Abstract
A genetic approach has been used to define the molecular basis for the different patterns of virulence and central nervous system cell tropism exhibited by reovirus types 1 and 3. Intracerebral inoculation of reovirus type 3 into newborn mice causes a necrotizing encephalitis (without ependymal damage) that is uniformly fatal. Animal inoculated with reovirus type 1 generally survive and may develop epedymal cell damage (without neuronal necrosis) and hydrocephalus. Using recombinant clones derived from crosses between reovirus types 1 and 3, we have been able to determine that the S1 genome segment is responsible for the differing cell tropism of reovirus serotypes and is the major determinant of neurovirulence. The type 1 S1 genome segment is responsible for ependymal damage with subsequent hydrocephalus; the type 3 S1 genome segment is responsible for neuronal necrosis and neurovirulence. We postulate that these differences are due to the specific interaction of the σ1 outer capsid polypeptide (the protein coded for by the S1 genome segment) with receptors on the surface of either ependymal cells or neuronal cells.
Keywords: cell tropism, neurovirulence, ependyma and neurons, reovirus recombinants
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNET M., LIND P. E. Genetics of virulence in influenza viruses. Nature. 1954 Apr 3;173(4405):627–630. doi: 10.1038/173627a0. [DOI] [PubMed] [Google Scholar]
- FRASER K. B. Features of the MEL x NWS recombination systems in influenza A virus. III. The pathogenicity of recombinant neuro-MEL viruses grown in mouse brain and chick embryo brain. Virology. 1959 Oct;9:191–201. doi: 10.1016/0042-6822(59)90114-x. [DOI] [PubMed] [Google Scholar]
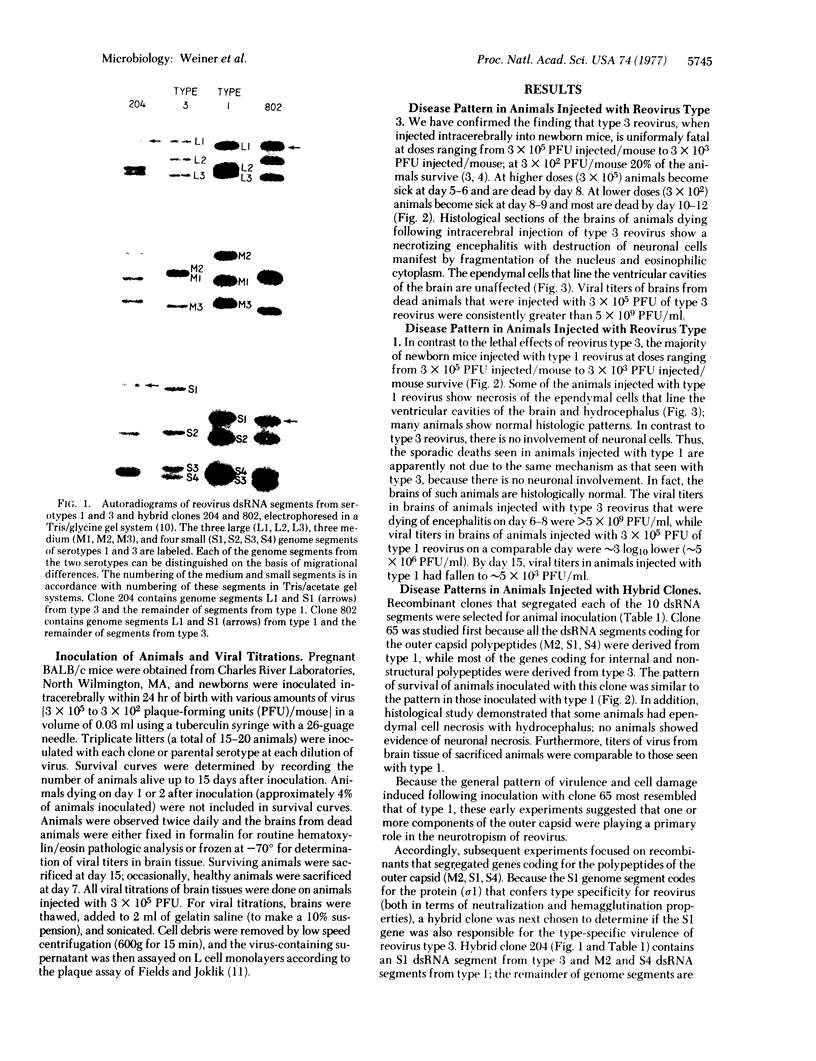
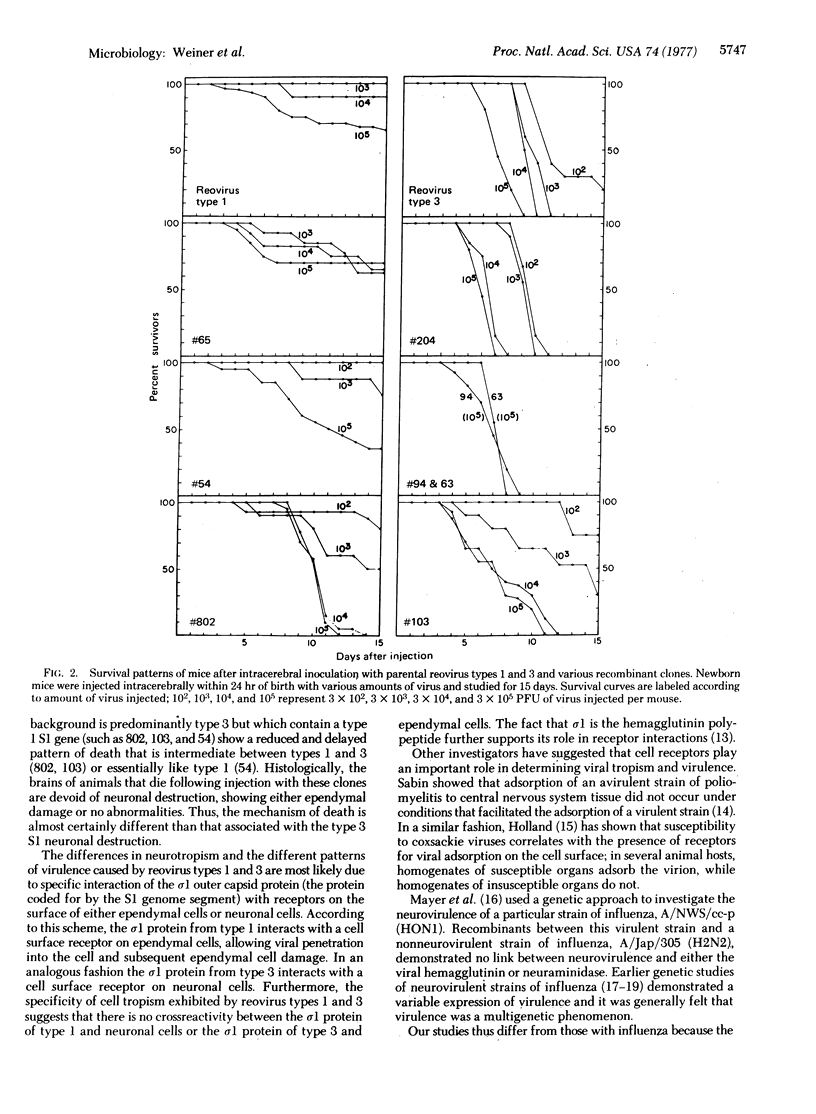
- Fields B. N., Joklik W. K. Isolation and preliminary genetic and biochemical characterization of temperature-sensitive mutants of reovirus. Virology. 1969 Mar;37(3):335–342. doi: 10.1016/0042-6822(69)90217-7. [DOI] [PubMed] [Google Scholar]
- GOTLIEB T., HIRST G. K. The experimental production of combination forms of virus. VI. Reactivation of influenza viruses after inactivation by ultraviolet light. Virology. 1956 Apr;2(2):235–248. doi: 10.1016/0042-6822(56)90019-8. [DOI] [PubMed] [Google Scholar]
- Gonatas N. K., Margolis G., Kilham L. Reovirus type 3 encephalitis: observations of virus-cell interactions in neural tissues. II. Electron microscopic studies. Lab Invest. 1971 Feb;24(2):101–109. [PubMed] [Google Scholar]
- HOLLAND J. J. Receptor affinities as major determinants of enterovirus tissue tropisms in humans. Virology. 1961 Nov;15:312–326. doi: 10.1016/0042-6822(61)90363-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Margolis G., Kilham L. Hydrocephalus in hamsters, ferrets, rats, and mice following inoculations with reovirus type I. II. Pathologic studies. Lab Invest. 1969 Sep;21(3):189–198. [PubMed] [Google Scholar]
- Masters C., Alpers M., Kakulas B. Pathogenesis of reovirus type 1 hydrocephalus in mice. Significance of aqueductal changes. Arch Neurol. 1977 Jan;34(1):18–28. doi: 10.1001/archneur.1977.00500130038008. [DOI] [PubMed] [Google Scholar]
- Mayer V., Schulman J. L., Kilbourne E. D. Nonlinkage of neurovirulence exclusively to viral hemagglutinin or neuraminidase in genetic recombinants of A-NWS (HON1) influenza virus. J Virol. 1973 Feb;11(2):272–278. doi: 10.1128/jvi.11.2.272-278.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEN L. Reoviruses in animals other than man. Ann N Y Acad Sci. 1962 Nov 30;101:461–465. doi: 10.1111/j.1749-6632.1962.tb18886.x. [DOI] [PubMed] [Google Scholar]
- ROSEN L. Serologic grouping of reoviruses by hemagglutination-inhibition. Am J Hyg. 1960 Mar;71:242–249. doi: 10.1093/oxfordjournals.aje.a120107. [DOI] [PubMed] [Google Scholar]
- Raine C. S., Fields B. N. Reovirus type 3 encephalitis--a virologic and ultrastructural study. J Neuropathol Exp Neurol. 1973 Jan;32(1):19–33. doi: 10.1097/00005072-197301000-00002. [DOI] [PubMed] [Google Scholar]
- Ramig R. F., Cross R. K., Fields B. N. Genome RNAs and polypeptides of reovirus serotypes 1, 2, and 3. J Virol. 1977 Jun;22(3):726–733. doi: 10.1128/jvi.22.3.726-733.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Zweerink H. J., Joklik W. K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Fields B. N. Neutralization of reovirus: the gene responsible for the neutralization antigen. J Exp Med. 1977 Nov 1;146(5):1305–1310. doi: 10.1084/jem.146.5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]