ABSTRACT
Background
Two steroid-free, over-the-counter skin protectant products have been developed for the care and treatment of atopic dermatitis (AD)—Eucerin Eczema Relief Body Crème (Body Cream) for daily skin moisturization and Eucerin Eczema Relief Instant Therapy cream (Instant Therapy) for treatment of AD flare-ups. We tested the efficacy and tolerability of these formulations in infants and children with AD.
Methods
Study 1: Body Cream was applied twice daily to the lower legs of 64 children with a history of AD (aged 3 months to 12 years) for 14 days. Study 2: Instant Therapy was applied to active lesions and surrounding skin of 29 children (aged 3 months to 12 years) with active atopic lesions. Assessments were performed at baseline and Days 7 and 14. Symptoms were assessed using the Atopic Dermatitis Severity Index in Study 2.
Results
Body Cream significantly improved skin hydration and reduced itching, burning/stinging, erythema, and tactile roughness. Instant Therapy significantly improved skin hydration and AD symptoms, notably pruritus, erythema, and lichenification. Both products were safe and well tolerated.
Discussion
Body Cream and Instant Therapy were effective and well tolerated in the treatment of AD in children. These products provide steroid-free, nonprescription therapy for the maintenance and treatment of acute eczema and were proven effective and safe in infants as young as 3 months.
Key words: Atopic dermatitis, Infants, Children, Eczema, OTC, Steroid-free, Topical
Eczema is a common inflammatory dermatitis characterized by dry, irritated skin with a recurring itchy rash involving erythema, edema, and pruritus (Berke, Singh, & Guralnick, 2012; Hanifin & Rajka, 1980; Ring et al., 2012; Sabin, Peters, & Peters, 2012). The most common type of eczema, atopic dermatitis (AD), affects 15%–30% of children and 2%–10% of adults (Eichenfield et al., 2012; Ellis, Mancini, Paller, Simpson, & Eichenfield, 2012; Hanifin & Rajka, 1980; Sabin et al., 2012). Typically, AD appears within the first 5 years of life (Berke et al., 2012), with approximately 70% of children outgrowing the condition by early adolescence (Sabin et al., 2012); however, patients retain a lifelong predisposition for skin that is easily irritated.
AD presents as a condition with quiescent periods interrupted by periodic flare-ups. There are three clinical presentations of AD: subacute, acute, and chronic. Subacute eczema is characterized by dry, pruritic, scaly skin with erythematous papules and plaques (Berke et al., 2012; Sabin et al., 2012). Acute eczema is characterized by red, pruritic papules and serous exudates (Sabin et al., 2012). Secondary lesions can occur because of scratching, resulting in excoriations and crusted lesions. Chronic irritation and uncontrolled scratching can result in lichenification of the skin (Sabin et al., 2012).
Pediatric skin differs from adult skin, having characteristics that may make it more prone to AD. The stratum corneum in infants is extremely thin and shows both increased exogenous water absorption rates and water desorption in the first year of life compared with adults, suggesting an inability to hold water (Nikolovski, Stamatas, Kollias, & Wiegand, 2008). Over the first 1–4 years, the stratum corneum gains thickness and shows increased ability to hold water, with infant and toddler skin actually having higher water content than adult skin, despite lower natural moisturizing factor levels. However, the smaller and less dense corneocytes and keratinocytes, thinner total epidermis, inability to maintain hydration homeostasis, and higher ratio of surface area to volume in the skin of young children all reflect skin with compromised barrier integrity (Fernandes, Machado, & Oliveira, 2011; Nikolovski et al., 2008). This compromised barrier results in water loss and xerotic skin, which is easily irritated and difficult to heal. To overcome the itch–scratch cycle evident in AD, the skin needs to be adequately hydrated, and the itch needs to be controlled to allow the skin to heal (Sabin et al., 2012).
Current European and U.S. guidelines recommend the daily application of emollient moisturizers for both the prevention and treatment of AD (Eichenfeld et al., 2014; Hanifin et al., 2004; Ring et al., 2012). Emollient therapy can improve hydration, prevent transepidermal water loss (TEWL; Eichenfield et al., 2012), and relieve itching (Ellis et al., 2003). Topical corticosteroids are the primary prescription product used to control flare-ups of AD, with short-term oral steroids (up to 1 week) used to treat major exacerbations. However, long-term use of topical or oral steroids is not recommended for the treatment of AD, particularly in children, because of the risk for cutaneous side effects, rebound flare-ups, suppression of growth rate, and reduced bone density (Hanifin et al., 2004; Ring et al., 2012). In addition, reports indicate that approximately 50% of parents request steroid-sparing medications for their children with moderate-to-severe eczema because of concerns about adverse events (Hon et al., 2006; Kojima et al., 2013).
Development of steroid-free, over-the-counter (OTC) skin care products that can support the barrier and help manage bothersome symptoms is a major goal for eczema therapy. We have developed two new OTC products for the management and treatment of AD. The first is a Body Cream designed for daily use to maintain hydration of the skin, support skin barrier function, and reduce TEWL. An Instant Therapy cream has been designed to specifically treat acute AD flare-ups, calming the skin and supporting hydration and barrier repair. Both products contain colloidal oatmeal, licochalcone, ceramide 3, and castor oil. In addition, Instant Therapy contains menthoxypropanediol, a novel cooling agent to calm itch and provide a cooling sensation.
Here, we report on two controlled clinical studies conducted to test the efficacy and tolerability of the Body Cream and Instant Therapy in infants and children with AD.
METHODS
Overview of Clinical Studies
Two single-center, 2-week clinical studies were conducted to assess the efficacy and tolerability of two emollient creams—one designed for daily moisturization (Eucerin Eczema Relief Body Crème: Body Cream) and the other for acute therapy of AD lesions (Eucerin Eczema Relief Instant Therapy cream: Instant Therapy). Both formulations were developed by Beiersdorf, Inc. (Wilton, Connecticut), for the treatment of AD.
Patient Enrollment and Eligibility
In Study 1, male and female infants, toddlers, and children aged 3 months to 12 years with a history of atopic skin disease were enrolled at a clinical research center in Colorado Springs, CO. Study 1 subjects with one or more active atopic skin sites of mild-to-moderate severity were analyzed as an active eczema subgroup. In Study 2, conducted in Carrollton, TX, all subjects had at least one active atopic skin site of mild-to-moderate intensity. The age range was the same as for Study 1. All parents/guardians of subjects signed an informed consent form approved by the Institutional Review Board and a disclosure agreement as required by the Health Insurance Portability and Accountability Act (HIPAA).
Study Design
No products were applied to the treatment test areas for ≥2 days before the treatment test period. In Study 1, Body Cream was applied to both legs (knee to ankle; all subjects) and to the designated active eczema lesion and surrounding unaffected skin (subgroup only) twice daily for 14 days. In Study 2, Instant Therapy was applied to designated lesions twice daily for 14 days (all subjects) in the morning and evening after cleansing. Assessments were conducted on Days 7 and 14.
Efficacy and Tolerability Assessments
Corneometer measurements
Corneometer CM 825 (Courage and Khazaka, Germany) electrical capacitance measurements were made at the outer aspect of both legs and the active lesion and their adjacent sites to measure product hydration effects on the skin surface; increases in average measurements relative to baseline indicated improved skin hydration.
Skin irritation assessments
A 4-point scale (0 = none, 3 = severe) was used by clinicians to grade erythema, edema, and tactile roughness of the outer legs. Parents graded itching and burning/stinging of legs and active lesions using the same scale.
Product assessment questionnaire
Parents/guardians completed a product evaluation regarding skin condition and each test material’s efficacy and characteristics at Day 14.
Imaging procedures
Digital photographs were taken of designated active lesions and the surrounding, unaffected skin using a Nikon D7000 digital SLR camera.
Atopic Dermatitis Severity Index (Study 2 Only)
Designated active lesions were evaluated using the Atopic Dermatitis Severity Index (ADSI; van Leent et al., 1998), assessing the following components of the target lesion(s): erythema, pruritus, exudation, excoriation, and lichenification. Each component was evaluated according to a 4-point grading system (0 = none, 3 = severe) with a total ADSI score of 0–15.
Tolerability assessments
Global tolerability of the test material for each subject was evaluated by a clinician according to a 4-point scale (1 = excellent, 4 = poor). Adverse events were recorded.
Statistical Analysis
The per-protocol population was used for the efficacy and tolerance testing. For skin irritation assessments and ADSI, statistical change from baseline was calculated using Wilcoxon’s signed-rank test. Statistical change in corneometer measurements used a paired t test. For the product evaluation questionnaire, a binomial (sign) test was used to determine if the proportion of the combined designated favorable responses was equal to the combined designated negative responses for each question. Statistical analyses were performed using SAS software version 9.30 series (SAS Institute, Inc., Cary, NC).
RESULTS
Subject Demographics
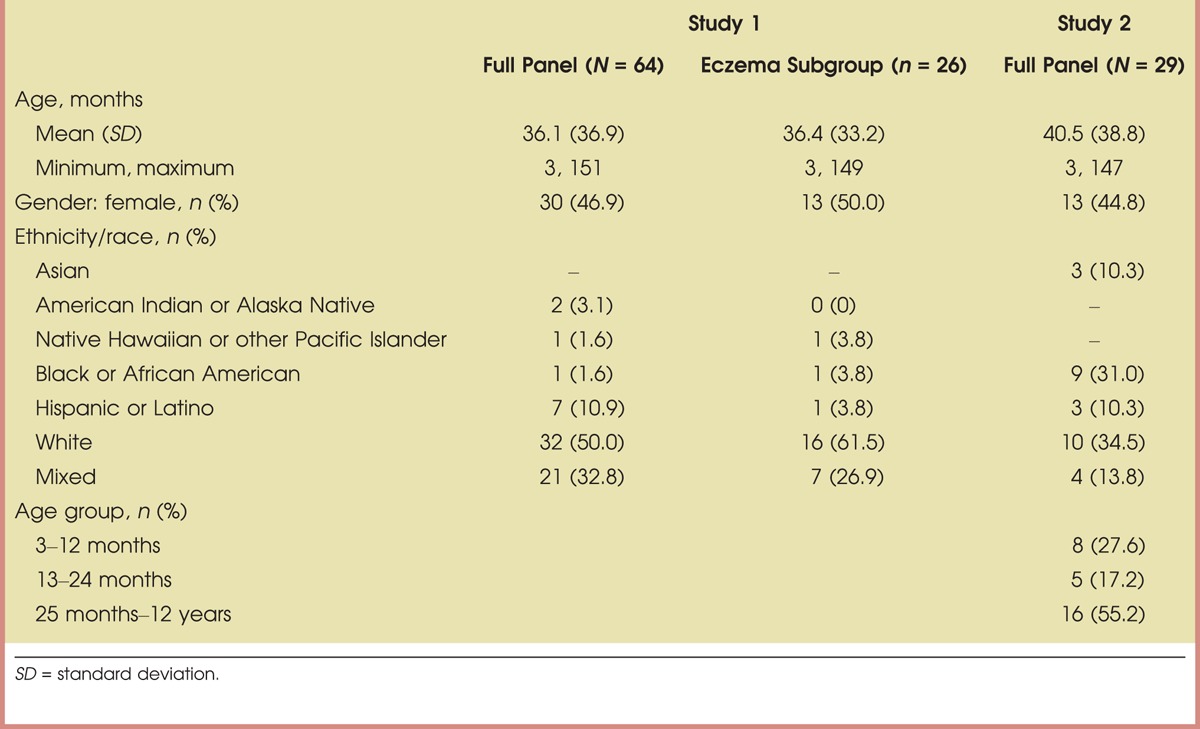
Sixty-four infants and children aged 3–151 months with a history of AD completed Study 1; 26 subjects had active lesions at enrollment and were included in the subgroup assessments. In Study 2, 29 infants and children aged 3–147 months with mild-to-moderate active AD completed the study. Demographic information is summarized in Table 1.
TABLE 1.
Summary Demographics: Per-Protocol Populations for Body Cream Moisturizing Therapy (Study 1) and Instant Therapy Cream (Study 2)
Study 1: Body Cream Assessment
Skin hydration
Body Cream significantly improved stratum corneum moisture content of the lower legs after 7 and 14 days of twice-daily application compared with baseline (p ≤ .001; Figure 1A). Corneometer values were significantly increased by 33.6% at Day 7 and 40.8% at Day 14. Application of Body Cream for 2 weeks also significantly improved corneometer measurements in the active lesion (91.6%) and surrounding skin (33.4%) compared with baseline (p < .001; Figure 1B).
FIGURE 1.
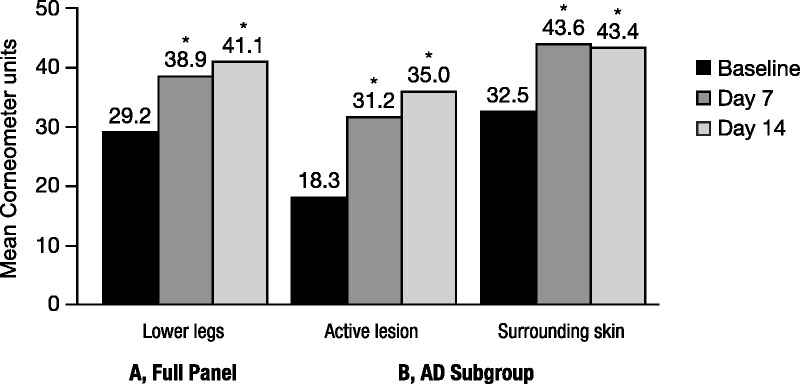
Improvement in skin hydration with Body Cream. Study 1: Corneometry measures after 14 days of twice-daily application. (A) Skin hydration levels were measured on the outer sides of each lower leg and averaged; N = 64. *p < .001 versus baseline. (B) Skin hydration levels of active lesion and surrounding skin in subgroup; N = 29. An increase in measurement indicates an improvement in skin hydration. *p < .001 versus baseline.
Skin irritation
Clinical grading of skin irritation indicated that Body Cream significantly reduced erythema and tactile roughness after 2 weeks. Tactile roughness scores were reduced by 81.7% at Day 7 and by 95% at Day 14 (p < .001; Figure 2). Body Cream significantly reduced erythema by 70% after 2 weeks of treatment (p = .016; Figure 2). Mean itching scores decreased by 63.6% at Day 7 and 84.8% by Day 14 (p < .001). Burning/stinging sensations were also reduced by 57.1% at Day 7 and by 85.7% at Day 14 (Figure 2). No edema was present at baseline; thus, there was no change for this assessment.
FIGURE 2.
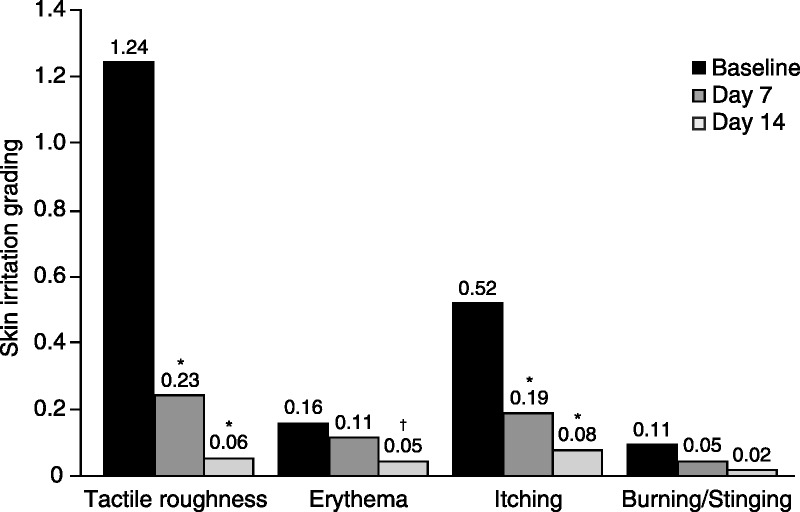
Skin irritation assessment. Tactile roughness, erythema, itching, and burning/stinging were graded on a 4-point scale (0 = none, 3 = severe); N = 64. *p < .001 versus baseline, †p < .05 versus baseline.
Symptoms of itching and burning/stinging also improved significantly in subjects with active lesions. The active subgroup baseline itch scores were notably higher than scores for the legs (1.42 vs. 0.52); the lesions showed significant reductions in itch at Days 7 (35.1% reduction, p = .031) and 14 (54.1% reduction, p = .003). Burning and stinging scores in the active subgroup decreased by 78.6% (p = .006).
Body Cream visibly improved the appearance of skin affected by AD after 14 days of twice-daily treatment (Figure 3).
FIGURE 3.
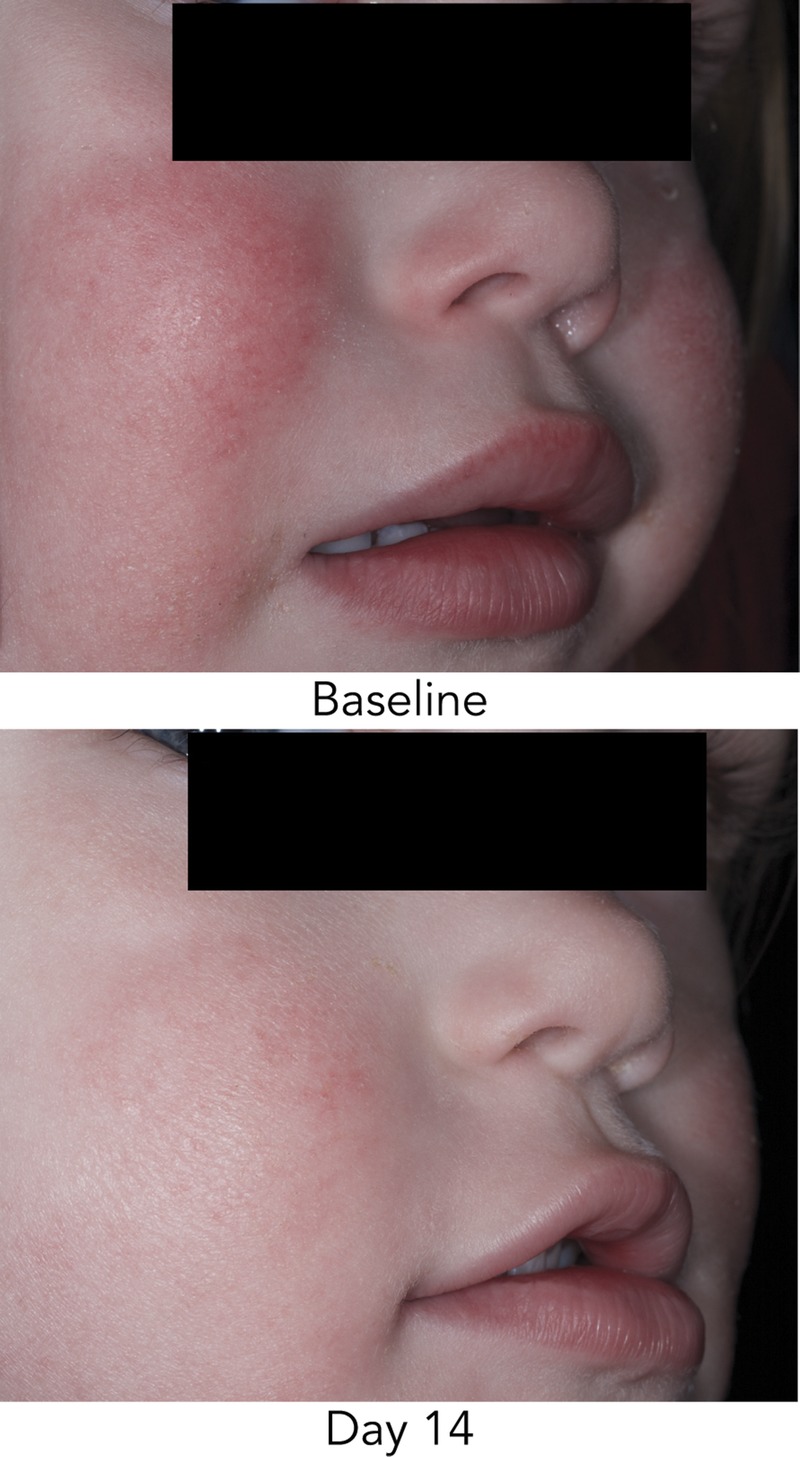
Improvement in appearance of active lesions after 2 weeks of Body Cream treatment. Improvements seen at Day 14 in erythema, dryness, and scaling on the face.
Product evaluation questionnaire
The questionnaire analysis showed that a statistically significant proportion of parents indicated agreement (strongly agree or agree) with statements supporting the efficacy and tolerability of Body Cream (Figure 4). Overall, 94% of parents/guardians confirmed that the product effectively moisturized their child’s skin, 94% stated that it did not irritate skin, 91% reported that it soothed skin, 88% thought that it protected their child’s skin from dryness, and 100% agreed that it was gentle enough to use every day.
FIGURE 4.
Product assessment questionnaire of Body Cream. Parents completed a questionnaire on the qualities of Body Cream at Day 14. Percentages show the combined responses that agreed or strongly agreed with the statements; <5% disagreed with any statement, and the remainder neither agreed nor disagreed. The proportion of all responses indicating agreement was statistically significant.
Tolerability
All subjects were graded as experiencing either “good” or “excellent” tolerability to Body Cream at Day 14. One subject experienced a mild transient erythematous rash on the lower legs; however, the subject continued daily application of the test product to the site, and the rash resolved after several days of treatment.
Study 2: Instant Therapy Assessment
Skin hydration
Instant Therapy significantly improved hydration levels of active lesions by 70.1% (p < .001) and surrounding skin by 29.3% (p = .001) at Day 14 (Figure 5). Active lesion hydration with Instant Therapy was significantly greater compared with the surrounding skin (p = .04), although the moisture level of the surrounding skin was within normal range.
FIGURE 5.
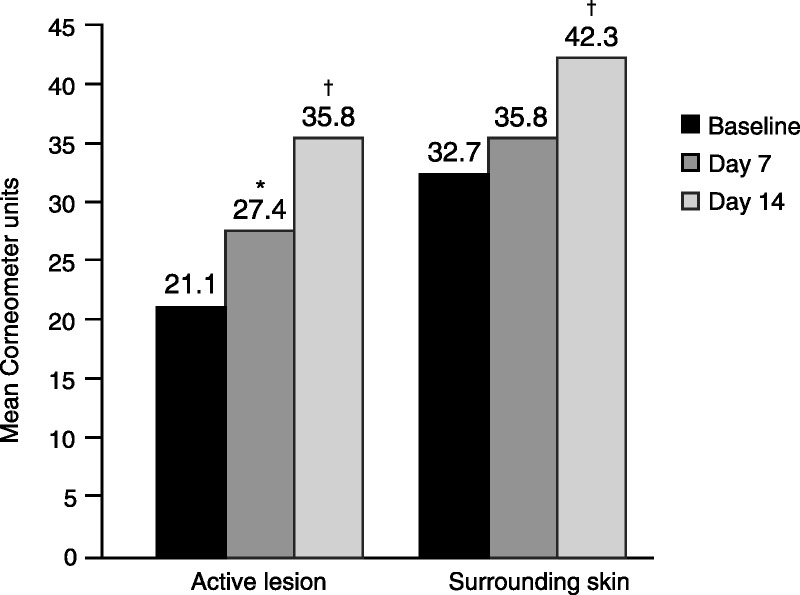
Improvement in skin hydration with Instant Therapy. Study 2: Corneometry results for active lesions and surrounding skin; N = 29. An increase in measurement indicates an improvement in skin hydration. *p < .05 versus baseline, †p ≤ .001 versus baseline.
ADSI clinical grading
Instant Therapy application significantly improved AD severity, decreasing total ADSI scores by 41.8% and 58.4% at Days 7 and 14, respectively (p < .001; Figure 6). Improvement in overall score was observed in 93.1% of subjects at Week 2. Treatment with Instant Therapy significantly improved the individual symptoms of AD, with the greatest improvements observed for pruritus (64.9%), erythema (54.0%), and lichenification (57.1%) at Day 14 (p < .001) and excoriation (73.6%) at Day 7 (p = .004; Figure 6). The visible improvement in the appearance of active lesions and the surrounding skin after 2 weeks of Instant Therapy can be seen in Figure 7; before and after photographs show examples of improvements in erythema, excoriation, lichenification, and lesion intensity after 2 weeks of treatment.
FIGURE 6.
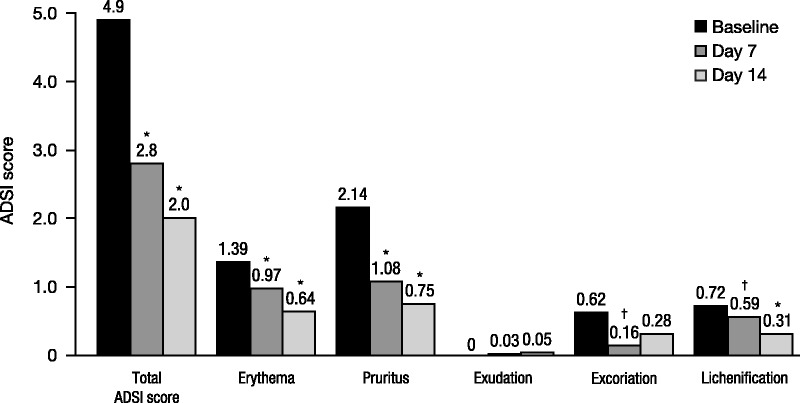
Improvement in Atopic Dermatitis Severity Index (ADSI) scores in Study 2. Individual ADSI symptoms were graded on a scale of 0 (none) to 3 (severe); sum provides total ADSI (maximum of 15); N = 29. *p < .001 versus baseline, †p < .05 versus baseline.
FIGURE 7.
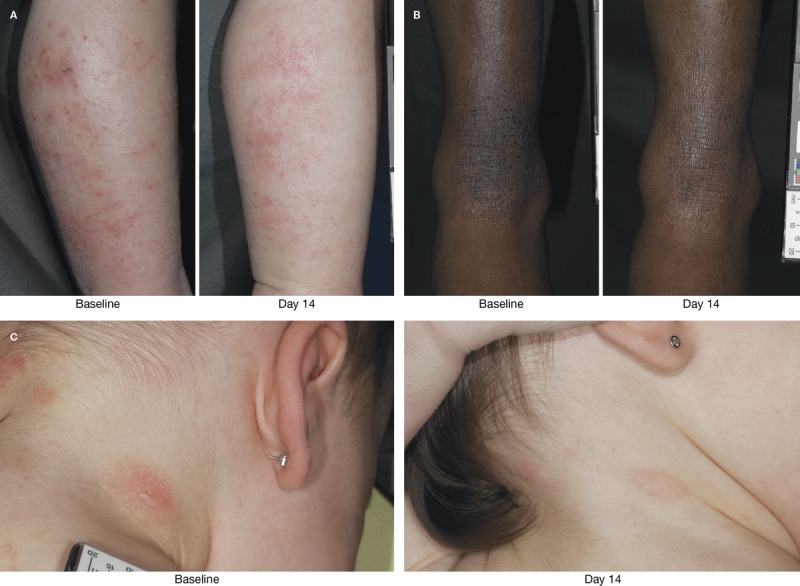
Improvement in active lesions after 2 weeks of Instant Therapy treatment. Improvements seen at Day 14 in erythema and exudation of the lower lateral leg (A), lichenification of the lower leg (B), and erythema and scaling on the back of the neck (C).
Product evaluation questionnaire
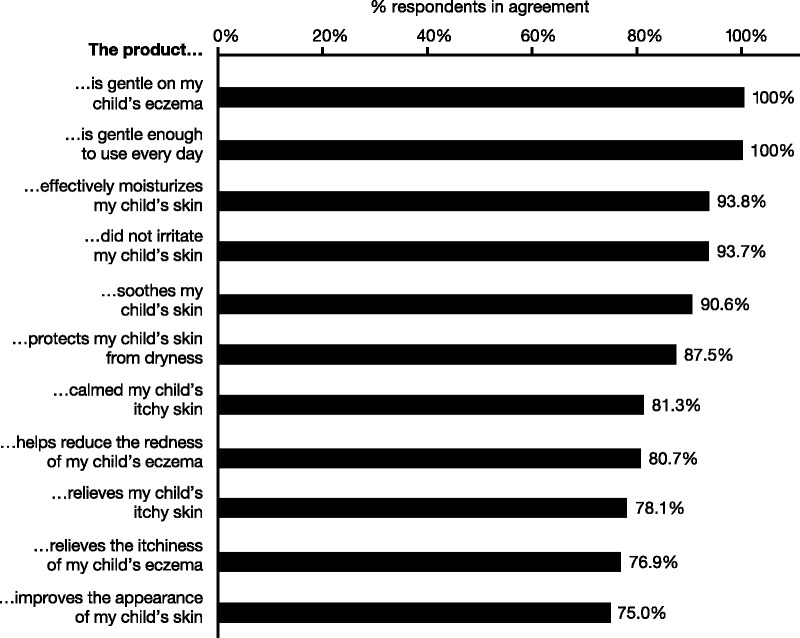
A statistically significant proportion of parents indicated agreement (strongly agree or agree) with statements that Instant Therapy was effectively able to moisturize their child’s itchy skin (93%), was able to calm skin (83%), works immediately to soothe skin (76%), relieves itchy skin (86%), protects against dryness (79%), reduces redness (90%), and was gentle enough to use every day (90%; Figure 8).
FIGURE 8.
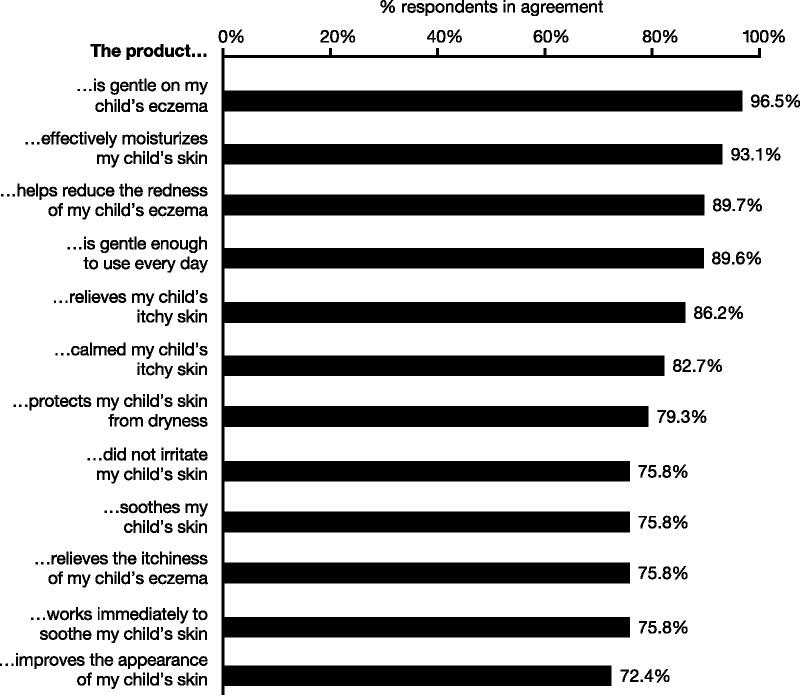
Product assessment questionnaire of Instant Therapy. Parents completed a questionnaire on the qualities of Instant Therapy at Day 14. Percentages show the combined responses that agreed or strongly agreed with the statements; <5% disagreed with any statement, and the remainder neither agreed nor disagreed. The proportion of all responses indicating agreement was statistically significant.
Tolerability
All subjects experienced either “good” or “excellent” tolerability to the Instant Therapy after 2 weeks of twice-daily treatment. One subject was reported as experiencing eczema, which resolved after 5 days. The subject continued product use and completed the study without additional medication. The investigator judged the event as having an unknown relationship to the test material.
DISCUSSION
AD is a highly prevalent skin condition in infants and children. Current guidelines for maintenance therapy of atopic skin recommend daily application of emollient therapy, whereas corticosteroids are typically used to control flare-ups (Eichenfield et al., 2014; Hanifin et al., 2004; Ring et al., 2012). However, approximately 50% of parents were reported to request nonsteroidal medications to treat their children’s moderate-to-severe eczema (Hon et al., 2006; Kojima et al., 2013). Parents’ reluctance to use topical steroids suggests that many children do not receive their prescribed steroid treatment until the condition has escalated (Hon et al., 2006; Kojima et al., 2013). Hence, there is a need for steroid-free moisturizing skin care treatments that are safe and well tolerated in young children with compromised skin barriers.
Both the Body Cream and Instant Therapy were well tolerated in children as young as 3 months. Only one adverse event, a mild erythematous rash, was considered possibly related to treatment with the Body Cream; however, it was transient and resolved while treatment continued. No adverse events were related to treatment with the Instant Therapy. None of the subjects discontinued the study because of adverse events or for any other reason.
Daily hydration and protection of the skin may help to reduce the frequency of AD flares. Body Cream and Instant Therapy significantly improved the hydration of skin, particularly that of active lesions, as shown by the corneometry results. Similar studies in adults have shown that these products significantly improved barrier function, as evidenced by a decrease in TEWL (Weber et al., 2014).
In addition, the daily application of the Body Cream significantly improved the symptoms of AD, including erythema, tactile roughness, and itching.
The efficacy and tolerability shown by the Body Cream and Instant Therapy were observed without the use of concomitant prescription therapy. Although there are other OTC preparations available in the United States for the treatment of AD, very few have been studied in pediatric subjects, and several of those studies permitted the continuation of topical steroid treatment throughout the treatment period (Del Rosso & Kircik, 2013; Simpson & Dutronc, 2011; Simpson et al., 2012). Allowing concomitant treatment makes it difficult to assess the true efficacy of the emollient formulation.
Symptoms of AD can have a dramatically negative impact on quality of life. Constant itching and discomfort can disrupt sleep as well as daytime work, school, and leisure activities (Kiebert et al., 2002). Itching can also exacerbate the existing inflammation, preventing the skin from healing. Both Body Cream and Instant Therapy significantly improved the symptoms of AD, most notably itching. Parents confirmed the efficacy of both products, with more than 90% agreeing that the products effectively moisturized their child’s itchy skin, calmed and soothed itch, protected against dryness, reduced redness, and were gentle enough to use every day. In addition, the skin showed overall visible improvements, which can be seen in Figures 3 and 7.
The efficacy and tolerability of the Body Cream and Instant Therapy are because of their formulations. Both product formulations are acidic, supporting the optimal pH of healthy skin and counteracting pH disturbances observed in atopic patients. The acidic milieu of the stratum corneum suppresses Staphylococcus aureus bacterial growth and is necessary for the functioning of enzymes that drive lipid synthesis and maturation and the desquamation process (Rippke, Schreiner, Doering, & Maibach, 2004). The formulas also contain ingredients known to soothe skin and enhance barrier repair, including colloidal oatmeal, ceramide 3, licochalcone, and castor seed oil. Oatmeal, a skin protectant active for eczema approved by the US Food and Drug Administration, contains several dermatologically active constituents with moisturizing, soothing, anti-inflammatory, barrier protective, and antioxidant properties. Oatmeal has been shown to have clinical efficacy as adjunctive therapy in reducing symptoms of AD, including itch (Fowler, Nebus, Wallo, & Eichenfield, 2012). Ceramides are one of three stratum corneum lipid constituents of the skin barrier, deficiencies of which have been linked to AD (Kezic et al., 2014). In the Eucerin formulations, ceramide 3 is an important constituent that combats the skin barrier defect, preventing TEWL and dryness. Licochalcone has been shown to have anti-inflammatory effects on the skin that may reduce skin irritation, erythema, and itching (Chu et al., 2012). Castor seed oil contains natural emollients that deeply penetrate the skin, moisturizing the skin and reducing TEWL and dryness. The Instant Therapy cream contains twice the ceramide content of the Body Cream as well as menthoxypropanediol, a cooling agent that provides rapid relief of itching and burning sensations upon contact (Akira, Michio, & Yoshida, 1984). Both formulations calm and soothe itch and other symptoms of AD and support barrier repair.
CONCLUSIONS
AD causes great discomfort for many children as they experience constant itch and subsequent scratching, inflamed lesions, and lichenification. Symptoms have a considerable negative impact on the quality of life for both children and parents. Providing parents with OTC options that are effective and well tolerated will help maintain the adequate hydration and protection needed for children with AD. The Eucerin Body Crème and Instant Therapy products have been specifically formulated to provide hydration and support barrier repair as well as to reduce symptoms of AD, allowing affected skin to heal. The studies reported here indicate that these products were effective and well tolerated in infants and children with AD as young as 3 months without the use of concomitant therapy. These formulations have also been tested in adults, with similar improvements in hydration and symptoms of AD (Weber et al., 2014). These products provide effective OTC treatments for eczema that are gentle enough for daily use in infants and children.
Acknowledgment
The authors would like to thank Jodie Macoun, PhD, of Evince Communications for her support in the preparation of this article.
Footnotes
This project was sponsored and funded by Beiersdorf, Inc. (Wilton, CT). The sponsor was involved in study design; the collection, analysis, and interpretation of data; the writing of study reports; and the decision to submit the manuscript for publication. Assistance in the preparation of this manuscript was provided by Evince Communications (editorial support and preparation of tables and figures).
The results were presented in poster form at the 2014 Winter Clinical Dermatology Conference, January 17–22, in Hawaii; the 13th Annual Caribbean Dermatology Symposium, January 21–25, in Aruba; and The Dermatology Nurses’ Association’s 32nd Annual Convention, May 1–4, 2014, in Orlando, Florida.
T. M. W. is an employee of Beiersdorf, Inc., the manufacturer of Eucerin Eczema Relief Body Crème and Eucerin Eczema Relief Instant Therapy. A. F., G. N., and A. M. S. are employees of Beiersdorf AG. J. H. H., M. E., T. J. S., and I. F. declare no conflicts of interest.
REFERENCES
- Akira A., Michio M., Yoshida T. inventors; Takasago Perfumery Co., Ltd, assignee. (1984). 3-Levo-Menthoxypropane-1,2-diol. U.S. Patent 4,459,425. July 10, 1984. [Google Scholar]
- Berke R., Singh A., Guralnick M. (2012). Atopic dermatitis: An overview. American Family Physician, 86 (1), 35– 42. [PubMed] [Google Scholar]
- Chu X., Ci X., Wei M., Yang X., Cao Q., Guan M., Deng X. (2012). Licochalcone a inhibits lipopolysaccharide-induced inflammatory response in vitro and in vivo. Journal of Agricultural and Food Chemistry, 60 (15), 3947– 3954. [DOI] [PubMed] [Google Scholar]
- Del Rosso J. Q., Kircik L. H. (2013). The integration of physiologically-targeted skin care in the management of atopic dermatitis: Focus on the use of a cleanser and moisturizer system incorporating a ceramide precursor, filaggrin degradation products, and specific “skin-barrier-friendly” excipients. Journal of Drugs in Dermatology, 12 (7), S85– S91. [PubMed] [Google Scholar]
- Eichenfield L. F., Ellis C. N., Mancini A. J., Paller A. S., Simpson E. L. (2012). Perspectives in atopic dermatitis: Optimizing outcomes. Seminars in Cutaneous Medicine and Surgery, 31 (Suppl. 3), S3– S5. [DOI] [PubMed] [Google Scholar]
- Eichenfield L. F., Tom W. L., Berger T. G., Krol A., Paller A. S., Schwarzenberger K., Sidbury R. (2014). Guidelines of care for the management of atopic dermatitis: Section 2. Management and treatment of atopic dermatitis with topical therapies. Journal of the American Academy of Dermatology, 71 (1), 116– 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis C., Luger T., Abeck D., Allen R., Graham-Brown R. A., De Prost Y., … ICCAD II Faculty. (2003). International Consensus Conference on Atopic Dermatitis II (ICCAD II): Clinical update and current treatment strategies. British Journal of Dermatology, 148 (Suppl 63), 3– 10. [DOI] [PubMed] [Google Scholar]
- Ellis C. N., Mancini A. J., Paller A. S., Simpson E. L., Eichenfield L. F. (2012). Understanding and managing atopic dermatitis in adult patients. Seminars in Cutaneous Medicine and Surgery, 31 (Suppl. 3), S18– S22. [DOI] [PubMed] [Google Scholar]
- Fernandes J. D., Machado M. C., Oliveira Z. N. (2011). Children and newborn skin care and prevention. Anais Brasileiros de Dermatologia, 86 (1), 102– 110. [DOI] [PubMed] [Google Scholar]
- Fowler J. F., Nebus J., Wallo W., Eichenfield L. F. (2012). Colloidal oatmeal formulations as adjunct treatments in atopic dermatitis. Journal of Drugs in Dermatology, 11 (7), 804– 807. [PubMed] [Google Scholar]
- Hanifin J. M., Cooper K. D., Ho V. C., Kang S., Krafchik B. R., Margolis D. J., Van Voorhees A. S. (2004). Guidelines of care for atopic dermatitis, developed in accordance with the American Academy of Dermatology (AAD)/American Academy of Dermatology Association “Administrative Regulations for Evidence-Based Clinical Practice Guidelines.” Journal of the American Academy of Dermatology, 50 (3), 391– 404. [DOI] [PubMed] [Google Scholar]
- Hanifin J. M., Rajka G. (1980). Diagnostic features of atopic dermatitis. Acta Dermato-Venereologica, 92 (Suppl.), 42– 47. [Google Scholar]
- Hon K. L., Kam W. Y., Leung T. F., Lam M. C., Wong K. Y., Lee K. C., Ng P. C. (2006). Steroid fears in children with eczema. Acta Paediatrica, 95, 1451– 1455. [DOI] [PubMed] [Google Scholar]
- Kezic S., Novak N., Jakasa I., Jungersted J. M., Simon M., Brandner J. M., Weidinger S. (2014). Skin barrier in atopic dermatitis. Frontiers in Bioscience (Landmark Edition), 19, 542– 556. [DOI] [PubMed] [Google Scholar]
- Kiebert G., Sorensen S. V., Revicki D., Fagan S. C., Doyle J. J., Cohen J., Fivenson D. (2002). Atopic dermatitis is associated with a decrement in health-related quality of life. International Journal of Dermatology, 41 (3), 151– 158. [DOI] [PubMed] [Google Scholar]
- Kojima R., Fujiwara T., Matsuda A., Narita M., Matsubara O., Nonoyama S., Matsumoto K. (2013). Factors associated with steroid phobia in caregivers of children with atopic dermatitis. Pediatric Dermatology, 30 (1), 29– 35. [DOI] [PubMed] [Google Scholar]
- Nikolovski J., Stamatas G. N., Kollias N., Wiegand B. C. (2008). Barrier function and water-holding and transport properties of infant stratum corneum are different from adult and continue to develop through the first year of life. Journal of Investigative Dermatology, 128 (7), 1728– 1736. [DOI] [PubMed] [Google Scholar]
- Ring J., Alomar A., Bieber T., Deleuran M., Fink-Wagner A., Gelmetti C., … European Dermatology Forum (EDF); European Academy of Dermatology and Venereology (EADV); European Federation of Allergy (EFA); European Task Force on Atopic Dermatitis (ETFAD); European Society of Pediatric Dermatology (ESPD); Global Allergy and Asthma European Network (GA2LEN). (2012). Guidelines for treatment of atopic eczema (atopic dermatitis) part I. Journal of the European Academy of Dermatology and Venereology, 26 (8), 1045– 1060. [DOI] [PubMed] [Google Scholar]
- Rippke F., Schreiner V., Doering T., Maibach H. I. (2004). Stratum corneum pH in atopic dermatitis: Impact on skin barrier function and colonization with staphylococcus aureus. American Journal of Clinical Dermatology, 5 (4), 217– 223. [DOI] [PubMed] [Google Scholar]
- Sabin B. R., Peters N., Peters A. T. (2012). Chapter 20: Atopic dermatitis. Allergy and Asthma Proceedings, 33 (Suppl 1), S67– S69. [DOI] [PubMed] [Google Scholar]
- Simpson E., Dutronc Y. (2011). A new body moisturizer increases skin hydration and improves atopic dermatitis symptoms among children and adults. Journal of Drugs in Dermatology, 10 (7), 744– 749. [PubMed] [Google Scholar]
- Simpson E., Trookman N. S., Rizer R. L., Preston N., Colón L. E., Johnson L. A., Gottschalk R. W. (2012). Safety and tolerability of a body wash and moisturizer when applied to infants and toddlers with a history of atopic dermatitis: Results from an open-label study. Pediatric Dermatology, 29 (5), 590– 597. [DOI] [PubMed] [Google Scholar]
- van Leent E. J., Gräber M., Thurston M., Wagenaar A., Spuls P. I., Bos J. D. (1998). Effectiveness of the ascomycin macrolactam SDZ ASM 981 in the topical treatment of atopic dermatitis. Archives of Dermatology, 134 (7), 805– 809. [DOI] [PubMed] [Google Scholar]
- Weber T. M., Babcock M. J., Herndon J. H., Jr., Schoelermann A. M., Filbry A., Scherdin U., Rippke F. (2014). Steroid-free emollient formulation reduces symptoms of eczema and improves quality of life. Journal of Drugs in Dermatology, 13 (5), 589– 595. [PubMed] [Google Scholar]


