Abstract
BACKGROUND/OBJECTIVES
Although single, high doses of vitamin D effectively maintain vitamin D sufficiency in several populations, no studies have evaluated healthy adults over winter, during which vitamin D status declines. This study investigated whether high-dose vitamin D3 given once to healthy adults before winter will (1) prevent the wintertime decline in vitamin D status, (2) promote vitamin D sufficiency 1 year following the dose and (3) prevent the rise of parathyroid hormone (PTH) concentrations.
SUBJECTS/METHODS
In this double-blind, placebo-controlled trial, we assessed plasma 25(OH)D and PTH concentrations at baseline, 5, 90 and 365 days after drug administration in 28 healthy adults. In all, >80% of subjects returned at each time point.
RESULTS
At baseline, the young, healthy participants had a mean plasma 25(OH)D concentration of 17.5 ± 6.1 ng/ml. Only two subjects exhibited plasma 25(OH)D concentrations >30 ng/ml. At 5 days, subjects randomized to vitamin D3 had a higher mean plasma 25(OH)D concentration compared with the placebo group (39.1 vs 19.1 ng/ml, P<0.001). Plasma 25(OH)D concentrations returned to baseline at 90 and 365 days in the vitamin D3 group and remained unchanged in the placebo group. PTH and calcium concentrations were unrelated to changes in 25(OH)D levels and similar between groups over time.
CONCLUSIONS
A dose of 250 000 IU of vitamin D3 given once in November resulted in a robust increase in plasma 25(OH)D after 5 days, but it was unable to sustain this increase after 90 days. A larger or more frequent dosing regimen may be needed for long-term vitamin D sufficiency.
INTRODUCTION
Vitamin D status is best assessed by measurement of the serum 25-hydroxyvitamin D (25(OH)D) concentration, the major circulating form of vitamin D.1 Exposure to ultraviolet-B (UVB) radiation from the sun is the major determinant of serum 25(OH)D concentration in humans,2 as UVB radiation is necessary for the conversion of 7-dehydrocholesterol to vitamin D in the skin.3 Changes in vitamin D status throughout the year occur in part owing to seasonal changes in the zenith angle of the earth to the sun, which cause less UVB radiation to penetrate the Earth's surface during the winter.4 As a result, circulating serum 25 (OH)D concentrations are generally lowest in the late winter and early spring and highest in late summer and early fall,5–11 with greater seasonal variation occurring with increasing latitude.5
Bolus administration of large, oral vitamin D doses offers a means to mitigate the winter-related decline in serum 25(OH)D concentrations and potentially stabilize vitamin D status throughout the year. This approach may be advantageous because several large clinical trials report low adherence to daily doses of oral vitamin D.12–14 Vitamin D given as a single, large, bolus dose has demonstrated higher adherence rates compared with daily and monthly dosing regimens.15 Large doses offer consistent efficacy and safety in improving vitamin D status and suppressing markers of inflammation and bone health, such as parathyroid hormone (PTH), in a variety of populations.16 Vitamin D supplemented in large doses has a sustained effect; once ingested, some vitamin D is converted to 25(OH)D, whereas the remainder is redistributed into fat, from which it is slowly released over time.17 Owing to the long circulating half-life of 25(OH)D in blood (2–3 weeks),18,19 vitamin D administered in daily, weekly and monthly dosing can sustain the same circulating concentrations of 25(OH)D over an equivalent period of time.20
Large, oral, bolus doses of vitamin D have been examined in several studies, with the greatest efficacy shown with the use of >200 000 IU of vitamin D and using cholecalciferol rather than ergocalciferol.16 However, no study to our knowledge has assessed the effect of a single, oral dose on vitamin D status over winter months in a healthy, young adult population. In the present study, we evaluated whether a single 250 000 IU oral dose of vitamin D3 administered before winter prevents a wintertime decline in circulating plasma 25(OH)D and promotes optimal concentrations of 25(OH)D and PTH throughout the entire year in a healthy, young, adult population.
SUBJECTS AND METHODS
This study was approved by the Emory University Institutional Review Board (project approval number: IRB00061103) and registered with clinicaltrials.gov (NCT01924910).
Recruitment
We recruited healthy adults between August and December 2012 with flyer advertisements on Emory University campus (Atlanta, GA, 30322) and Institutional Review Board-approved emails. All participants were between the ages of 18 and 65 years and gave written informed consent before participation in the study. We assessed each subject's eligibility via a questionnaire at enrollment. All participants were in good health by self-report and were not pregnant or breast-feeding. Subjects were excluded because of conditions that could pose safety risks following high-dose vitamin D, including granulomatous conditions, kidney or liver disease, diabetes, certain medications (anticonvulsants, barbiturates, steroids, >1000 mg/day calcium supplementation), calcium or bone abnormalities (hyperparathyroidism, Paget's disease, osteoporosis), thyrotoxicosis, history of malignancy or complete immobilization. Skin pigment was classified using a Fitzpatrick skin type assessment.21
Design
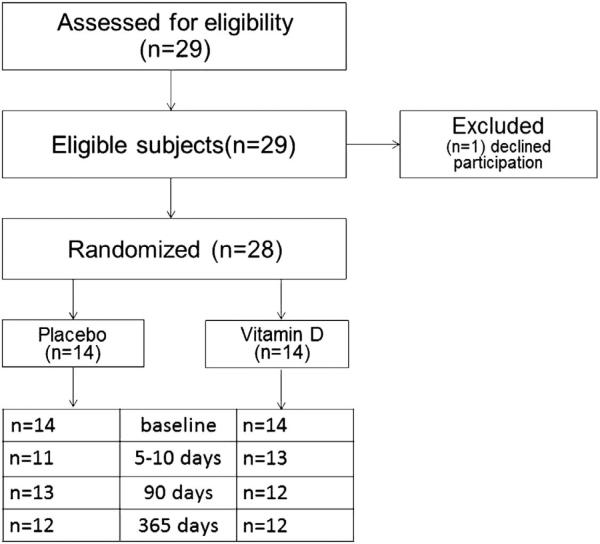
Twenty-eight participants were randomized into treatment and placebo groups, summarized in Figure 1. Subjects were randomized into five blocks of six (three placebo and three treatment in each block) by the Emory Investigational Drug Service. The vitamin D3 and placebo pills were purchased from Biotech, Pharmacal (Fayetteville, AK, USA). They were identical in size, shape and color. Each vitamin D capsule contained 50 000 IU of vitamin D3 and each placebo pill contained only the vehicle substance. Participants’ blood was drawn for baseline values in November 2012, and then they were directly observed to take either 250 000 IU (6.25 g contained in five pills) of oral vitamin D3 (n = 14) or five pills of an identical placebo (n = 14). Additional blood draws occurred after 5–10 days, 90 days and 365 days. For safety purposes, serum calcium was assessed between 5 and 10 days, and questions about hypercalcemia symptoms were administered at each visit. Vitamin D sufficiency was defined as plasma 25(OH)D concentration ≥ 30 ng/ml.
Figure 1.
CONSORT Diagram. Twenty-nine adults were deemed eligible for inclusion by our screening questionnaire and provided informed consent for participation in the study. One subject declined participation following consent and before administration of the vitamin D dose or placebo. The remaining 28 subjects were randomized into blocks of six. In each block, three subjects received placebo and three received vitamin D. The subjects demonstrated good follow-up (>80% at 5, 90 and 365 days) with attrition randomly in both groups, aside from one subject who did not return following the dose in the placebo group.
Analytical methods
Blood samples were processed and stored at − 80 °C for analysis. Analysis was completed in two cumulative batches to reduce inter-assay variability. Plasma 25(OH)D and PTH concentrations were analyzed using IDS-iSYS immunoassay (Immunodiagnostic Systems, Inc., Fountain Hills, Arizona). External quality control assessment for vitamin D analysis was conducted in partnership with the Vitamin D External Quality Assessment Scheme (DEQAS, site #606). The calculated intra-assay coefficient of variance (CV) was 4.33% based on samples selected to be retested (n = 12), and inter-assay CV was 10.1–13%, using previous results from the same diagnostics machine.22 Serum calcium was analyzed by Emory Medical Laboratory services using the Unicel D×C 800 instrument (Beckman-Coulter, Fullerton, CA, USA).
Statistical analysis
Group comparisons involving categorical and continuous outcomes at baseline were evaluated with χ2-tests and t-tests, respectively. Measurements of the primary end points were taken on the same participant at four time points. Statistical analyses of repeated measurements were performed using a linear mixed-effects model, which provided separate estimates of the mean by time on intervention and control groups. Any difference in response profiles among treatments was reflected in the group-by-time interaction term, indicating that group differences varied with time. The model-based means are unbiased with unbalanced and incomplete data, provided that the missing data are noninformative (missing at random). A compound symmetry form in the repeated measurements was assumed for each outcome. In addition, because the repeated measurements had skewed distributions, they were log-transformed. Geometric means were then calculated; comparisons of the geometric means between groups were made using the geometric mean ratio.
Assuming a standard deviation of 10 ng/ml in the change in mean plasma 25(OH)D at a significance level of 0.05 using a two-sided two-sample t test, 28 subjects (14 in each arm) would provide greater than 90% power to detect an increase in 25(OH)D of 13 ng/ml compared with placebo between baseline and post-intervention. Assuming about 10% participant withdrawal or dropout, 30 subjects were to be recruited. All statistical calculations were performed using SAS version 9.3 (Cary, NC, USA). Confidence intervals were determined using a confidence level of 95%; statistical tests were two-sided and were performed at a 5% level of significance.
RESULTS
Subject demographics
Twenty-eight subjects were enrolled: 14 randomized to vitamin D3 and 14 to placebo. There were no significant differences in the baseline characteristics of the treatment and placebo groups (Table 1). Both study arms were primarily female, white (skin pigmentation between 2 and 4 in 86% of subjects by the Fitzpatrick skin assessment) and age <30 years. Data collected by self-report indicated that individuals had a normal BMI and spent <10 h outdoors per week (range 2–20 h). At baseline, two participants had 25(OH)D concentrations >30 ng/ml, five had plasma 25(OH)D between 20 and 30 ng/ml and the remainder had plasma 25(OH)D concentrations <20 ng/ml. Five of the subjects were receiving daily vitamin D supplementation at baseline (400 to 1000 IU); only one subject receiving prior supplementation was vitamin D sufficient (plasma 25(OH)D ≥ 30 ng/ml), whereas all others had 25(OH)D concentrations <20 ng/ml and as low as 10.2 ng/ml. No subjects reported intake of calcium supplements, treatment for serious illness, tobacco use or alcohol consumption greater than moderate amounts of alcohol according to the 2010 Dietary Guidelines for Americans (>1 drink/day for female subjects, >2 drinks /day for male subjects).23 Study participants demonstrated good follow-up at each time point (83.3%, 87.5% and 83.3% at 5, 90 and 365 days, respectively), with attrition nearly equally distributed between the groups (Figure 1).
Table 1.
Participant demographics
| Baseline characteristics | Study arm |
|
|---|---|---|
| Vitamin D (n = 14) | Placebo (n = 14) | |
| Age, year, mean (s.d.) | 28.2 (6.7) | 26.5 (5.2) |
| Female, n (%) | 12 (86) | 10 (71) |
| White, n (%) | 9 (64) | 9 (64) |
| Weight, kg, mean (s.d.) | 66.6 (9.3) | 65.5 (10.2) |
| Height, m, mean (s.d.) | 1.7 (0.1) | 1.7 (0.1) |
| BMI, mean (s.d.) | 23.7 (2.9) | 22.3 (2.2) |
| Fitzpatrick scale, n | ||
| Type 2 | 5 | 6 |
| Type 3 | 4 | 4 |
| Type 4 | 2 | 2 |
| Serum calcium level, mg/dl, mean (s.d.) | 9.3 (0.3) | 9.2 (0.3) |
| Hours outdoors, week, mean (s.d.) | 9.0 (5.2) | 7.0 (5.4) |
| Current vitamin D supplementation, n | 4 | 1 |
Study participants (n = 28) at baseline were between ages 18 and 65 years and were healthy by self-report. Participants were not pregnant or chronically ill and worked at indoor occupations. None of the participants reported smoking or excessive alcohol consumption. Hours outdoors, height, weight and details of current vitamin D supplementation were collected by self-report. There were no significant differences between the groups at baseline. Few participants (n = 2) were vitamin D sufficient (25(OH)D >30 ng/ml), and many (n = 21) had 25(OH)D concentrations < 20ng/ml. Of five participants taking vitamin D supplementation (400-1000IU daily) at the start of the study, only one was vitamin D sufficient.
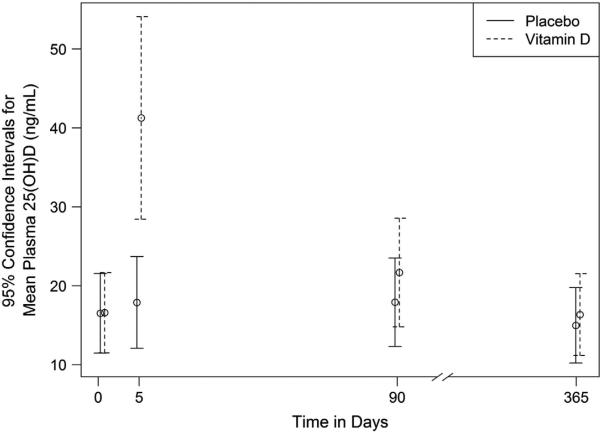
Impact of bolus vitamin D3 on plasma 25(OH)D concentrations The plasma 25(OH)D response to 250 000 IU of vitamin D3 measured at baseline, 5 days, 90 days and 365 days is presented graphically in Figure 2. At baseline, both study groups demonstrated similar plasma 25(OH)D concentrations, with geometric means of 16.58 and 16.51 ng/ml for the treatment and placebo groups, respectively (geometric mean ratio = 1.00, P = 0.97). Within 5–10 days from baseline, the plasma 25(OH)D concentration geometric mean was 41.27 ng/ml (95% CI 34.85–48.87) in the vitamin D3 group and 17.88 ng/ml (95% CI 14.97–21.36) in the placebo group, respectively (geometric mean ratio = 2.31, P<0.001). However, 25(OH)D concentrations were similar between study groups at the 90- and 365-day time points, respectively (Figure 2).
Figure 2.
Geometric mean plasma 25(OH)D at each visit. Plasma 25-hydroxyvitamin D (25(OH)D) concentrations were measured at baseline, 5 days, 90 days and 365 days following an oral dose of 250 000 IU of vitamin D3. The plasma 25(OH)D concentration increased 130% relative to placebo at the 5-day time point (GMR 2.31, P<0.0001), but it did not sustain this significant increase at additional time points. There was no significant change in either group from baseline measured over the winter months (comparing baseline and 90-day time points). Geometric means at each visit are plotted, together with their 95% confidence intervals.
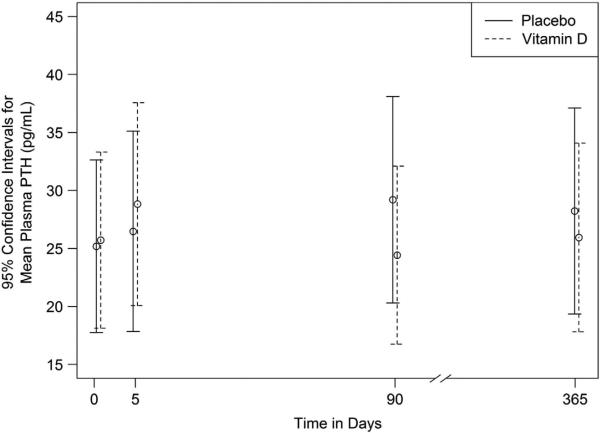
Impact of bolus vitamin D3 on plasma PTH concentrations
PTH concentrations were measured at baseline, 5 days, 90 days and 365 days, and are shown in Figure 3. At baseline, both the vitamin D and placebo groups had nearly equal PTH concentrations (geometric mean ratio 1.02, P = 0.86), with no study participants outside a normal range (8–51 ng/l). Geometric means at each visit are plotted, together with their 95% confidence intervals. There were no significant differences in PTH concentrations between groups at any of the study time points after vitamin D3 administration.
Figure 3.
Geometric mean plasma PTH at each visit. Plasma parathyroid hormone (PTH) concentrations were measured at baseline, 5 days, 90 days and 365 days following an oral dose of 250 000 IU vitamin D3. At baseline, no participants had abnormal PTH concentrations. There was no significant difference between PTH concentrations at baseline and any additional time points or between groups. Geometric means at each visit are plotted, together with their 95% confidence intervals.
Adverse events
There were no signs of hypervitaminosis D following the administration of this dose. Serum calcium between groups was not significantly different after 5 days (P = 0.73), with all individuals remaining within the reference range of our laboratory. One study participant in the vitamin D group developed a nontoxic goiter 11 months after receiving the treatment.
DISCUSSION
In our population of young, healthy, adults, a single dose of 250 000 IU of vitamin D3 produced a robust and significant increase in plasma 25(OH)D within 5 days, but fell to baseline values within 90 days of receiving the dose. We observed no corresponding changes in plasma PTH concentration and no changes in plasma calcium levels during the study.
Our results reproduced the expected physiologic effect of a large, oral bolus on vitamin D status; oral doses of vitamin D3 >100 000 IU tend to cause a peak in circulating 25(OH)D concentrations between 7 and 30 days following the dose16 followed by a gradual decline.24–26 However, the dose used in our study appears to be inadequate to promote vitamin D sufficiency over the winter months and throughout the following year in the population we studied. Although vitamin D3 supplementation with doses >100 000 IU and doses >200 000 IU are generally able to sustain 25(OH)D concentrations >20 ng/ml and >30 ng/ml, respectively, in the short term27–37 such increases are only sustained at 3-month time points in studies that used either larger doses of vitamin D3 (500 00034 and 600 000 IU33,36) or administered lower doses (100 00038 and 250 000 IU of vitamin D330) to relatively vitamin D-sufficient populations (27.1 and 30.5 ng/ml, respectively). In contrast, in our study population at baseline, only two participants were vitamin D sufficient, whereas five were insufficient and 21 were deficient (plasma 25(OH)D concentration <20 ng/ml). The degree of vitamin D deficiency observed in our sample, which comprised participants who spent <10 h outdoors per week (range 2–20 h/week), has been reported elsewhere. It is well-documented that indoor workers are vitamin D deficient compared with those who work outdoors39–42 and that vitamin D status is correlated with hours spent outdoors.40 However, the participants in this study had a lower 25(OH)D concentration at baseline than indoor workers in other studies,39,41,42 and spent less time outdoors by self-report than the mean reported in another study of indoor workers.40
Our findings also reinforced the idea that many people receiving daily vitamin D supplementation may still not have optimal vitamin D status. Of five participants receiving vitamin D supplementation at baseline, only one was vitamin D sufficient, whereas the others had 25(OH)D concentrations <20 ng/ml. Although it is likely that the adherence to daily dosing was highly variable in these selected participants, this finding may also indicate that daily supplementation with 400–1000 IU is inadequate in vitamin D-deficient populations. As suggested by Vieth et al.,43 doses as high as 1 700 IU daily may be necessary to maintain blood 25(OH)D concentrations >32 ng/ml.
Overall, it is likely, given the vitamin D deficiency of our population, that a higher dose of vitamin D3, or potentially even additional doses in insufficient individuals, could be necessary to promote optimal concentrations of vitamin D over a 3- to 4-month period following the initial bolus. Providing 50 000 IU of vitamin D3 at monthly intervals sustained 25(OH)D concentrations >20 ng/ml in 96.2% of a healthy, young, vitamin D-insufficient population when administered over 6 months,44 and concentrations averaging >30 ng/ml when administered over 3–5 months in vitamin D-insufficient elderly individuals.32 Preceding 50 000 IU monthly boluses by a 500 000 IU loading dose yielded even better results, showing vitamin D status to increase safely during the first month.32 Murdoch et al.45 showed efficacy with a similar dosing strategy; 100 000 IU doses of vitamin D3 administered monthly (following one or two 200 000 IU loading doses) promoted continued vitamin D sufficiency and prevented the decline in winter concentrations of vitamin D. Ultimately, although a variety of potential strategies may provide more sustained circulating 25 (OH)D concentrations, it appears that the magnitude of the dose necessary may be linked to the baseline vitamin D status of the population.
Strengths of our study include 100% adherence to the initial dose and >80% follow-up at each of the three time points, with attrition between groups randomly distributed. Subjects were also fairly healthy by self-report, with few reported medications (oral contraceptives, selective serotonin reuptake inhibitors, proton-pump inhibitors, albuterol inhalers and short courses of antibiotics), and no chronic illnesses that would compromise study results or add additional variables. The major limitation of the study is sample size precluding analysis of any potential confounders of the vitamin D3 response (for example, BMI, skin pigmentation and baseline vitamin D status). Our results in a young, healthy population also cannot be generalized to the overall population, older individuals or those with underlying disease states.
CONCLUSION
A single, 250 000 IU dose of vitamin D3 given before winter resulted in a very robust and significant increase in serum 25(OH) D after 5–10 days but was unable to prevent the seasonal decline of plasma 25(OH)D after 3 months. Although this dose may have been adequate for those who were vitamin D-sufficient at baseline, vitamin D-deficient individuals may require additional doses, either as a loading dose or maintenance doses. Importantly, this study showed a dose of 250 000 IU of vitamin D3 given at once to be safe in a healthy, young adult population, and may thus help inform vitamin D dosing regimens in future studies.
ACKNOWLEDGEMENTS
This work was supported in part by National Institutes of Health grants T32 DK007298-32S1 (JAA), K23 AR054334 (VT), K24 DK096574 (TRZ), and UL1 TR000454 (VT, TRZ). Clinical Trial Registration Number: clinicaltrials.gov: NCT01924910.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
MDK and VT were responsible for the design of the study; MDK and VT recruited subjects and coordinated the study; MDK, VT, JAA and DL were responsible for data collection; JNGB and DW were responsible for statistical analysis; MDK, VT, JAA, DL, JNGB, DW and TRZ evaluated the data analysis results; MDK wrote the draft of the manuscript; and all authors contributed to revisions of the manuscript.
REFERENCES
- 1.Alvarez JA, Zughaier SM, Law J, Hao L, Wasse H, Ziegler TR, Tangpricha V. Effects of high-dose cholecalciferol on serum markers of inflammation and immunity in patients with early chronic kidney disease. Eur J Clin Nutr. 2013;67:264–269. doi: 10.1038/ejcn.2012.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 3.Lehmann B, Genehr T, Knuschke P, Pietzsch J, Meurer M. UVB-induced conversion of 7-dehydrocholesterol to 1alpha,25-dihydroxyvitamin D3 in an in vitro human skin equivalent model. J Invest Dermatol. 2001;117:1179–1185. doi: 10.1046/j.0022-202x.2001.01538.x. [DOI] [PubMed] [Google Scholar]
- 4.Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92:4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 6.Thuesen B, Husemoen L, Fenger M, Jakobsen J, Schwarz P, Toft U, et al. Determinants of vitamin D status in a general population of Danish adults. Bone. 2012;50:605–610. doi: 10.1016/j.bone.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Wolfenden LL, Judd SE, Shah R, Sanyal R, Ziegler TR, Tangpricha V. Vitamin D and bone health in adults with cystic fibrosis. Clin Endocrinol (Oxf) 2008;69:374–381. doi: 10.1111/j.1365-2265.2008.03216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bird ML, Hill KD, Robertson IK, Ball MJ, Pittaway J, Williams AD. Serum [25(OH)D] status, ankle strength and activity show seasonal variation in older adults: relevance for winter falls in higher latitudes. Age Ageing. 2013;42:181–185. doi: 10.1093/ageing/afs067. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Parra E, Avila PJ, Mahillo-Fernandez I, Lentisco C, Gracia C, Egido J, et al. High prevalence of winter 25-hydroxyvitamin D deficiency despite supplementation according to guidelines for hemodialysis patients. Clin Exp Nephrol. 2012;16:945–951. doi: 10.1007/s10157-012-0642-2. [DOI] [PubMed] [Google Scholar]
- 10.Rockell JE, Skeaff CM, Venn BJ, Williams SM, Green TJ. Vitamin D insufficiency in New Zealanders during the winter is associated with higher parathyroid hormone concentrations: implications for bone health? N Z Med J. 2008;121:75–84. [PubMed] [Google Scholar]
- 11.Kashi Z, Saeedian F, Akha O, Gorgi M, Emadi S, Zakeri H. Vitamin D deficiency prevalence in summer compared to winter in a city with high humidity and a sultry climate. Endokrynol Pol. 2011;62:249–251. [PubMed] [Google Scholar]
- 12.Boonen S, Vanderschueren D, Haentjens P, Lips P. Calcium and vitamin D in the prevention and treatment of osteoporosis - a clinical update. J Intern Med. 2006;259:539–552. doi: 10.1111/j.1365-2796.2006.01655.x. [DOI] [PubMed] [Google Scholar]
- 13.Unson CG, Litt M, Reisine S, Mahoney-Trella P, Sheperd T, Prestwood K. Adherence to calcium/vitamin D and estrogen protocols among diverse older participants enrolled in a clinical trial. Contem Clin Trials. 2006;27:215–226. doi: 10.1016/j.cct.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Sanfelix-Genoves J, Gil-Guillen VF, Orozco-Beltran D, Giner-Ruiz V, Pertusa-Martinez S, Reig-Moya B, et al. Determinant factors of osteoporosis patients' reported therapeutic adherence to calcium and/or vitamin D supplements: a cross-sectional, observational study of postmenopausal women. Drugs Aging. 2009;26:861–869. doi: 10.2165/11317070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Stephens WP, Klimiuk PS, Berry JL, Mawer EB. Annual high-dose vitamin D prophylaxis in Asian immigrants. Lancet. 1981;2:1199–1202. doi: 10.1016/s0140-6736(81)91439-2. [DOI] [PubMed] [Google Scholar]
- 16.Kearns MD, Alvarez JA, Tangpricha V. Large, single-dose, oral vitamin d supplementation in adult populations: a systematic review. Endocr Prac. 2013;20:341–351. doi: 10.4158/EP13265.RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heaney RP, Armas LA, Shary JR, Bell NH, Binkley N, Hollis BW. 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr. 2008;87:1738–1742. doi: 10.1093/ajcn/87.6.1738. [DOI] [PubMed] [Google Scholar]
- 18.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19:73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–2072. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ish-Shalom S, Segal E, Salganik T, Raz B, Bromberg IL, Vieth R. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. J Clin Endocrinol Metab. 2008;93:3430–3435. doi: 10.1210/jc.2008-0241. [DOI] [PubMed] [Google Scholar]
- 21.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 22.Osunkwo I, Ziegler TR, Alvarez J, McCracken C, Cherry K, Osunkwo CE, et al. High dose vitamin D therapy for chronic pain in children and adolescents with sickle cell disease: results of a randomized double blind pilot study. Br J Haematol. 2012;159:211–215. doi: 10.1111/bjh.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.United States Department of Agriculture; Human Nutrition Information Service; Dietary Guidelines Advisory Committee, United States; Agricultural Research Service . Report of the Dietary Guidelines Advisory Committee on the dietary guidelines for Americans, 2010: to the Secretary of Agriculture and the Secretary of Health and Human Services. vi. United States Department of Agriculture; United States Department of Health and Human Services; Washington, DC: 2010. p. 445. [Google Scholar]
- 24.Cipriani C, Romagnoli E, Pepe J, Russo S, Carlucci L, Piemonte S, et al. Long-term bioavailability after a single oral or intramuscular administration of 600 000 IU of ergocalciferol or cholecalciferol: implications for treatment and prophylaxis. J Clin Endocrinol Metab. 2013;98:2709–2715. doi: 10.1210/jc.2013-1586. [DOI] [PubMed] [Google Scholar]
- 25.Davie MW, Lawson DE, Emberson C, Barnes JL, Roberts GE, Barnes ND. Vitamin D from skin: contribution to vitamin D status compared with oral vitamin D in normal and anticonvulsant-treated subjects. Clinical Sci. 1982;63:461–472. doi: 10.1042/cs0630461. [DOI] [PubMed] [Google Scholar]
- 26.Weisman Y, Schen RJ, Eisenberg Z, Amarilio N, Graff E, Edelstein-Singer M, et al. Single oral high-dose vitamin D3 prophylaxis in the elderly. J Am Geriatr Soc. 1986;34:515–518. doi: 10.1111/j.1532-5415.1986.tb04243.x. [DOI] [PubMed] [Google Scholar]
- 27.Witham MD, Dove FJ, Dryburgh M, Sugden JA, Morris AD, Struthers AD. The effect of different doses of vitamin D(3) on markers of vascular health in patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2010;53:2112–2119. doi: 10.1007/s00125-010-1838-1. [DOI] [PubMed] [Google Scholar]
- 28.Premaor MO, Scalco R, da Silva MJ, Froehlich PE, Furlanetto TW. The effect of a single dose versus a daily dose of cholecalciferol on the serum 25-hydroxycholecalciferol and parathyroid hormone levels in the elderly with secondary hyperparathyroidism living in a low-income housing unit. J Bone Miner Metab. 2008;26:603–608. doi: 10.1007/s00774-008-0858-0. [DOI] [PubMed] [Google Scholar]
- 29.Leventis P, Kiely PD. The tolerability and biochemical effects of high-dose bolus vitamin D2 and D3 supplementation in patients with vitamin D insufficiency. Scand J Rheumatol. 2009;38:149–153. doi: 10.1080/03009740802419081. [DOI] [PubMed] [Google Scholar]
- 30.Grossmann RE, Zughaier SM, Kumari M, Seydafkan S, Lyles RH, Liu S, et al. Pilot study of vitamin D supplementation in adults with cystic fibrosis pulmonary exacerbation: a randomized, controlled trial. Dermatoendocrinol. 2012;4:191–197. doi: 10.4161/derm.20332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amrein K, Sourij H, Wagner G, Holl A, Pieber TR, Smolle KH, et al. Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: a randomized, double-blind, placebo-controlled pilot study. Criti Care. 2011;15:R104. doi: 10.1186/cc10120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bacon CJ, Gamble GD, Horne AM, Scott MA, Reid IR. High-dose oral vitamin D3 supplementation in the elderly. Osteoporos Int. 2009;20:1407–1415. doi: 10.1007/s00198-008-0814-9. [DOI] [PubMed] [Google Scholar]
- 33.Rossini M, Gatti D, Viapiana O, Fracassi E, Idolazzi L, Zanoni S, et al. Short-term effects on bone turnover markers of a single high dose of oral vitamin D(3). J Clin Endocrinol Metab. 2012;97:E622–E626. doi: 10.1210/jc.2011-2448. [DOI] [PubMed] [Google Scholar]
- 34.Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303:1815–1822. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 35.Stoll D DJ, Lamy O, Hans D, Krieg MA, Aubry-Rozier B. Can one or two high doses of oral vitamin D3 correct insufficiency in a non-supplemented rheumatologic population? Osteoporos Int. 2012;24:495–500. doi: 10.1007/s00198-012-1962-5. [DOI] [PubMed] [Google Scholar]
- 36.Tellioglu A, Basaran S, Guzel R, Seydaoglu G. Efficacy and safety of high dose intramuscular or oral cholecalciferol in vitamin D deficient/insufficient elderly. Maturitas. 2012;72:332–338. doi: 10.1016/j.maturitas.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Romagnoli E, Mascia ML, Cipriani C, Fassino V, Mazzei F, D'Erasmo E, et al. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J Clin Endocrinol Metab. 2008;93:3015–3020. doi: 10.1210/jc.2008-0350. [DOI] [PubMed] [Google Scholar]
- 38.Ilahi M, Armas LA, Heaney RP. Pharmacokinetics of a single, large dose of cholecalciferol. Am J Clin Nutr. 2008;87:688–691. doi: 10.1093/ajcn/87.3.688. [DOI] [PubMed] [Google Scholar]
- 39.Cinar N, Harmanci A, Yildiz BO, Bayraktar M. Vitamin D status and seasonal changes in plasma concentrations of 25-hydroxyvitamin D in office workers in Ankara, Turkey. Eur J Intern Medicine. 2014;25:197–201. doi: 10.1016/j.ejim.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Azizi E, Pavlotsky F, Vered I, Kudish AI. Occupational exposure to solar UVB and seasonal monitoring of serum levels of 25-hydroxy vitamin D3: a case-control study. Photochem Photobiol. 2009;85:1240–1244. doi: 10.1111/j.1751-1097.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- 41.Devgun MS, Paterson CR, Johnson BE, Cohen C. Vitamin D nutrition in relation to season and occupation. Am J Clin Nutr. 1981;34:1501–1504. doi: 10.1093/ajcn/34.8.1501. [DOI] [PubMed] [Google Scholar]
- 42.Pazaitou-Panayiotou K, Papapetrou PD, Chrisoulidou A, Konstantinidou S, Doumala E, Georgiou E, et al. Height, whole Body Surface Area, gender, working outdoors, and sunbathing in previous summer are important determinants of serum 25-hydroxyvitamin D levels. Exp Clin Endocrinol Diabetes. 2012;120:14–22. doi: 10.1055/s-0031-1285912. [DOI] [PubMed] [Google Scholar]
- 43.Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85:649–650. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 44.Brunel E, Schnitzler M, Foidart-Dessalle M, Souberbielle JC, Cavalier E. A Double-Blind, Placebo Controlled, Randomized Trial to Assess the Impact of a Monthly Administration of 50,000 IU of Vitamin D3 for 6 Months on Serum Levels of 25-Hydroxyvitamin D in Healthy Young Adults. Int J Endocrinol. 2013;2013:652648. doi: 10.1155/2013/652648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murdoch DR, Slow S, Chambers ST, Jennings LC, Stewart AW, Priest PC, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308:1333–1339. doi: 10.1001/jama.2012.12505. [DOI] [PubMed] [Google Scholar]