol. 2013;88:261–264. doi: 10.1002/ajh.23397. [DOI] [PubMed] [Google Scholar]
- 2.Allen RP, Barker PB, Wehrl F, Song HK, Earley CJ. MRI measurement of brain iron in patients with restless legs syndrome. Neurology. 2001;56:263–265. doi: 10.1212/wnl.56.2.263. [DOI] [PubMed] [Google Scholar]
- 3.Allen RP, Earley CJ. Augmentation of the restless legs syndrome with carbidopa/levodopa. Sleep. 1996;19:205–213. doi: 10.1093/sleep/19.3.205. [DOI] [PubMed] [Google Scholar]
- 4.Beard JL, Chen Q, Connor J, Jones BC. Altered monamine metabolism in caudate-putamen of iron-deficient rats. Pharmacol Biochem Behav. 1994;48:621–624. doi: 10.1016/0091-3057(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 5.Beard JL, Connor JR. Iron status and neural functioning. Annu. Rev. Nutr. 2003 doi: 10.1146/annurev.nutr.23.020102.075739. 020102.075739. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Shachar D, Youdim M. Intranigral iron injection induces behavioral and biochemical "parkinsonism" in rats. J Neurochem. 1991;57:2133–2135. doi: 10.1111/j.1471-4159.1991.tb06432.x. [DOI] [PubMed] [Google Scholar]
- 7.Bianco LE, Unger EL, Earley CJ, Beard JL. Iron deficiency alters the daynight variation in monoamine levels in mice. Chronobiol Int. 2009;26:447–463. doi: 10.1080/07420520902820905. [DOI] [PubMed] [Google Scholar]
- 8.Bianco LE, Wiesinger J, Earley CJ, Jones BC, Beard JL. Iron deficiency alters dopamine uptake and response to L-DOPA injection in Sprague-Dawley rats. J Neurochem. 2008;106:205–215. doi: 10.1111/j.1471-4159.2008.05358.x. [DOI] [PubMed] [Google Scholar]
- 9.Bolan EA, Kivell B, Jaligam V, Oz M, Jayanthi LD, Han Y, Sen N, Urizar E, Gomes I, Devi LA, Ramamoorthy S, Javitch JA, Zapata A, Shippenberg TS. D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2- dependent and phosphoinositide 3 kinase-independent mechanism. Mol Pharmacol. 2007;71:1222–1232. doi: 10.1124/mol.106.027763. [DOI] [PubMed] [Google Scholar]
- 10.Bungay PM, Newton-Vinson P, Isele W, Garris PA, Justice JB. Microdialysis of dopamine interpreted with quantitative model incorporating probe implantation trauma. J Neurochem. 2003;86:932–946. doi: 10.1046/j.1471-4159.2003.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chefer VI, Zapata A, Shippenberg TS, Bungay PM. Quantitative no-net-flux microdialysis permits detection of increases and decreases in dopamine uptake in mouse nucleus accumbens. J Neurosci Methods. 2006;155:187–193. doi: 10.1016/j.jneumeth.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Connor JR, Boyer PJ, Menzies SL, Dellinger B, Allen RP, Earley CJ. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology. 2003;61:304–309. doi: 10.1212/01.wnl.0000078887.16593.12. [DOI] [PubMed] [Google Scholar]
- 13.Connor JR, Menzies SL, St. Martin SM, Mufson EJ. A histochemical study of iron, transferrin, and ferritin in Alzheimer's diseased brains. J Neurosci Res. 1992;31:75–83. doi: 10.1002/jnr.490310111. [DOI] [PubMed] [Google Scholar]
- 14.Connor JR, Wang XS, Allen RP, Beard JL, Wiesinger JA, Felt BT, Earley CJ. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain. 2009;132:2403–2412. doi: 10.1093/brain/awp125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conrad ME, Umbreit JN. Iron absorption and transport-an update. Am J Hematol. 2000;64:287–298. doi: 10.1002/1096-8652(200008)64:4<287::aid-ajh9>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 16.Cooper JR, Bloom FE, Roth RH. The Biochemical Basis of Neuropharmacology. New York: Oxford University Press; 2008. [Google Scholar]
- 17.Earley CJ. Clinical practice. Restless legs syndrome. N Engl J Med. 2003;348:2103–2109. doi: 10.1056/NEJMcp021288. [DOI] [PubMed] [Google Scholar]
- 18.Earley CJ, Kuwabara H, Wong DF, Gamaldo C, Salas R, Brasic J, Ravert HT, Dannals RF, Allen RP. The dopamine transporter is decreased in the striatum of subjects with restless legs syndrome. Sleep. 2011;34:341–347. doi: 10.1093/sleep/34.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Earley CJ, Kuwabara H, Wong DF, Gamaldo C, Salas RE, Brasic JR, Ravert HT, Dannals RF, Allen RP. Increased synaptic dopamine in the putamen in restless legs syndrome. Sleep. 2013;36:51–57. doi: 10.5665/sleep.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erikson KM, Jones BC, Beard JL. Iron deficiency alters dopamine transporter functioning in rat striatum. J Nutr. 2000;130:2831–2837. doi: 10.1093/jn/130.11.2831. [DOI] [PubMed] [Google Scholar]
- 21.Erikson KM, Jones BC, Hess EJ, Zhang Q, Beard JL. Iron deficiency decreases dopamine D1 and D2 receptors in rat brain. Pharmacol Biochem Behav. 2001;69:409–418. doi: 10.1016/s0091-3057(01)00563-9. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Borreguero D. Augmentation: understanding a key feature of RLS. Sleep Med. 2004;5:5–6. doi: 10.1016/j.sleep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Godau J, Schweitzer KJ, Liepelt I, Gerloff C, Berg D. Substantia nigra hypoechogenicity: definition and findings in restless legs syndrome. Mov Disord. 2007;22:187–192. doi: 10.1002/mds.21230. [DOI] [PubMed] [Google Scholar]
- 24.Haeger P, Alvarez A, Leal N, Adasme T, Nunez MT, Hidalgo C. Increased hippocampal expression of the divalent metal transporter 1 (DMT1) mRNA variants 1B and +IRE and DMT1 protein After NMDA-receptor stimulation or spatial memory training. Neurotox Res. 2010;17:238–247. doi: 10.1007/s12640-009-9096-z. [DOI] [PubMed] [Google Scholar]
- 25.Jellen LC, Unger EL, Lu L, Williams R, Rousseau S, Wang X, Earley CJ, Allen RP, Miles M, Jones BC. Systems genetic analysis of the effects of iron deficiency in mouse brain. Neurogenetics. 2012;13:147–157. doi: 10.1007/s10048-012-0321-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee B-Y, Farace E, Vesek J, Connor J, Yang Q. In vivo measurement of iron deficiency in restless legs syndrome(RLS) with voxel-based R2 relaxometry. Proc Intl Soc Mag Reson Med. 2007;15:2170. [Google Scholar]
- 27.Mao A, Freeman KA, Tallarida RJ. Transient loss of dopamine autoreceptor control in the presence of highly potent dopamine agonists. Life Sci. 1996;59:PL317–PL324. doi: 10.1016/0024-3205(96)00525-5. [DOI] [PubMed] [Google Scholar]
- 28.Mefford IN. Application of high performance liquid chromatography with electrochemical detection to neurochemical analysis: measurement of catecholamines, serotonin and metabolites in rat brain. J Neurosci Methods. 1981;3:207–224. doi: 10.1016/0165-0270(81)90056-x. [DOI] [PubMed] [Google Scholar]
- 29.Moos T. Brain iron homeostasis. Dan Med Bull. 2002;49:279–301. [PubMed] [Google Scholar]
- 30.Nakamichi N, Ohno H, Nakamura Y, Hirai T, Kuramoto N, Yoneda Y. Blockade by ferrous iron of Ca2+ influx through N-methyl-D-aspartate receptor channels in immature cultured rat cortical neurons. J Neurochem. 2002;83:1–11. doi: 10.1046/j.1471-4159.2002.01042.x. [DOI] [PubMed] [Google Scholar]
- 31.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 32.Prikhojan A, Brannan T, Yahr MD. Intrastriatal iron perfusion releases dopamine: an in-vivo microdialysis study. J Neural Transm. 2002;109:645–649. doi: 10.1007/s007020200053. [DOI] [PubMed] [Google Scholar]
- 33.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 34.Refshauge C, Kissinger PT, Dreiling R, Blank L, Freeman R, Adams RN. New high performance liquid chromatographic analysis of brain catecholamines. Life Sci. 1974;14:311–322. doi: 10.1016/0024-3205(74)90061-7. [DOI] [PubMed] [Google Scholar]
- 35.Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23:209–216. doi: 10.1016/s0166-2236(99)01543-x. [DOI] [PubMed] [Google Scholar]
- 36.Rizzo G, Manners D, Testa C, Tonon C, Vetrugno R, Marconi S, Plazzi G, Pizza F, Provini F, Malucelli E, Gramegna LL, Lodi R. Low brain iron content in idiopathic restless legs syndrome patients detected by phase imaging. Mov Disord. 2013 doi: 10.1002/mds.25576. [DOI] [PubMed] [Google Scholar]
- 37.Robinson MB. Regulated trafficking of neurotransmitter transporters: common notes but different melodies. J Neurochem. 2002;80:1–11. doi: 10.1046/j.0022-3042.2001.00698.x. [DOI] [PubMed] [Google Scholar]
- 38.Rocchitta G, Migheli R, Mura MP, Grella G, Esposito G, Marchetti B, Miele E, Desole MS, Miele M, Serra PA. Signaling pathways in the nitric oxide and iron-induced dopamine release in the striatum of freely moving rats: Role of extracellular Ca2+ and L-type Ca2+ channels. Brain Research. 2005;1047:18–29. doi: 10.1016/j.brainres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Santiago M, Matarredona ER, Granero L, Cano J, Machado A. Neurotoxic relationship between dopamine and iron in the striatal dopaminergic nerve terminals. Brain Res. 2000;858:26–32. doi: 10.1016/s0006-8993(99)02485-3. [DOI] [PubMed] [Google Scholar]
- 40.Schmidauer C, Sojer M, Seppi K, Stockner H, Hogl B, Biedermann B, Brandauer E, Peralta CM, Wenning GK, Poewe W. Transcranial ultrasound shows nigral hypoechogenicity in restless legs syndrome. Ann Neurol. 2005;58:630–634. doi: 10.1002/ana.20572. [DOI] [PubMed] [Google Scholar]
- 41.See RE, Sorg BA, Chapman MA, Kalivas PW. In vivo assessment of release and metabolism of dopamine in the ventrolateral striatum of awake rats following administration of dopamine D1 and D2 receptor agonists and antagonists. Neuropharmacology. 1991;30:1269–1274. doi: 10.1016/0028-3908(91)90022-4. [DOI] [PubMed] [Google Scholar]
- 42.Smith AD, Justice JB. The effect of inhibition of synthesis, release, metabolism and uptake on the microdialysis extraction fraction of dopamine. J Neurosci Methods. 1994;54:75–82. doi: 10.1016/0165-0270(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 43.Trenkwalder C, Walters A, Hening W, et al. Circadian rhythm of patients with the idiopathic restless legs syndrome. Sleep Research. 1995;24:360. [Google Scholar]
- 44.Unger EL, Beard JL, Jones BC. Iron regulation in C57BLI6 and DBA/2 mice subjected to iron overload. Nutr Neurosci. 2007;10:89–95. doi: 10.1080/10284150701350653. [DOI] [PubMed] [Google Scholar]
- 45.Unger EL, Earley CJ, Thomsen LL, Jones BC, Allen RP. Effects of IV iron isomaltoside-1000 treatment on regional brain iron status in an iron-deficient animal. Neuroscience. 2013;246:179–185. doi: 10.1016/j.neuroscience.2013.04.049. [DOI] [PubMed] [Google Scholar]
- 46.Unger EL, Wiesinger JA, Hao L, Beard JL. Dopamine D2 receptor expression is altered by changes in cellular iron levels in PC12 cells and rat brain tissue. J Nutr. 2008;138:2487–2494. doi: 10.3945/jn.108.095224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiesinger JA, Buwen JP, Cifelli CJ, Unger EL, Jones BC, Beard JL. Down-regulation of dopamine transporter by iron chelation in vitro is mediated by altered trafficking, not synthesis. J Neurochem. 2007;100:167–179. doi: 10.1111/j.1471-4159.2006.04175.x. [DOI] [PubMed] [Google Scholar]
- 48.Yin L, Unger EL, Jellen LC, Earley CJ, Allen RP, Tomaszewicz A, Fleet JC, Jones BC. Systems genetic analysis of multivariate response to iron deficiency in mice. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1282–R1296. doi: 10.1152/ajpregu.00634.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]