Abstract
Purpose
RAS mutations have been reported to be a potential prognostic factor in patients with colorectal liver metastases (CLM). However, the impact of RAS mutations on response to chemotherapy remains unclear. We sought to determine the association between RAS mutations and response to preoperative chemotherapy and their impact on survival in patients undergoing curative resection of CLM.
Methods
RAS mutational status was assessed and its relation to morphologic response and pathologic response was investigated in 184 patients meeting inclusion criteria. Predictors of survival were assessed. The prognostic impact of RAS mutational status was then analyzed using two different multivariate models including either radiologic morphologic response (model 1) or pathologic response (model 2).
Results
Optimal morphologic response and major pathologic response were more common in patients with wild-type RAS (32.9% and 58.9%, respectively) than in patients with RAS mutations (10.5% and 36.8%; P =.006 and .015, respectively). Multivariate analysis confirmed that wild-type RAS was a strong predictor of optimal morphologic response (odds ratio [OR], 4.38; 95% CI, 1.45-13.2) and major pathologic response (OR,2.79; 95% CI, 1.29-6.04). RAS mutations were independently correlated with both overall survival and recurrence free-survival (hazard ratios, 3.25 and 2.02, respectively, in model 1, and 3.19 and 2.23, respectively, in model 2). Subanalysis revealed that RAS mutational status clearly stratified prognosis in patients with inadequate response to preoperative chemotherapy.
Conclusion
RAS mutational status can be used to complement the current prognostic indicators for patients undergoing curative resection of CLM after preoperative modern chemotherapy.
Keywords: RAS mutation, response to chemotherapy, prognosis, colorectal liver metastasis
INTRODUCTION
Recent developments in chemotherapy and multidisciplinary approaches have changed the prognosis of patients with colorectal liver metastases (CLM).[1, 2] As a result, traditional prognostic factors such as disease-free interval, tumor number, tumor size, serum carcinoembryonic antigen level, and lymph node status have become less reliable.[3-8] Our group has previously reported that pathologic[9] and radiologic morphologic responses[10, 11] to preoperative chemotherapy are potent prognostic indicators for patients undergoing resection of CLM. However, most patients would be classified as inadequate responders according to these criteria, with 70% experiencing a suboptimal morphologic response[11] and 55% experiencing a minor pathologic response.[9] Moreover, the molecular bases for these prognostic indicators remain unclear.
During the last decade, the study of somatic gene mutations has become a focus of colorectal cancer research.[12-16] Especially, the mutational status of the RAS oncogene family (KRAS, NRAS, and the rarer HRAS) has been shown to be a strong predictor of response to anti-epidermal growth factor receptor (EGFR) agents.[16-18] Furthermore, two recent studies have suggested that KRAS mutation is a predictor of worse survival in patients undergoing resection of CLM regardless of the use of bevacizumab.[19, 20] Additionally, our group previously reported that KRAS mutation is related to more aggressive tumor biology.[21] However, the prognostic impact of RAS oncogene family mutation remains unclear, and little is known about the association between RAS mutational status and radiologic morphologic and pathologic responses to chemotherapy.
This study aimed to identify predictors of survival after curative resection of CLM, to define associations between RAS mutations and pathologic and/or morphologic response to chemotherapy, and finally, to assess whether RAS mutational status further stratifies patient prognosis within subgroups defined according to type of response to chemotherapy.
PATIENTS AND METHODS
Study Population
We searched the prospectively maintained hepatobiliary database at The University of Texas MD Anderson Cancer Center to identify patients who had undergone curative resection of CLM (without concomitant radiofrequency ablation) after a fluorouracil-based irinotecan regimen or a fluorouracil-based oxaliplatin regimen that included bevacizumab. Patients previously treated for hepatic and extrahepatic colorectal cancer metastases were excluded from our analysis, as were those who had received pre- or postoperative anti-EGFR agents, those who died within 90 days after hepatectomy, and those who had a viable tumor cell rate <5% in their CLM specimen. This study was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center (PA12-0736).
Somatic Gene Mutation Profiling
DNA extracted from formalin-fixed, paraffin-embedded resected CLM was analyzed with Sequenom MassArray technology (Sequenom, Inc, San Diego, CA) using a protocol developed in one of our institutional core facilities.[22] Tumor position was identified on slides with standard hematoxylin-eosin staining and microdissected. A total of 17 point mutations at codons 12, 13, 61, and 146 were tested in the RAS gene family (including KRAS and NRAS). Sequenom’s MassArray system utilizes polymerase chain reaction amplification and single-base primer extension for mutation detection.[23-25]
Assessment of Radiologic Morphologic Response to Chemotherapy
Computed tomographic scans were performed using a triphasic liver protocol or single-phase technique, as previously described.[10] The radiologic morphologic response of CLM was assessed by two independent radiologists (K.H. and E.M.L.) blinded to clinical data, according to the following previously described criteria: group 1, homogeneous low attenuation with a thin, sharply defined tumor-liver interface; group 3, heterogeneous attenuation with a thick, poorly defined tumor-liver interface; and group 2, intermediate morphology that cannot be classified as either group 1 or group 3.[10, 11] Optimal radiologic morphologic response to chemotherapy was defined as a change in morphology from group 3 or 2 to group 1. Suboptimal morphologic response was defined as a change in morphology from group 3 to group 2 or the absence of remarkable changes in morphology. In patients with multiple tumors, morphologic response was determined according to the response seen in the majority of tumors or, in patients with two CLM, in the largest one.
Assessment of Pathologic Response to Chemotherapy
Hematoxylin-eosin-stained specimens sectioned into 5-mm-thick slices were evaluated by a gastrointestinal pathologist who was blinded to clinical data. The extent of residual carcinoma was assessed semiquantitatively as the percentage of the total tumor surface area composed of residual viable tumor cells, as previously described. Pathologic response was scored as minor if residual viable tumor cells made up ≥50% of the total tumor surface area and major if residual viable tumor cells made up < 50%% of the total tumor surface area.[9]
Statistical Analysis
To identify predictors of optimal morphologic response and major pathologic response, categorical variables were compared using the χ2 test or Fisher’s exact test, and continuous variables were compared using the Mann-Whitney U test, where appropriate. Overall survival (OS) was measured from the date of hepatectomy until the date of death or last follow-up. Recurrence-free survival (RFS) was measured from the date of hepatectomy until the date of radiographic detection of any recurrence or last follow-up. Survival curves were generated using the Kaplan-Meier method, and survival differences were evaluated with the log-rank test.
Based on results from previous studies, 11 variables were identified as the most important factors affecting survivals after liver resection for CLM and consequently included in univariate analysis of predictors of OS and RFS: disease-free interval between primary tumor and CLM diagnosis, Primary tumor location (colon vs rectum), Primary tumor nodal status, presence of extrahepatic metastases, number of preoperative chemotherapy cycles, number and diameter of CLM, positive surgical margin, pathologic and morphologic response to chemotherapy, and RAS mutational status.
To identify independent prognostic factors for RFS and OS, all variables included in the univariate analysis (except pathologic response in model 1 and morphologic response in model 2) were subsequently included in a multivariate regression analysis, performed using the Cox proportional hazards model with backward elimination for variables with P < .1. Given the strong colinearity between pathologic and morphologic responses to chemotherapy, prognostic factors influencing survival were analyzed using two different multivariate models that included either pathologic response (model 1) or morphologic response (model 2).[10, 11] To identify factors associated with optimal morphologic or major pathologic response, a multivariate analysis was performed using the logistic regression model for clinical variables with P < .1 in univariate analysis. Statistical analyses were performed using IBM SPSS software (version 19.0; SPSS Inc., IL, USA). All statistical tests were two-sided, and significance was set at P < .05.
RESULTS
Patient Characteristics and comparison of patients according to RAS mutational status
Of the 308 patients initially eligible for this study, we excluded 36 patients who lacked tissue samples for DNA extraction owing to complete pathologic response, 30 patients whose DNA samples were of insufficient quality for Sequenom analysis, and 58 patients whose computed tomographic images were of insufficient quality or whose lesions were too small (<10 mm after preoperative chemotherapy) for morphologic response to be determined. Baseline characteristics of the remaining 184 patients included in the current study are summarized in Table 1. RAS mutations were seen in 38 patients (21% of the population; 32 had KRAS mutation and 6 had NRAS mutation). There was no significant difference in demographic and clinical characteristics between the patients with RAS mutation and those without, except for optimal morphologic and major pathologic response, which were significantly more frequent among RAS wild-type patients, compared to RAS mutants. BRAF mutation was observed only in 1 patient with RAS wild type.
Table 1.
Baseline Demographic and Clinical Characteristics of the study population and comparison of characteristics according to RAS mutational status.*
| Characteristic | Overall study population (n=184) |
RAS mutational status |
p value | ||
|---|---|---|---|---|---|
| Mutant (n=38) |
Wild-type (n=146) |
||||
| Age, years | |||||
| Median | 58 | 58 | 57 | .473 | |
| Range | 25–84 | 33-81 | 25-84 | ||
|
| |||||
| Sex | |||||
| Male | 107 | (58) | 18 (47) | 89 (61) | .130 |
| Female | 77 | (42) | 20 (53) | 57 (39) | |
|
| |||||
| Primary tumor location | |||||
| Rectum | 37 | (20) | 6(16) | 31 (21) | .476 |
| Colon | 147 | (80) | 32 (84) | 115 (79) | |
|
| |||||
| Primary tumor nodal status | |||||
| Negative | 52 | (28) | 10 (26) | 42 (29) | .765 |
| Positive | 132 | (72) | 28 (74) | 104 (71) | |
|
| |||||
| Extrahepatic metastases | |||||
| Yes | 16 | (9) | 4(11) | 12(8) | .653 |
| No | 168 | (91) | 36 (89) | 134 (92) | |
|
| |||||
| DFI, years | |||||
| <1 | 122 | (66) | 23 (61) | 99 (68) | .398 |
| ≥1 | 62 | (34) | 15 (39) | 47 (32) | |
|
| |||||
| Multiple CLM | |||||
| Yes | 62 | (34) | 14 (37) | 48 (33) | .645 |
| No | 122 | (66) | 24 (63) | 98 (67) | |
|
| |||||
| CLM size at surgery, cm | |||||
| Median | 2.5 | 2.5 | 2.5 | .713 | |
| Range | 1–15 | 1.2-13.5 | 1-15 | ||
|
| |||||
| Fluorouracil-based chemotherapy regimen |
|||||
| Oxaliplatin | 137 | (74) | 25 (66) | 112 (77) | .209 |
| Irinotecan | 47 | (36) | 13 (34) | 34 (23) | |
|
| |||||
| Number of preoperative chemotherapy cycles |
|||||
| Median | 6 | 8 | 6 | .597 | |
| Range | 5–18 | 6-18 | 5-18 | ||
|
| |||||
| Morphologic response | |||||
| Optimal | 52 | (28) | 4(11) | 48 (33) | .006 |
| Suboptmal | 132 | (72) | 34 (89) | 96 (67) | |
|
| |||||
| Pathologic response | |||||
| Major | 100 | (54) | 14 (37) | 86 (59) | .015 |
| Minor | 84 | (46) | 24 (63) | 60 (41) | |
|
| |||||
| Portal vein embolization | 39 | (21) | 10 (26) | 29 (20) | .363 |
|
| |||||
| Hepatectomy | |||||
| Major resection | 123 | (67) | 25 (66) | 98 (67) | .876 |
| Minor resection | 61 | (33) | 13 (34) | 48 (33) | |
|
| |||||
| Operating time, min | |||||
| Median | 231 | 235 | 230 | .880 | |
| Range | 55–535 | 62-460 | 55-535 | ||
|
| |||||
| Intraoperative transfusion | 14 | (8) | 3(8) | 11(8) | .915 |
|
| |||||
| Surgical margin | |||||
| Positive | 23 | (12) | 7(18) | 16(11) | .215 |
| Negative | 161 | (88) | 31 (82) | 130 (89) | |
|
| |||||
| Morbidity | |||||
| None | 100 | (54) | 19 (50) | 81 (55) | .168 |
| Minor | 51 | (28) | 8(21) | 43 (30) | |
| Major | 33 | (18) | 11 (29) | 22(15) | |
|
| |||||
| RAS mutational status | |||||
| Wild-type | 146 | (79) | |||
| Mutant | 38 | (21) | |||
| KRAS | 32/38 | (84) | |||
| Codon 12 | 28/32 | (88) | |||
| Codon 13 | 2/32 | (6) | |||
| Codon 61 | 2/32 | (6) | - | - | - |
| Codon 146 | 0/32 | (0) | |||
| NRAS | 6/38 | (16) | |||
| Codon 12 | 3/6 | (50) | |||
| Codon 13 | 0/6 | (0) | |||
| Codon 61 | 3/6 | (50) | |||
| Codon 146 | 0/6 | (0) | |||
Abbreviations: DFI, disease-free interval; CLM, colorectal liver metastases.
Values in table are number of patients (%) unless otherwise specified.
Predictors of Pathologic and Morphologic Response to Chemotherapy
Optimal morphologic response was observed in 52 of the 184 patients (28%), and major pathologic response was observed in 100 patients (54%). Tumor cell viability differed according to morphologic response (median viability, 30% [range, 5%-70%] in patients with optimal response vs. 50% [range, 5%-90%] in patients with suboptimal response [P < .001]). Optimal morphologic response and major pathologic response were more common in patients with wild-type RAS (32.9% and 58.9%, respectively) than in patients with RAS mutations (10.5% and 36.8%; P =.006 and .015, respectively). Multivariate analysis showed that wild-type RAS (odds ratio [OR], 4.38; 95% confidence interval [CI], 1.45-13.2) and largest CLM size ≤3 cm (OR, 1.98; 95% CI, 1.02-3.87) was associated with optimal morphologic response. Wild-type RAS (OR, 2.79; 95% CI, 1.29-6.04) and CLM size ≤3 cm (OR 2.39; 95% CI, 1.25-4.59) was also associated with major pathologic response (Table 2).
Table 2.
Univariate and Multivariate Analysis of Predictors of Optimal Morphologic and Major Pathologic Response
| Factor | Morphologic Response |
Pathologic Response |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Optimal Response (n = 52) (%) |
Univariate Analysis P Value |
Multivariate Analysis |
Major Response (n = 100) (%) |
Univariate Analysis P Value |
Multivariate Analysis |
|||||
| P | OR | 95% CI | P | OR | 95% CI | |||||
| DFI, years | ||||||||||
| <1 (n = 122) | 34 (27.9) | .868 | 61 (50) | .097 | ||||||
| ≥1 (n = 62) | 18 (29) | 39 (62.9) | ||||||||
|
| ||||||||||
| Primary tumor location | ||||||||||
| Rectum (n = 37) | 11 (29.7) | .824 | 19 (51.4) | .682 | ||||||
| Colon (n = 147) | 41 (27.9) | 81 (55.1) | ||||||||
|
| ||||||||||
| Primary tumor nodal status |
||||||||||
| Positive (n= 132) | 36 (27.3) | .635 | 67 (50.8) | .119 | ||||||
| Negative (n = 52) | 16(30.8) | 33 (63.5) | ||||||||
|
| ||||||||||
| Preoperative chemotherapy |
||||||||||
| >6 cycles (n = 66) | 14(21.2) | .112 | 35 (53) | .788 | ||||||
| ≤6 cycles (n = 118) | 38 (32.2) | 65 (55.1) | ||||||||
|
| ||||||||||
| Multiple CLM | ||||||||||
| Yes (n = 122) | 36 (29.5) | .598 | 66 (54.1) | .924 | ||||||
| No (n = 62) | 16(25.8) | 34 (54.8) | ||||||||
|
| ||||||||||
| Largest CLM at diagnosis |
||||||||||
| ≤3 cm (n = 72) | 26 (36.1) | .058 | .044 | 1.98 | 1.02-3.87 | 46 (63.9) | .037 | .009 | 2.39 | 1.25-4.59 |
| >3 cm (n = 112) | 26 (23.2) | 54 (48.2) | ||||||||
|
| ||||||||||
| RAS mutational status | ||||||||||
| Wild-type (n = 146) | 48 (32.9) | .006 | .008 | 4.38 | 1.45-13.15 | 86 (58.9) | .015 | .009 | 2.79 | 1.29-6.04 |
| Mutant (n = 38) | 4 (10.5) | 14(36.8) | ||||||||
Abbreviations: OR, odds ratio; DFI, disease-free interval; CLM, colorectal liver metastases.
When classifying patients according to percentage of viable tumor cells, RAS mutation rate decreased as the percentage of viable tumor cells declined. RAS mutation rate was 33% in patients with more than 80% of viable tumor, 29% in those with viable tumors cells ranging between 61 and 80%, 26% among patients with 41-60% of viable tumor cells, 15% in those with 21-40% of viable cells, and 13% in those with 5-20% viable cells (P =.021) .
Risk Factors for Decreased OS and RFS
Multivariate analysis showed that independent predictors of decreased OS were RAS mutation (hazard ratio [HR], 3.25), minor pathologic response (HR, 1.78), and having undergone >6 preoperative chemotherapy cycles (HR, 1.70) in model 1 and RAS mutation (HR, 3.19), suboptimal morphologic response (HR, 2.32), and positive nodal status for the primary tumor (HR, 1.95) in model 2 (Table 3). Multivariate analysis for RFS revealed that independent predictors of decreased RFS were RAS mutation (HR, 2.02), having undergone >6 chemotherapy cycles (HR, 1.57), and minor pathologic response (HR: 1.78) in model 1 and RAS mutation (HR, 2.23), suboptimal morphologic response (HR, 1.81), having undergone > 6 chemotherapy cycles (HR, 1.44), and largest CLM >5 cm (HR, 1.89) in model 2.
Table 3.
Univariate and Multivariate Analysis of Predictors of Overall Survival (OS)
| Factor | 5-year OS, % |
Median OS, mo |
Univariate Analysis |
Multivariate Analysis* |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
||||||||||
| P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | |||
| DFI, years | |||||||||||
| <1 (n= 122) | 49.6 | 71.4 | .252 | 1.39 | 0.79-2.44 | ||||||
| ≥1 (n= 62) | 65.9 | 79.7 | |||||||||
|
| |||||||||||
| Primary tumor location | |||||||||||
| Rectum (n= 37) | 47.8 | 71.4 | .254 | 1.41 | 0.79-2.54 | ||||||
| Colon (n= 147) | 57.3 | 74.8 | |||||||||
|
| |||||||||||
| Primary tumor nodal status |
|||||||||||
| Positive (n= 132) | 47.4 | 72.8 | .031 | 2.06 | 1.07-3.97 | .114 | 1.71 | 0.88-3.35 | .049 | 1.95 | 1.01-3.81 |
| Negative (n= 52) | 74.1 | 77.8 | |||||||||
|
| |||||||||||
| Extrahepatic metastases | |||||||||||
| Yes (n= 16) | 31.5 | 83.9 | .765 | 1.16 | 0.42-3.23 | ||||||
| No (n= 168) | 56 | 73.3 | |||||||||
|
| |||||||||||
| Preoperative chemotherapy |
|||||||||||
| >6 cycles (n= 66) | 42.6 | 63.7 | .022 | 1.82 | 1.09-3.03 | .044 | 1.70 | 1.01 - 2.86 | .088 | 1.57 | 0.93-2.64 |
| ≤6 cycles (n= 118) | 64.5 | 81.4 | |||||||||
|
| |||||||||||
| Multiple CLM | |||||||||||
| Yes (n= 122) | 53.3 | 74.6 | .775 | 1.08 | 0.62-1.88 | ||||||
| No (n= 62) | 60.3 | 73.4 | |||||||||
|
| |||||||||||
| Largest CLM at surgery | |||||||||||
| >5 cm (n= 26) | 48.6 | 61.7 | .199 | 1.54 | 0.80-2.96 | ||||||
| ≤5 cm (n= 158) | 56.4 | 76.5 | |||||||||
|
| |||||||||||
| Positive margin | |||||||||||
| Yes (n= 23) | 65.8 | 76.8 | .959 | 1.02 | 0.44-2.38 | ||||||
| No (n= 161) | 54.6 | 74.4 | |||||||||
|
| |||||||||||
| Pathologic response | |||||||||||
| Minor (n= 84) | 44.6 | 61.5 | .005 | 2.11 | 1.25-3.56 | .033 | 1.78 | 1.05 - 3.03 | - | - | - |
| Major (n= 100) | 64.2 | 85.3 | |||||||||
|
| |||||||||||
| Morphologic response | |||||||||||
| Suboptimal (n= 132) | 49.2 | 68.7 | .007 | 2.78 | 1.31-5.85 | - | - | - | .029 | 2.32 | 1.09-4.97 |
| Optimal (n= 52) | 72.9 | 89 | |||||||||
|
| |||||||||||
| RAS mutational status | |||||||||||
| Mutant (n= 38) | 23.2 | 43.4 | <.001 | 3.57 | 1.99-6.40 | <.001 | 3.25 | 1.80 - 5.87 | <.001 | 3.19 | 1.75-5.78 |
| Wild-type (n= 146) | 61.6 | 81.2 | |||||||||
Abbreviations: HR, hazard ratio; DFI, disease-free interval; CLM, colorectal liver metastasis.
In Model 1, all factors except morphologic response were entered in the multivariate analysis. In Model 2, all factors except pathologic response were entered in the multivariate analysis.
Survival Outcomes
After a median follow-up of 33 months, 64 patients had died (61 of cancer and three of other causes), 40 were alive with disease recurrence, and 80 were alive with no evidence of disease at last follow-up. OS rates for the entire cohort were 97.8% at 1 year, 72.6% at 3 years, and 52.4% at 5 years. The 5-year OS rate was higher for patients with major pathologic response (64.2%) than for those with minor pathologic response (44.6%; P = .005) (Fig 1A), higher for patients with optimal morphologic response (72.9%) than for those with suboptimal morphologic response (42.9%; P = .007) (Fig 1C), and higher for patients with wild-type RAS (61.6%) than for those with RAS mutation (23.2%; P < .001; Table 3). Likewise, the 5-year RFS rate was higher for patients with major pathologic response (35.8%) than for those with minor pathologic response (16.5%; P < .001), higher for patients with optimal morphologic response (39.8%) than for those with suboptimal morphologic response (22.2%; P = .002), and higher for patients with wild-type RAS (31.7%) than for those with RAS mutation (NA; P < .001).
Fig 1.
(A-D) Cumulative overall survival curves for patients with major and minor pathologic response overall (A) and stratified according to RAS mutational status (B). Cumulative overall survival curves for patients with optimal and suboptimal morphologic response overall (C) and stratified according to RAS mutational status (D).
Additional subanalysis revealed that among patients with minor pathologic response (Fig 1B), patients with RAS mutation had a significantly worse 3-year OS rate than patients with wild-type RAS (30.9% v 72.3%). Similarly, among patients with suboptimal morphologic response (Fig 1D), patients with RAS mutation had a significantly worse 3-year OS rate than patients with wild-type RAS (41.8% v 76.8%).
DISCUSSION
In this study, we analyzed 184 patients who underwent curative resection after receiving a fluorouracil-based irinotecan regimen or a fluorouracil-based oxaliplatin regimen that included bevacizumab. RAS mutational status independently predicted both morphologic response and pathologic response to this type of chemotherapy. RAS mutational status also independently predicted both OS and RFS, and further subanalysis revealed that RAS mutational status stratified survival outcomes in patients with suboptimal morphologic response or minor pathologic response to chemotherapy.
In the era of modern chemotherapy, pathologic response[9, 26, 27] and morphologic response[10, 11] have been proposed as new endpoints to predict long-term outcomes in patients undergoing resection of CLM after chemotherapy. However, these response criteria can be used only after chemotherapy, and the molecular bases for differences in susceptibility to chemotherapy remain unclear. RAS mutation is a well-known predictor of lack of response to anti-EGFR agents in both patients with advanced metastatic colorectal cancer[16, 18] and patients with metastases limited to the liver.[28] Additionally, a growing body of evidence suggests that the RAS mutational status of CLM might be associated with tumor aggressiveness.[19-21]
In this study, optimal morphologic response was less common in patients with RAS mutation (10.5%) than those with wild-type RAS (32.9%). Likewise, major pathologic response was less common in patients with RAS mutation (36.8%), than in those with wild-type RAS (56.8%). As confirmed in the present study, there was an association between RAS mutation rate and percentage of viable tumor cells after preoperative chemotherapy. These results suggest that RAS mutational status would be a strong predictor of response to chemotherapy not only in patients receiving anti-EGFR antibodies as previously reported, but also in patients receiving preoperative modern chemotherapy containing bevacizumab.
Another important result was that the prognosis of patients with optimal morphologic or major pathologic response was favorable independent of RAS mutational status. In contrast, among patients with suboptimal morphologic or minor pathologic response to chemotherapy, those with RAS mutation had a significantly lower OS rate than did those with wild-type RAS (Fig 1). These results indicate that RAS mutational status is not only associated with response to preoperative chemotherapy, but is also a strong prognostic factor in patients with poor response to chemotherapy.
The current data, if confirmed in larger studies, could be used to predict clinical outcomes precisely, not only after resection but also when resection is being considered. Among patients with multiple ill-located metastases necessitating extended, risky resections, a combination of radiologic response of CLM and RAS mutational status (determined by analyzing the primary tumor or by using biopsy specimens from the primary tumor or CLM) could help select patients who might benefit from a more aggressive surgical approach. In this study, among patients with a suboptimal morphologic response, those with wild-type RAS had a 3-year OS rate of 76.8%, which was significantly higher than the OS rate for patients with RAS mutation (41.8%; P < 0.001) (Fig 1C). Thus, while patients with a combination of suboptimal morphologic response and RAS mutation should generally be considered candidates for small/not technically demanding liver resection if negative margins can be reasonably expected, the same patients should be considered more cautiously for complex hepatectomy because the risk related to extensive hepatectomy may outweigh the limited potential survival benefit (Fig 2).
Fig 2.
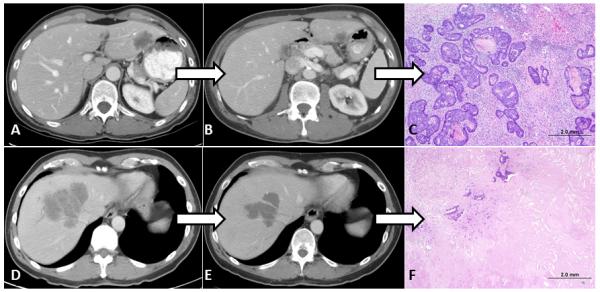
(A-C) A 57-year-old patient with a single CLM in segments 2-3 and RAS mutation. (A) Before initiation of treatment. (B) Suboptimal radiologic morphologic response after 4 months of preoperative FOLFOX (leucovorin, fluorouracil, and oxaliplatin) with bevacizumab. (C) Minor pathologic response (75% viable tumor cells in CLM) after resection. The patient died of disease 18 months after undergoing a partial left hepatectomy. (D-F) A 68-year-old patient with a single large CLM involving the origin of the right and middle hepatic veins and wild-type RAS. (D) Before initiation of treatment. (E) Optimal morphologic response after 3 months of preoperative FOLFOX with bevacizumab. (F) Major pathologic response (<10% viable tumor cells in CLM) after resection. As of this writing, the patient is alive and disease-free more than 5 years after extended right hepatectomy.
One criticism of this study is that RAS mutational status was determined on resected CLM and not on the primary tumor and that the two tumor sites might differ in mutational status. However, a growing body of evidence suggests a high rate of concordance in somatic gene mutational status between primary tumor and related metastases, strengthening the validity of our results.[29-33] Other potential criticisms of this study include its retrospective nature and the selected population. Although this study was conducted using data from a prospectively constructed database of patients undergoing resection of CLM, a number of patients were excluded from the initial population because of a lack of sufficient tumor for DNA extraction and Sequenom analysis. However, even after exclusion of patients with extremely good pathologic response to chemotherapy, morphologic and pathologic response independently predicted survival, consistent with results from previous studies based on large cohorts of patients[9, 11]. Moreover, RAS mutational status remained a powerful prognostic factor. Although OS may have been affected by anti-EGFR therapy after recurrence, the impact of RAS status on RFS suggests that anti-EGFR therapy has a minimal confounding effect.
In conclusion, we investigated the genetic backgrounds affecting pathologic response and morphologic response in patients treated with bevacizumab and found that RAS mutational status is associated with both morphologic and pathologic response to chemotherapy. RAS mutations, pathologic response, and morphologic response independently predict survival outcomes in patients undergoing resection of CLM after preoperative chemotherapy. RAS mutations clearly stratify survival outcomes in patients with inadequate response to chemotherapy. Finally, RAS mutational status should be used as a complementary molecular marker of response to chemotherapy and of prognosis both before and after preoperative chemotherapy for CLM. Wild-type RAS status may encourage some surgeons to pursue resection in patients with borderline resectable disease and suboptimal response to chemotherapy. As such, the current results may contribute to personalized care in the treatment of CLM.
ACKNOWLEDGEMENTS
The authors would like to recognize Ms. Ruth Haynes for administrative support in the preparation of this manuscript.
Funding sources: This research was supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant, CA016672.
REFERENCES
- 1.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopetz S, Vauthey JN. Perioperative chemotherapy for resectable hepatic metastases. Lancet. 2008;371:963–965. doi: 10.1016/S0140-6736(08)60429-8. [DOI] [PubMed] [Google Scholar]
- 3.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. discussion 318-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwatsuki S, Dvorchik I, Madariaga JR, et al. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999;189:291–299. doi: 10.1016/s1072-7515(99)00089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 6.Rees M, Tekkis PP, Welsh FK, et al. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: A multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 7.Jarnagin WR. Clinical scoring systems for stratifying risk after resection of hepatic colorectal metastases: still relevant? Ann Surg Oncol. 2011;18:2711–2713. doi: 10.1245/s10434-011-1821-1. [DOI] [PubMed] [Google Scholar]
- 8.Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 9.Blazer DG, 3rd, Kishi Y, Maru DM, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–5351. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 10.Chun YS, Vauthey JN, Boonsirikamchai P, et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009;302:2338–2344. doi: 10.1001/jama.2009.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shindoh J, Loyer EM, Kopetz S, et al. Optimal morphologic response to preoperative chemotherapy: an alternate outcome end point before resection of hepatic colorectal metastases. J Clin Oncol. 2012;30:4566–4572. doi: 10.1200/JCO.2012.45.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bos JL, Fearon ER, Hamilton SR, et al. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987;327:293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- 13.Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 14.Andreyev HJ, Norman AR, Cunningham D, et al. Kirsten ras mutations in patients with colorectal cancer: the multicenter “RASCAL” study. J Natl Cancer Inst. 1998;90:675–684. doi: 10.1093/jnci/90.9.675. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe T, Wu TT, Catalano PJ, et al. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med. 2001;344:1196–1206. doi: 10.1056/NEJM200104193441603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 17.Lievre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 18.Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 19.Nash GM, Gimbel M, Shia J, et al. KRAS mutation correlates with accelerated metastatic progression in patients with colorectal liver metastases. Ann Surg Oncol. 2010;17:572–578. doi: 10.1245/s10434-009-0605-3. [DOI] [PubMed] [Google Scholar]
- 20.Stremitzer S, Stift J, Gruenberger B, et al. KRAS status and outcome of liver resection after neoadjuvant chemotherapy including bevacizumab. Br J Surg. 2012;99:1575–1582. doi: 10.1002/bjs.8909. [DOI] [PubMed] [Google Scholar]
- 21.Andreou A, Aloia TA, Brouquet A, et al. Margin status remains an important determinant of survival after surgical resection of colorectal liver metastases in the era of modern chemotherapy. Ann Surg. 2013;257:1079–1088. doi: 10.1097/SLA.0b013e318283a4d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabriel S, Ziaugra L, Tabbaa D. SNP genotyping using the Sequenom MassARRAY iPLEX platform. Curr Protoc Hum Genet. 2009 doi: 10.1002/0471142905.hg0212s60. Chapter 2: Unit 2 12. [DOI] [PubMed] [Google Scholar]
- 24.Thomas RK, Baker AC, Debiasi RM, et al. High-throughput oncogene mutation profiling in human cancer. Nat Genet. 2007;39:347–351. doi: 10.1038/ng1975. [DOI] [PubMed] [Google Scholar]
- 25.Pearce M, Ehrich M. Somatic Mutation Analysis in Tumor Samples using the Sequenom. In MassARRAY® System. San Diego: Sequenom. 2011 [Google Scholar]
- 26.Rubbia-Brandt L, Giostra E, Brezault C, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299–304. doi: 10.1093/annonc/mdl386. [DOI] [PubMed] [Google Scholar]
- 27.Poultsides GA, Bao F, Servais EL, et al. Pathologic response to preoperative chemotherapy in colorectal liver metastases: fibrosis, not necrosis, predicts outcome. Ann Surg Oncol. 2012;19:2797–2804. doi: 10.1245/s10434-012-2335-1. [DOI] [PubMed] [Google Scholar]
- 28.Folprecht G, Gruenberger T, Bechstein WO, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 29.Santini D, Loupakis F, Vincenzi B, et al. High concordance of KRAS status between primary colorectal tumors and related metastatic sites: implications for clinical practice. Oncologist. 2008;13:1270–1275. doi: 10.1634/theoncologist.2008-0181. [DOI] [PubMed] [Google Scholar]
- 30.Knijn N, Mekenkamp LJ, Klomp M, et al. KRAS mutation analysis: a comparison between primary tumours and matched liver metastases in 305 colorectal cancer patients. Br J Cancer. 2011;104:1020–1026. doi: 10.1038/bjc.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Italiano A, Hostein I, Soubeyran I, et al. KRAS and BRAF mutational status in primary colorectal tumors and related metastatic sites: biological and clinical implications. Ann Surg Oncol. 2010;17:1429–1434. doi: 10.1245/s10434-009-0864-z. [DOI] [PubMed] [Google Scholar]
- 32.Etienne-Grimaldi MC, Formento JL, Francoual M, et al. K-Ras mutations and treatment outcome in colorectal cancer patients receiving exclusive fluoropyrimidine therapy. Clin Cancer Res. 2008;14:4830–4835. doi: 10.1158/1078-0432.CCR-07-4906. [DOI] [PubMed] [Google Scholar]
- 33.Oudejans JJ, Slebos RJ, Zoetmulder FA, et al. Differential activation of ras genes by point mutation in human colon cancer with metastases to either lung or liver. Int J Cancer. 1991;49:875–879. doi: 10.1002/ijc.2910490613. [DOI] [PubMed] [Google Scholar]



