Abstract
Minihepcidins are small peptides that mimic biological activity of the iron-regulatory hormone hepcidin. Structurally, they contain thiol-free-cysteine residue in position 7 which is crucial for their bioactivity. Nonetheless, free sulfhydryl group is not desirable in pharmaceutical entities as it may lead to dermatological side effects. Moreover free thiol moiety is quite reactive and depending on conditions/reagents may be alkylated and/or oxidized giving various Cys-derivatives: S-alkyl cysteines, sulfoxides, sulfones, disulfides, cysteinesulfinic and cysteic acids. To limit such reactivity and maintain bioactivity of minihepcidin(s) we used thiol-protection strategy based on activated vinyl thioethers. Novel S-protected analogs of physiologically active minihepcidin PR73 were synthesized and tested in vitro showing activity comparable to parental molecule. The most active compound, PR73SH was also tested in vivo showing activity profile analogous to PR73. Collectively, our findings suggest that S-vinyl-derivatization of minihepcidin(s) may be a suitable approach in the development of physiologically active agonists of hepcidin.
Keywords: Minihepcidins, Peptides, S-Alkylation of peptides, Iron
Hepcidin is a key regulator of iron homeostasis1 and its abnormal production has been associated with several common iron disorders2. This 25 amino acids long peptide hormone is mainly produced in the liver3 although other tissues were also implicated4;5. The iron-regulatory function of hepcidin is mediated through its receptor, ferroportin (Fpn)6, which is the sole known cellular iron exporter in vertebrates7. Mechanistically, hepcidin’s binding to Fpn causes the receptor internalization and subsequent proteasomal degradation, effectively reducing systemic iron availability, by decreasing both, the absorption of iron (duodenum) and release of recycled iron (macrophages)8.
Hepcidin and related pathways, have recently emerged as an attractive target for the development of novel therapeutics for iron disorders, with diverse group of leading compounds under development, such as monoclonal antibodies, proteins, peptides, oligonucleotides and small organic molecules9–11.
We previously described minihepcidins, rationally designed peptide-based hepcidin agonists, with potent in vitro and in vivo bioactivity12;13, which are currently under commercial development by Merganser Biotech LLC. Collectively, available data strongly suggest that minihepcidins may be useful for the prevention of iron overload, or as an auxiliary in combination with phlebotomy or chelation for the treatment of existing iron overload. Structurally, these analogs contain unprotected free-cysteine residue in position 7, which is crucial for their bioactivity. However, drugs containing/releasing free sulfhydryl group(s) may be problematic in pharmaceutical development due to either (1) decreased stability associated with inherent free-thiol reactivity (S-alkylation/oxidation), and/or (2) dermatological side effects (e.g. skin eruptions)14;15. Therefore, we decided to test whether minihepcidins can be efficiently S-derivatized and retain their bioactivity. Based on previous findings we concluded that most obvious –S-S-R derivative(s) (e.g. S-tertbutylthio), although viable, may not represent an optimal protecting strategy, as such analog (C7-SStBut) showed significantly lower activity compared to parental free-SH peptide (hep9)12. As an alternative protective moiety we decided to use 1,2-double substituted vinyl-sulfides which may be efficiently synthesized from corresponding electron-deficient alkynes and unprotected free-cysteine containing peptides in aqueous media16 (Figure 1). Notably, similar cysteine-protecting/labeling synthetic schemes employing, both mono-17;18 and double-substituted alkynes17–19, were recently reported. Analogous reaction(s) employing allenes were also successfully used to create vinylthioether-linkage20;21. In our case, all employed electron-deficient alkynes were commercially available and symmetrical (X1=X2), except for PR73SE (X1=phenyl, X2=−SO2NH2). For structural details please see Figure 216. Parental minihepcidin PR73 was synthesized as previously described12. S-alkylation reaction was carried out in the mixture of 80% 1,4-dioxane in water due to physical properties of PR73 (e.g. relatively high hydrophobicity resulting from the presence of N-palmitylamide) and proceeded efficiently over 25 min (room temp.). The use of acetylenedicarboxylic acid as alkylating reagent lead to partially decarboxylated analog PR73SD, which is consistent with previously reported data22. Obtained analogs were purified by RP-HPLC and characterized by matrix-assisted laser desorption ionization spectrometry (MALDI-MS) as well as analytical RP-HPLC23 (Table 1).
Figure 1.
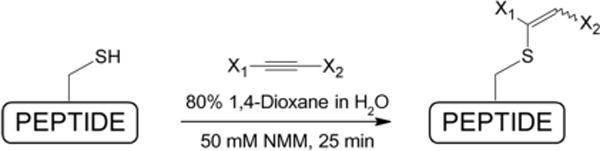
General synthetic scheme for derivatization of minihepcidin PR73.
Figure 2.
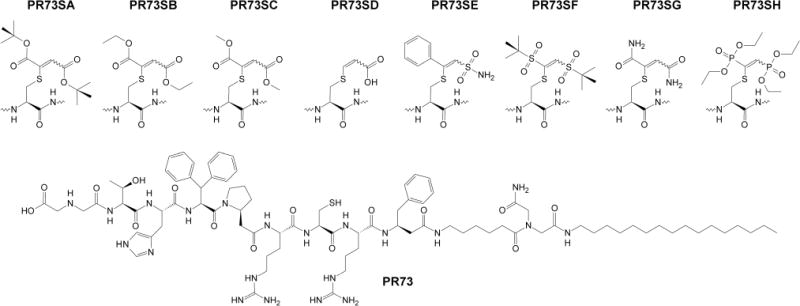
General structure of S-derivatized PR73 analogs.
Table 1.
Analytical and in vitro activity data for S-alkylated PR73 analogs.
| Peptide | Composition | MW Calc/Found | RT [min] | EC50 [nM] TREX-hFpn-GFP cells |
|---|---|---|---|---|
| PR73 | C86H133N21O15S | 1733.19/1734.34 | 47.11 | 4.2±0.3 |
| PR73SA | C98H151N21O19S | 1959.46/1959.80 | 52.47 | 6.3±1.2 |
| PR73SB | C94H143N21O19S | 1903.35/1904.58 | 49.44 | 10.4±1.2 |
| PR73SC | C92H139N21O19S | 1875.30/1876.60 | 48.32 | 12.6±1.8 |
| PR73SD | C89H135N21O17S | 1803.24/1803.66 | 46.60 | 218.1±13.4 |
| PR73SE | C94H140N22O17S2 | 1914.40/1915.02 | 48.52 | 34.0±5.4 |
| PR73SF | C96H151N21O19S3 | 1999.56/1999.80 | 52.89* | 10.0±3.4 |
| PR73SG | C90H137N23O17S | 1845.28/1846.59 | 49.33* | 8.4±2.5 |
| PR73SH | C96H153N21O21P2S | 2031.40/2031.33 | 52.18* | 1.1±0.1 |
Analytical RP-HPLC was performed using an analytical reversed-phase C4 XBridge™ BEH300 column, 4.6×150 mm, 3.5 μm (Waters, Milford, MA), or (*) an analytical reversed-phase C18 SymmetryShield™ column, 4.6×250 mm, 5 μm (Waters, Milford, MA).
Novel S-protected analogs were tested in vitro using a previously described cellular assay based on Fpn degradation25 (results summarized in Table 1). Generally, all new analogs showed high potency in the low nanomolar range. However only one analog (PR73SH, EC50=1.1±0.1 nM) showed bioactivity higher than parental PR73 (EC50=4.2±0.3 nM). Interestingly, chemical making of S-substituent doesn’t appear to have clear, defined impact on bioactivity, rather overall steric hindrance plays the major role, with the most bulky substituents having favorable properties. The hydrophobicity may also play some role, as the activity increases in the carboxy-esters-substituent(s) order:
Moreover, the geometry of the vinyl substituents (planar versus tetrahedral) does not appear to significantly influence activity, as planar analog PR73SA has fairly similar potency to its tetragonal counterpart (PR73SF). Considering that remaining tetragonal analog PR73SH shows highest activity, and the fact that all 3 analogs (e.g. PR73SA, PR73SF and PR73SH) are chemically fairly similar having the same number of substituent(s)-carbon-atoms (2×4=8), overall volume/space occupied by S-attached moiety appears again as important factor, with the activity increasing from most compact (PR73SF) to most bulky (PR73SH) substituent(s). Consistently, PR73SD which has the most hydrophilic and least bulky substituent shows the lowest potency (EC50=218.1±13.4 nM).
Based on in vitro results, we selected PR73SH as suitable candidate for animal studies26, which were carried out as previously described12;13;25. We compared directly PR73SH and PR73 in vivo activity by assaying serum iron levels at 3 time points: (6, 24 and 48 hours) and concentrations that were previously shown to be sufficient for PR73 to exert potent bioactivity (50–100 nmoles/mouse). Because disulfide bond (–S-S-) crosslinking between minihepcidin C7 and Fpn C326 seems to be essential for their interaction12, we considered that protection of free –SH group in PR73SH might results in a “delayed” onset of activity, analogous to previously observed effects12 and/or extended time of bioactivity. Nonetheless, PR73SH activity mirrored the parental PR73 activity profile, with decreased serum iron observed at 6 and 24 h time points, but not at 48 h time point (Figure 3B). Since no significant activity difference between PR73 and PR73SH was observed in either, in vitro or in vivo experiments, we conclude that proposed free-cysteine derivatization scheme of minihepcidins is viable option in future development of these promising drug candidates. Notably, PR73SH appears also to be remarkably stable in mildly oxidizing conditions as prolonged storage of the compound in DMSO (10 mM solution) at room temperature for 30 days shows very limited levels of decomposition or sulfide oxidation (99.5±0.5% of stability, determined by LC/MS/MS experiments). Interestingly, compounds containing similar functional group(s) described to date17–19 show diverse bio-stability levels.
Figure 3.
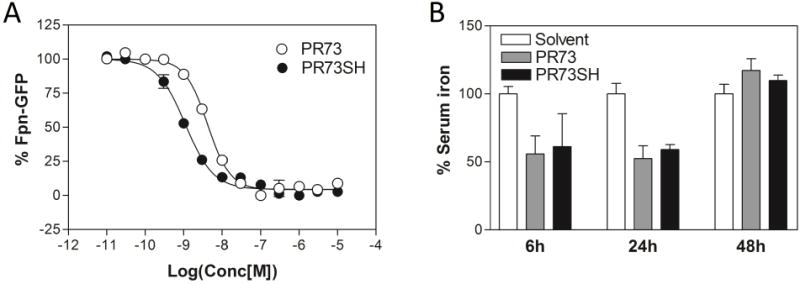
Comparison of in vitro and in vivo activity of PR73 and PR73SH: (A) the representative examples of in vitro dose response curves obtained for PR73 and PR73SH analogs using ferroportin degradation assay, (B) in vivo activity of PR73 and PR73SH at 6, 24 and 48h time-points.
In conclusion, a new scheme of free-thiol derivatization in peptides was described and applied in synthesis of S-substituted analogs of potent minihepcidin PR73. Various 1,2-double substituted vinyl-sulfides of the peptide (PR73SA-PR73SH) were tested in vitro showing activity comparable to the parental compound (PR73), with the most potent analog PR73SH being slightly more active then original peptide. PR73SH was additionally tested in vivo showing activity profile similar to the parental free-thiol containing analog (PR73). Considering its simplicity, the protocol described in our study may be useful as a general method of free-thiol derivatization in pharmaceutically relevant peptides.
Acknowledgments
This project was partially supported by funds from the Adams and Burnham endowments provided by the Dean’s Office of the David Geffen School of Medicine at UCLA (PR) and NIH grant R01 DK90554 (TG and EN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Ganz T. Blood. 2003;102:783. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- 2.Ganz T, Nemeth E. Annu Rev Med. 2011;62:347. doi: 10.1146/annurev-med-050109-142444. [DOI] [PubMed] [Google Scholar]
- 3.Park CH, Valore EV, Waring AJ, Ganz T. J Biol Chem. 2001;276:7806. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 4.Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B, Iannelli A, Staccini-Myx A, Casanova D, Ben A, Saint-Paul M, Huet PM, Sadoul JL, Gugenheim J, Srai SK, Tran A, Le Marchand-Brustel Y. Gastroenterology. 2006;131:788. doi: 10.1053/j.gastro.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Zechel S, Huber-Wittmer K, von Bohlen, H O. J Neurosci Res. 2006;84:790. doi: 10.1002/jnr.20991. [DOI] [PubMed] [Google Scholar]
- 6.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Science. 2004;306:2090. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 7.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. Cell Metab. 2005;1:191. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Ganz T, Nemeth E. Biochim Biophys Acta. 2012;1823:1434. doi: 10.1016/j.bbamcr.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fung E, Nemeth E. Haematologica. 2013;98:1667. doi: 10.3324/haematol.2013.084624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poli M, Asperti M, Ruzzenenti P, Regoni M, Arosio P. Front Pharmacol. 2014;5:86. doi: 10.3389/fphar.2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruchala P, Nemeth E. Trends Pharmacol Sci. 2014;35:155. doi: 10.1016/j.tips.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preza GC, Ruchala P, Pinon R, Ramos E, Qiao B, Peralta MA, Sharma S, Waring A, Ganz T, Nemeth E. J Clin Invest. 2011;121:4880. doi: 10.1172/JCI57693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramos E, Ruchala P, Goodnough JB, Kautz L, Preza GC, Nemeth E, Ganz T. Blood. 2012;120:3829. doi: 10.1182/blood-2012-07-440743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitamura K, Aihara M, Osawa J, Naito S, Ikezawa Z. J Dermatol. 1990;17:44. doi: 10.1111/j.1346-8138.1990.tb01608.x. [DOI] [PubMed] [Google Scholar]
- 15.Kitamura K, Aihara M, Ikezawa Z. J Dermatol. 1990;17:168. doi: 10.1111/j.1346-8138.1990.tb01620.x. [DOI] [PubMed] [Google Scholar]
-
16.Synthesis of S-alkylated analogs PR73SA-PR73SH. S-alkylated analogs were derived in one step reaction from parental peptide PR73 PR73 was synthesized as previously described12 Peptide was solubilized in 80% 1,4-dioxane in water, containing 50 mM N-methylmorpholine (NMM) (~2 mg/mL) and subsequently a proper alkyne derivative was added (2 eq.) Mixture was vigorously stirred for 25 min and subsequently lyophilized Obtained solid residue was purified by preparative reverse-phase high performance liquid chromatography (RP-HPLC) and its purity evaluated by matrix-assisted laser desorption ionization spectrometry (MALDI-MS) as well as analytical RP-HPLC (see Table 1) Alkyne derivatives were for
PR73SA – Di-tert-butyl acetylenedicarboxylate,
PR73SB – Diethyl acetylenedicarboxylate,
PR73SC – Dimethyl acetylenedicarboxylate,
PR73SD – Acetylenedicarboxylic acid,
PR73SE – Pifithrin-μ,
PR73SF – 1,2-Bis(tert-but ylsulfonyl)acetylene,
PR73SG – Acetylenedicarboxamide,
PR73SH – Bis(diethoxyphosphoryl)acetylene.
- 17.Shiu HY, Chan TC, Ho CM, Liu Y, Wong MK, Che CM. Chem Eur J. 2009;15:3839. doi: 10.1002/chem.200800669. [DOI] [PubMed] [Google Scholar]
- 18.Shiu HY, Chong HC, Leung YC, Wong MK, Che CM. Chem Eur J. 2010;16:3308. doi: 10.1002/chem.200903121. [DOI] [PubMed] [Google Scholar]
- 19.Koniev O, Leriche G, Nothisen M, Remy JS, Strub JM, Schaeffer-Reiss C, Van DA, Baati R, Wagner A. Bioconjug Chem. 2014;25:202. doi: 10.1021/bc400469d. [DOI] [PubMed] [Google Scholar]
- 20.Abbas A, Xing B, Loh TP. Angew Chem Int Ed Engl. 2014;53:7491. doi: 10.1002/anie.201403121. [DOI] [PubMed] [Google Scholar]
- 21.Chan AO, Tsai JL, Lo VK, Li GL, Wong MK, Che CM. Chem Commun (Camb) 2013;49:1428. doi: 10.1039/c2cc38214h. [DOI] [PubMed] [Google Scholar]
- 22.Ranjit S, Duan Z, Zhang P, Liu X. Org Lett. 2010;12:4134. doi: 10.1021/ol101729k. [DOI] [PubMed] [Google Scholar]
- 23.Analytical RP-HPLC was performed on a Varian ProStar 210 HPLC system equipped with ProStar 325 Dual Wavelength UV-Vis detector with the wavelengths set at 220 nm and 280 nm (Varian Inc., Palo Alto, CA). Mobile phases consisted of solvent A, 0.1% TFA in water, and solvent B, 0.1% TFA in acetonitrile. Analyses of peptides were performed with an analytical reversedphase C4 XBridge™ BEH300 column, 4.6×150 mm, 3.5 μm (Waters, Milford, MA), or (*) an analytical reversed-phase C18 SymmetryShield™ column, 4.6×250 mm, 5 μm (Waters, Milford, MA)applying linear gradient of solvent B from 0 to 100% over 100 min (flow rate: 1 ml/min).
- 24.In vitro ferroportin (Fpn) degradation assay was carried out as previously descried25. Briefly, HEK293:TREX-Fpn-GFP, a cell line stably transfected with the human ferroportin-GFP construct under the control of doxycycline-inducible promoter, was plated on poly-D-lysine–coated plates in the presence of 20 μM FAC. Fpn expression was induced with 500 ng/mL doxycycline treatment for 24 hours. Then, doxycycline was washed off, and cells were treated with peptides for 24 hours. Cells were then trypsinized and resuspended at 1 × 106 cells/mL, and the intensity of green fluorescence was analyzed by flow cytometry using FACScan (fluorescence activated cell scanner) Analytic Flow Cytometer (Becton Dickinson, San Jose, CA) with CellQuest version 3.3 so ftware. Cells not induced with doxycycline to express Fpn-GFP were used to establish a gate to exclude background fluorescence. Cells induced with doxycycline, but not treated with any peptides, were used as the positive control. Each peptide treatment was repeated independently 3 to 6 times. The results were expressed as a fraction of the activity of hep25, according to the formula 1 − [(Fx − Fhep25)/(Funtreated − Fhep25)], where F was the mean of the gated green fluorescence and x was the peptide.
- 25.Nemeth E, Preza GC, Jung CL, Kaplan J, Waring AJ, Ganz T. Blood. 2006;107:328. doi: 10.1182/blood-2005-05-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Animal studies were carried out as previously described2,13,25. Animal studies were approved by the Animal Research Committee at UCLA. Briefly, C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and were maintained on NIH 31 rodent diet (iron content 336 mg/kg; Harlan Teklad, Indianapolis, IA). Mice were injected intraperitoneally either with 100 μL PBS (control) or with 50 or 100 nmoles peptide in 100 μL PBS. Mice were killed 6, 24, and 48 hours later, blood was collected by cardiac puncture, and serum was separated using Microtainer tubes (Becton Dickinson, Franklin Lakes, NJ). Serum iron was determined by using a colorimetric assay (Diagnostic Chemicals, Oxford, CT) which was modified for the microplate format so that 50 μL serum was used per measurement. The results were expressed as the percentage of decrease in serum iron when compared with the average value of serum iron levels in PBS-injected mice.


