Spinocerebellar ataxias are clinically and genetically heterogeneous neurodegenerative disorders.1 Over 50% of patients with inherited conditions never achieve genetic diagnosis, suggesting additional disease genes await recognition.1 Here we present the first observation of a functionally deleterious and therefore potentially pathogenic variant in the murine ataxia gene TRPC3 in a patient with adult-onset cerebellar ataxia.
A 40-year old white man of European ancestry presented with 2 years of progressive imbalance and ataxic gait. MRI brain showed only mild atrophy of the cerebellar vermis (Fig. A). Evaluation for acquired causes of ataxia1 was unremarkable as were nerve conduction/electromyogram studies. There was no obvious family history but he was estranged from his father. Dominant genetic conditions were considered, but testing for SCA1, SCA2, SCA3, SCA5, SCA6, SCA7, SCA8, SCA10, SCA12, SCA13, SCA14, SCA17, SCA28, and dentatorubral-pallidoluysian atrophy were negative. Given no identifiable etiology and incomplete family history, exome sequencing was performed for rare genetic causes.2 We identified a single variant of potential clinical significance,2 a heterozygous p.Arg762His (Chr4:122824185G>A, build hg19) in the TRPC3 gene, confirmed by Sanger sequencing (Supplemental Data). This position is highly conserved and the protein change was predicted damaging using five independent algorithms (Supplemental Data). The variant was not found in the patient’s unaffected mother, but with no paternal samples and no full siblings, we were unable to determine if inherited or de novo, thus limiting our ability to directly confirm the pathogenicity of this variant in this patient.
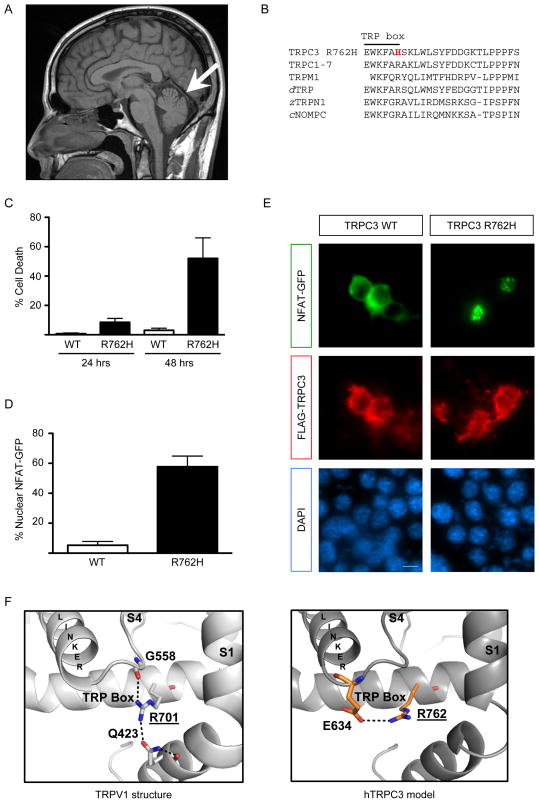
Figure. Functional analysis of TRPC3 p.Arg762His.
(A) Sagittal T1 magnetic resonance imaging of the brain shows a very mild atrophy of the cerebellar vermis (arrow) and no brainstem involvement.
(B) The TRPC3 p.Arg762His variant (R762H) lies within the TRP box (EWKFAR), part of the highly conserved TRP domain in TRP channels including all TRPCs, TRPMs (only TRPM1 shown), Drosophila dTRP, zebrafish zTRPN1, and the related C. elegans cNOMPC.
(C) Neuro-2a mouse neuroblastoma cells were transfected with FLAG-tagged TRPC3 constructs. Cells were fixed 24 or 48 hours after transfection and subjected to indirect immunofluorescence with antibodies against FLAG and the DNA dye DAPI. After 48 hours, overexpression of the p.Arg762His mutant caused significant cell death (mean + SEM; n=3; ANOVA, Bonferroni’s post-hoc test, p<0.01).
(D,E) Neuro-2a cells were transfected with FLAG-tagged TRPC3 constructs and GFP-tagged NFAT. Expression of the p.Arg762His mutant caused significant nuclear NFAT-GFP localization (mean + SEM; n=3; t-test, p<0.0005) (D). Cells were fixed 24 hours after transfection and subjected to indirect immunofluorescence with antibodies against FLAG, GFP and the DNA dye DAPI (E). Scale bar 20 μm.
(F) Structural homology modeling between the TRP box of TRPV1 (PDB ID: 3J5P) and TRPC3. TRPC3 p.Arg762 is homologous to the structurally important p.Arg701 residue in TRPV1. While the p.Arg701-interacting residues are not conserved in TRPC3, modeling suggests an interaction of p.Arg762 with the highly conserved p.Glu634 residue.
TRPC3, a transient receptor potential (TRP) family member, is a non-selective cation channel linked to key signaling pathways affected in cerebellar ataxia including mGluR1.3 The p.Arg762His variant is within the TRP domain of TRP3, a highly conserved region implicated in regulating channel gating (Fig. B).4 Given the variant’s bioinformatic results and key location, we directly assessed for effects on protein function in mouse neuronal cells. Mutant p.Arg762His channels were expressed similarly to wildtype (wt) TRPC3 at the plasma membrane (Supplemental Data) but significantly induced neuronal cell death (Fig. C), suggesting toxic gain-of-function. Consistent with increased channel activity, significantly increased nuclear localization of the calcium-sensitive transcription factor NFAT occurred upon overexpression of TRPC3 p.Arg762His (Fig. D,E). We also performed structural modeling based on the recently published high resolution structure of the related TRPV1 channel5 (Fig. F, Supplemental Data), which suggests p.Arg762 is important for channel gating and mutation would likely have a significant effect on channel function.
Genetic mouse models of Trpc3 exhibit cerebellar dysfunction and ataxia3, 6, 7 and TRPC3 is expressed in human cerebellum (Supplemental Figure), making TRPC3 an excellent candidate for cerebellar ataxia in humans. However, TRPC3 is likely an uncommon cause, as a previous study did not identify mutations in sporadic late-onset or episodic ataxia patients.8 Our functional studies show the p.Arg762His mutation behaves similarly in these same assays (Fig. C, D, E) to the pathogenic mouse Mwk Trpc3 mutation6 that causes ataxia via toxic gain-of-function. Although the functional data is suggestive, the p.Arg762His variant was detected in a single individual out of over 13,000 chromosomes (minor allele frequency 0.008%) in the NHLBI Exome Variant Server (http://evs.gs.washington.edu/EVS/), illustrating the need for additional clinical confirmation in other patients and/or families, as late-onset or incompletely penetrant mutations could still be encountered in databases of presumed normal variation if banking occurs before symptom onset. Thirty-five rare TRPC3 missense variants are present in this database (minor allele frequency < 0.1%) and we identified 2 bioinformatically predicted to be as pathogenic as the p.Arg762His mutation (Supplemental Table). However, neither variant localized to a functionally critical region within TRPC3 and their substitution had no functional effect in our assays (Supplemental Data). Toxic gain-of-function is further supported by the observation of rare nonsense variants in the population (Supplemental Table) and the absence of ataxia symptoms in individuals with heterozygous deletions.9
In summary, based on available clinical, bioinformatic, genomic, structural, and functional information, we conclude mutation of the murine ataxia gene TRPC3 has the potential to be a rare cause of adult-onset spinocerebellar ataxia in humans. We therefore recommend testing in additional populations with undiagnosed dominant disease to search for additional variants of potential pathogenicity to support this observation.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Mental Health (K08MH086297 to BLF), the National Institute for Neurological Disorders and Stroke (R01NS082094 to BLF), and the National Ataxia Foundation (Young Investigator Award to BLF). The authors wish to thank Hane Lee for assistance with exome analysis, the UCLA Clinical Genomics Center for performing exome sequencing, Xizhe Wang and Lloyd Baik for technical assistance, and Prof. Mark S.P. Sansom for resources and advice related to the structural modeling experiments. EBEB is the recipient of a Royal Society Research Fellowship.
Footnotes
Author Contributions
BLF performed clinical evaluations, analysis and interpretation of exome data, and bioinformatics. SMH performed structural modeling. EBEB performed cellular experiments, data analysis, and bioinformatics. All authors contributed to the writing and critical review of the manuscript.
Financial Disclosure Statement
Dr. Fogel is funded by NIH Grants K08MH086297 (NIMH) and R01NS082094 (NINDS), has received funding from the National Ataxia Foundation, has served as a guest editor for a special issue of Neurologic Clinics, and serves as a speaker for the American Physician Institute for Advanced Professional Studies.
Dr. Hanson is currently supported as a Postdoctoral Research Scholar in the lab of Dr. John D. Chodera at the Memorial Sloan Kettering Cancer Center.
Dr. Becker is funded by a Research Fellowship from the Royal Society, a Research Grant from the Royal Society, an Explorer Award from the Simons Foundation Autism Research Initiative, and the John Fell OUP Fund. Other ongoing work in Dr. Becker’s laboratory is funded by the Wellcome Trust. Dr. Becker has received research support from the Oxford Stem Cell Institute. Dr. Becker serves as a scientific review board member of the Simons Foundation Autism Research Initiative.
References
- 1.Shakkottai VG, Fogel BL. Clinical neurogenetics: autosomal dominant spinocerebellar ataxia. Neurol Clin. 2013;31(4):987–1007. doi: 10.1016/j.ncl.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fogel BL, Lee H, Deignan JL, et al. Exome Sequencing in the Clinical Diagnosis of Sporadic or Familial Cerebellar Ataxia. JAMA Neurol. 2014 doi: 10.1001/jamaneurol.2014.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker EB. The Moonwalker Mouse: New Insights into TRPC3 Function, Cerebellar Development, and Ataxia. Cerebellum. 2014;13(5):628–36. doi: 10.1007/s12311-014-0564-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 5.Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504(7478):107–112. doi: 10.1038/nature12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker EB, Oliver PL, Glitsch MD, et al. A point mutation in TRPC3 causes abnormal Purkinje cell development and cerebellar ataxia in moonwalker mice. Proc Natl Acad Sci U S A. 2009;106(16):6706–6711. doi: 10.1073/pnas.0810599106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sekerkova G, Kim JA, Nigro MJ, et al. Early onset of ataxia in moonwalker mice is accompanied by complete ablation of type II unipolar brush cells and Purkinje cell dysfunction. J Neurosci. 2013;33(50):19689–19694. doi: 10.1523/JNEUROSCI.2294-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker EB, Fogel BL, Rajakulendran S, et al. Candidate screening of the TRPC3 gene in cerebellar ataxia. Cerebellum. 2011;10(2):296–299. doi: 10.1007/s12311-011-0253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hickey SE, Biswas S, Thrush DL, et al. Multigeneration family with short stature, developmental delay, and dysmorphic features due to 4q27-q28. 1 microdeletion. Eur J Med Genet. 2013;56(9):521–525. doi: 10.1016/j.ejmg.2013.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.