Abstract
Introduction: Maternal nicotine use during pregnancy has a negative impact on the child. Numerous studies have demonstrated an association between smoking during pregnancy and psychological deficits. This study looks at deficits in executive functioning in preschool-aged children. Methods: The executive functioning of preschool children was assessed by asking parents to complete the parental form of the Behavior Rating Inventory of Executive Functions – Preschool Version (BRIEF-P, German version). The results for preschool children whose mothers had smoked during pregnancy (n = 71) were compared with those of a control group. In a subsample, parental assessments of children of smokers (n = 42) and non-smokers (n = 27) were complemented by the teacher form of the BRIEF-P (German version), which allowed inter-rater agreement (parents vs. preschool teachers) to be assessed. Results: An increased incidence of executive function deficits was noted in the children of smokers, based on parental assessment. Clinically relevant deficits were particularly evident with regard to inhibition, with inhibitory deficits in children of smokers found to be almost four times higher than in the control group (p = 0.006). Inhibitory deficits were reported both by parents and by preschool teachers. Discussion: The increased percentage of executive function deficits described here, particularly the increased inhibitory deficits, confirms the current state of research on smoking during pregnancy. Poor inhibition or impulse control is a key symptom of ADHD.
Key words: nicotine, pregnancy, executive functions, ADHD, BRIEF-P
Abstract
Zusammenfassung
Einleitung: Der Nikotinkonsum der Mutter während der Schwangerschaft hat verschiedene negative Folgen für ein Kind. Eine Vielzahl von Studien zeigt dabei auch einen Zusammenhang mit psychischen Verhaltensauffälligkeiten auf. In der vorliegenden Studie soll der Fokus auf Störungen in den exekutiven Funktionen im Kindergartenalter gelegt werden. Methoden: Es wurden die Eltern- und Erziehereinschätzungen zu exekutiven Funktionen im Verhaltensinventar zur Beurteilung exekutiver Funktionen für das Kindergartenalter (BRIEF-P) von 71 Kindergartenkindern, deren Mütter während der Schwangerschaft geraucht haben, mit einer Kontrollgruppe verglichen. Für einen Teil der Kinder von Raucherinnen (n = 42) und von Nichtraucherinnen (n = 27) konnten darüber hinaus die Erzieherinneneinschätzungen im BRIEF-P berücksichtigt werden. Ergebnisse: Im Elternurteil ergab sich für Kinder von Raucherinnen ein gehäuftes Auftreten von Auffälligkeiten in den exekutiven Funktionen. Insbesondere im Bereich der Inhibition zeigten sich fast 4-mal häufiger klinisch relevante Defizite als für die Kinder der Kontrollgruppe (p = 0,006). Die Auffälligkeiten in der Inhibition zeigen sich sowohl im Eltern- als auch im Erzieherurteil. Diskussion: Die hier beschriebene erhöhte Rate an Defiziten in den exekutiven Funktionen, insbesondere im Bereich der Inhibition, bestätigt den Forschungsstand zum Nikotinkonsum während der Schwangerschaft. Mangelnde Inhibition bzw. Impulskontrolle stellt ein Kernsymptom der ADHS dar.
Schlüsselwörter: Nikotin, Schwangerschaft, exekutive Funktionen, ADHS, BRIEF-P
Introduction
In recent years various measures have been implemented in Germany to reduce smoking and decrease the number of people in the general population who smoke. Despite these attempts, the percentage of women who smoke has remained fairly constant at 29.3 %. The percentage of women who smoke regularly is highest in the group aged between 18 and 29 years, where it amounts to 29.7 %. A further 10.3 % of this age group reported that they occasionally smoked. It was found that smoking correlated with social status: women with a lower social status were far more likely to be smokers 1. Around 10 % of non-smokers were regularly exposed to nicotine in their home environment 2. Even pregnancy does not appear to prevent many women from continuing to smoke: at least 11–13 % of all pregnant women in Germany stated that they smoked at least one cigarette per day; smoking in this context was again found to be correlated to socio-economic status and age 3, 4, 5.
Many studies have shown that nicotine use during pregnancy has numerous adverse effects on both the expectant mother and the unborn child 6, 7. In addition to various complications of pregnancy such as preterm placental abruption, hypertension, premature delivery and miscarriage, maternal smoking during pregnancy has been found to be linked to infertility over the longer term (cf. 8 for a summary of risks). For the unborn child, maternal smoking carries an increased risk of intrauterine growth retardation (low birth weight, restricted growth) 9, 10 and of long-term injury to health (cf. 8 for a summary of risks); but cognitive and behavioral development were also found to be impaired by intrauterine exposure to nicotine 6, 11. External behavioral problems such as ADHD (Attention Deficit Hyperactivity Disorder) 12, 13 or executive function deficits 14, 15 have been reported in connection with maternal smoking. In a systematic review of the literature, Clifford, Lang and Chen 16 additionally demonstrated that similar effects on child development could be detected even if the mother had only been a passive smoker.
The term “executive function” comprises various higher cognitive processes which enable a person to adapt flexibly to new and complex tasks and to independently regulate the performance of activities. Executive functions include abilities such as inhibitory control, the ability to plan actions, the ability to flexibly (re-)direct attention, the ability to recognize and correct mistakes and a certain immunity to distractions 17. Commonly listed key competences include working memory performance, inhibitory control and cognitive flexibility 18.
A number of studies have been carried out into the development of executive functioning in children, and the studies have demonstrated that executive functioning is a multidimensional construct (cf. 19 for a summary). It was not only found that the interaction between executive functioning, biological maturation and environmental experience was associated with childrenʼs readiness for school and school attainment 20, but also that socio-economic status had a significant impact on the development of these cognitive abilities 18, 21.
This study examined whether it is possible to detect the impact of maternal smoking during pregnancy on executive functioning of preschool-aged children and what connection there could be to the familyʼs socio-economic status. The study also looked at the correlation between parental perspective and the perspective of the childrenʼs preschool teachers with regard to descriptions of child behavior.
Method
Instruments used
The questionnaire used in this study was the Verhaltensinventar zur Beurteilung exekutiver Funktionen für das Kindergartenalter (BRIEF-P, 22), the German-language version of the Behavior Rating Inventory of Executive Function® – Preschool Version by Gioia, Espy and Isquith 23. The questionnaire is designed to be completed by the childʼs caregivers (parents, teachers). BRIEF-P consists of 63 statements about the behavior of younger children, which can be summarized by the term “executive functioning”. The caregivers used a three-point scale (“never”, “sometimes”, “often”) to indicate whether certain behaviors (e.g.: throws a tantrum if he is told “no”) constituted a problem in the past six months. The ratings by primary caregivers were used to determine characteristic behaviors in five primary areas (Inhibit, Shift, Emotional Control, Working Memory, Plan/Organize), which were then used to create three indices (Inhibitory Self-Control, Flexibility, Emergent Metacognition) as well as a General Executive Composite score. The assignment of primary rating scores from the BRIEF-P to the respective indices and to the aggregate score is shown in Fig. 1. To obtain the aggregate score, the total raw scores of the five subtests were converted into a t-value (mean = 50, SD = 10). The respective index scores were compiled from the scores of two subtests, the subtest Emotional Control being part of both the Inhibitory Self-Control Index and the Flexibility Index.
Fig. 1.
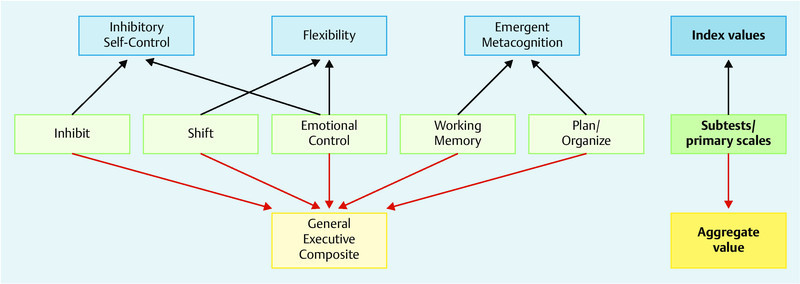
Consolidation of the primary rating scores from the BRIEF-P into three index scores and an aggregate score.
Internal consistency of the aggregate score of the BRIEF-P was α = 0.95 for the parental rating and α = 0.96 for the rating given by teachers. Gender-specific and age-specific standard t-values are available for BRIEF-P for children from the age of 2;0 to 6;11 years.
Parents were given an additional questionnaire which included questions about the pregnancy, the birth and the childʼs development as well as the familyʼs socio-economic status.
Study population
All data were collected as part of the German-language standardization of the Behavior Rating Inventory for Executive Function – Preschool Version (BRIEF-P) 22. Data collection was done from 2012 to 2013 in various daycare centers in six different German federal states under the direction of the Center for Clinical Psychology and Rehabilitation of the University of Bremen. Cooperating partners in kindergartens were contacted locally and information leaflets were handed out to preschool teachers and parents. Parents gave their consent to the use of data obtained from the questionnaire on the familyʼs socio-economic status and the childʼs early development as well as the use of the behavior rating given by the kindergarten teachers.
The statistical analyses below include the BRIEF-P parental ratings for 71 children (boys: n = 35, 49.3 %; girls: n = 36, 50.7 %), whose mothers had reported on the additional parental questionnaire on the childʼs development and the familyʼs socio-economic status that they had smoked during pregnancy. This study cohort was matched with 71 children from the standard BRIEF-P study population whose mothers had not smoked during pregnancy; matching was done using the characteristics “childʼs age” and “gender” and “maternal educational qualification”.
Executive function rating scores given by preschool teachers from the respective daycare centers and obtained using the teacher version of BRIEF-P were available in addition to parental rating scores for 69 children (n = 42 children of smokers, n = 27 children of non-smokers).
Statistical analysis
T-test was used to assess differences between groups for significance. Cohenʼs d was used to determine effect sizes for differences in means of study populations of the same size. In the BRIEF-P analysis, t-values > 65 were interpreted as suspicious. The t-values obtained were categorized for frequency analysis as follows: ≤ 65 = 0 = unremarkable, > 65 = 1 = suspicious. The odds ratio and 95 % confidence interval were calculated. Chi-square test was used to verify the significance of hypotheses in cross-tabulation.
The relationship between parental rating with BRIEF-P and rating by teachers using BRIEF-P was investigated. To do this, separate Pearson product-moment correlations were calculated for both groups. Differences between the correlation coefficients of both groups with regard to subtests, scales and total values were tested for significance using Fisher z-transformation. The paired correlation coefficients were then pooled. All analyses were done using SPSS Version 20.
Results
Description of the study population
Descriptions of the study population are given in Table 1 (maternal characteristics) and Table 2 (characteristics of the child).
Table 1 Description of the study population: maternal characteristics.
| Children of women who smoked (n = 71) | Children of women who did not smoke (n = 71) | ||||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | χ2 | df | p | |
| Note: POS = general polytechnic secondary school certificate (school-leaving certificate of the GDR, corresponds to intermediate school leaving certificate), t = t-value, df = degrees of freedom, p = two-tailed significance test | |||||||
| Maternal educational level | 0.795 | 2 | 0.672 | ||||
|
29 | 40.8 | 26 | 36.6 | |||
|
31 | 43.7 | 30 | 42.3 | |||
|
11 | 15.5 | 15 | 21,1 | |||
| Marital status | 5.358 | 1 | 0.021 | ||||
|
24 | 33.8 | 12 | 16.9 | |||
| Monthly family income (in Euros) | 15.097 | 3 | 0.002 | ||||
|
11 | 16.2 | 8 | 12.3 | |||
|
41 | 60.3 | 21 | 32.3 | |||
|
15 | 22.1 | 31 | 47.7 | |||
|
1 | 1.5 | 5 | 7.7 | |||
| Marital status X income | 17.957 | 3 | 0.000 | ||||
Table 2 Description of the study population: childrenʼs characteristics.
| Children of women who smoked (n = 71) | Children of women who did not smoke (n = 71) | ||||||
|---|---|---|---|---|---|---|---|
| Note: t = t-value, df = degrees of freedom, p = two-tailed significance test | |||||||
| mean | SD | mean | SD | t | df | p | |
| Age (in months) | 57.2 | 14.4 | 57.2 | 13.7 | − 0.012 | 140 | 0.990 |
| Birth weight (in grams) | 3 240.0 | 526.0 | 3 365.0 | 551.7 | 1.372 | 140 | 0.172 |
| N | % | N | % | χ2 | df | p | |
| Chronic respiratory disease | 6 | 8.5 | 2 | 2.8 | 2.119 | 1 | 0.145 |
Matching meant that the distribution of the characteristics “maternal educational achievement” (χ2 = 0.795, df = 2, p = 0.672) and “childʼs age” (t = − 0.012, df = 140, p = 0.990) were similar for both groups (smokers vs. non-smokers). In contrast, the distribution of the characteristic “family income” differed significantly between the smokers group and the non-smokers group. More than 75 % of the mothers who smoked reported a monthly family income of less than 2500 Euros; in the control group only around 45 % of families had a monthly income of less than 2500 Euros. There was also a significant difference with regard to marital status: 24 (33.8 %) women who smoked reported that they were single parents, while only 12 (16.9 %) women who reported that they did not smoke were living without a partner. This also indicates a significant association between family income and family status; mothers who were single parents were more likely to have a lower income (χ2 = 17.957, df = 3, p = 0.000).
The smokers were also questioned about the number of cigarettes they smoked per day while pregnant and whether or when they had stopped smoking. The results showed that 61.2 % of women in this group had smoked throughout their entire pregnancy. Those women who stopped smoking during pregnancy stopped on average towards the end of the 3rd month of pregnancy (SD = 1.9). The average number of cigarettes smoked was 8.6 per day (SD = 7.3, minimum = 1, maximum = 30, median = 5). Two smokers and one non-smoker additionally reported that they had also drunk alcohol on special occasions.
According to the parental questionnaire which looked at the childʼs development and the familyʼs socio-economic status, six of the 71 mothers (8.5 %) who had smoked during pregnancy also reported that their child suffered from asthma or from another chronic respiratory disease. Based on this, the children of women who smoked had a 3.2-fold higher risk (OR = 3.2, 95 % CI = 0.62–16.35) of developing a chronic respiratory illness. But according to the probability distribution (frequency of occurrence), the difference between these children (n = 2; 2.8 %) and the children of women who did not smoke was not statistically significant (χ2 = 2.119, df = 1, p = 0.145). In terms of birth weight it was found that the mean birth weight of children of mothers who smoked (mean birth weight = 3240.0, SD = 526.0) was 125 grams lower than that of children of non-smokers (mean birth weight = 3365.0 SD = 551.7); however, this difference was also not statistically significant (cf. Table 2). Other investigated variables of pregnancy and birth showed no differences between groups. No differences were found between groups with regard to the developmental milestones of early childhood (in particular language acquisition).
Executive function deficits in children whose mothers smoked during pregnancy are more obvious in the area of inhibitory control
As demonstrated in Table 3, a comparison of the mean values for parental rating using the BRIEF-P showed a significant difference for the primary scale Inhibit (t = − 2.777, df = 140, p = 0.006). The standardized difference in means could be interpreted as a medium effect size with d = − 0.45. The standardized differences in means for the remaining four primary scales did not differ significantly. However, analysis of the higher-level index scores showed that the standardized difference in means for Inhibitory Self-Control was statistically significant (t = 2.608, df = 140, p = 0.010). This index combines the two primary scales Inhibit and Emotional Control (cf. Fig. 1). Here too, the effect size was medium (d = − 0.42). The standardized difference in means calculated for the General Executive Composite was also statistically significant (t = − 2.060, df = 140, p = 0.041), although the effect size (d = − 0.34) was smaller than of the two above-mentioned differences. The aggregate score incorporates all five primary scales.
Table 3 Differences in parental assessment (BRIEF-P) of childrenʼs executive functioning between the children of mothers who smoked and the children of non-smokers.
| Children of women who did not smoke (n = 71) | Children of women who smoked (n = 71) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| mean | SD | mean | SD | Diff | t | df | p | d | |
| Note: ISCI = Inhibitory Self-Control Index, FI = Flexibility Index, EMI = Emergent Metacognition Index, GEC = General Executive Composite, Diff = difference in means, t = t-value, df = degrees of freedom, p = two-tailed test, with the analysis of index values and aggregate values done based on subscales but without adjusting the level of significance for multiple testing, d = effect size | |||||||||
| Inhibit | 50.4 | 9.5 | 55.0 | 10.1 | − 4.6 | − 2.777 | 140 | 0.006 | − 0.45 |
| Shift | 49.0 | 9.5 | 50.5 | 11.0 | − 1.5 | − 0.865 | 140 | 0.388 | − 0.14 |
| Emotional Control | 48.9 | 10.1 | 51.5 | 10.5 | − 2.6 | − 1.519 | 140 | 0.131 | − 0.25 |
| Working Memory | 51.2 | 10.2 | 53.7 | 10.7 | − 2.5 | − 1.416 | 140 | 0.159 | − 0.23 |
| Plan/Organize | 50.4 | 10.5 | 52.6 | 10.1 | − 2.2 | − 1.281 | 140 | 0.202 | − 0.22 |
| ISCI | 50.0 | 9.1 | 54.2 | 10.0 | − 4.2 | − 2.608 | 140 | 0.010 | − 0.42 |
| FI | 48.8 | 9.6 | 51.3 | 10.7 | − 2.5 | − 1.437 | 140 | 0.153 | − 0.23 |
| EMI | 51.2 | 10.2 | 53.4 | 10.4 | − 2.3 | − 1.305 | 140 | 0.194 | − 0.22 |
| GEC | 50.3 | 10.1 | 53.8 | 10.1 | − 3.5 | − 2.060 | 140 | 0.041 | − 0.34 |
In a clinical context t-values > 65 are interpreted as suspicious. A corresponding classification of t-values into the categories “suspicious” or “unremarkable” resulted in a greater percentage of suspicious values for the children of women who had smoked compared to the children from the control group in almost all areas (primary scales, indices) (cf. Table 4).
Table 4 Incidence of clinically suspicious ratings (t-value > 65) for children of women who smoked and children of women who did not smoke using the BRIEF-P.
| Children of women who did not smoke (n = 71) | Children of women who smoked (n = 71) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | χ2 | p | OR | CI (95 %) | ||
| Note: ISCI = Inhibitory Self-Control Index, FI = Flexibility Index, EMI = Emergent Metacognition Index, GEC = General Executive Composite, χ2 = χ2-value, p = two-tailed significance test, OR = odds ratio, CI = confidence interval. | |||||||||
| Inhibit | 4 | 5.6 | 13 | 18.3 | 5.413 | 0.020 | 3.75 | 1.16 | 12.15 |
| Shift | 2 | 2.8 | 7 | 9.9 | 2.966 | 0.085 | 3.77 | 0.76 | 18.84 |
| Emotional Control | 6 | 8.5 | 5 | 7.0 | 0.099 | 0.754 | 0.8 | 0.24 | 2.82 |
| Working Memory | 7 | 9.9 | 9 | 12.7 | 0.282 | 0.596 | 1.3 | 0.47 | 3.78 |
| Plan/Organize | 7 | 9.9 | 7 | 9.9 | 0.000 | 1.000 | 1.0 | 0.33 | 3.01 |
| ISCI | 4 | 5.6 | 11 | 15.5 | 3.652 | 0.056 | 3.1 | 0.93 | 10.16 |
| FI | 4 | 5.6 | 6 | 8.5 | 0.430 | 0.512 | 1.6 | 0.42 | 5.73 |
| EMI | 7 | 9.9 | 9 | 12.7 | 0.282 | 0.596 | 1.3 | 0.47 | 3.78 |
| GEC | 5 | 7.0 | 12 | 16.9 | 3.274 | 0.070 | 2.7 | 0.89 | 8.07 |
This difference was particularly evident and significant for the scale Inhibit: 18.3 % (n = 13) of the children of mothers who had smoked during pregnancy were assessed as clinically problematic; however, only 4.6 % of children in the control group (n = 4) were assessed as clinically problematic. The incidence of suspicious findings in the Inhibit scale was 3.8 times higher for children of mothers who smoked compared to the control group (OR = 3.8, 95 % CI = 1.16–12.15). Similarly, there were also more suspicious findings in the primary scale Shift for the children of mothers who smoked. The odds ratio was also 3.8, even though only 7 (9.9 %) of children were assessed as problematic. However, the difference in the distribution of values was not statistically significant. The odds ratio for the higher-level scale Inhibitory Self-Control was 3.1, indicating a higher rate of deficits among the children of mothers who smoked which narrowly missed statistical significance (p = 0.056). Findings were similar for the General Executive Composite score (OR = 2.7, p = 0.070).
Ratings by teachers also found more deficits in inhibition/impulse control in children of smokers
As not all children were assessed by their teacher with regard to executive functioning and as the sizes of the groups “children of smokers” and “children of non-smokers” differed, the following analyses must be interpreted with care. Analysis showed teacher ratings followed similar patterns to those of parental rating with regard to means and the distribution of suspicious t-values. None of the analyses found statistically significant differences (cf. Table 5).
Table 5 Differences in executive functions between the children of women who smoked and the children of non-smokers according to the ratings made by teachers using the BRIEF-P.
| Children of women who did not smoke (n = 27) | Children of women who smoked (n = 42) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| mean | mean | mean | SD | Diff | T | df | p | d | |
| Note: ISCI = Inhibitory Self-Control Index, FI = Flexibility Index, EMI = Emergent Metacognition Index, GEC = General Executive Composite, Diff = difference in means, t = t-value, df = degrees of freedom, p = two-tailed test, with the analysis of index values and aggregate values done based on subscales but without adjusting the level of significance for multiple testing, d = effect size. | |||||||||
| Inhibit | 51.0 | 51.0 | 55.2 | 11.2 | − 4.2 | − 1.511 | 67 | 0.135 | − 0.38 |
| Shift | 52.2 | 52.2 | 50.2 | 9.7 | 2.0 | 0.837 | 67 | 0.406 | 0.21 |
| Emotional Control | 52.1 | 52.1 | 52.3 | 9.9 | − 0.2 | − 0.093 | 67 | 0.926 | − 0.02 |
| Working Memory | 54.0 | 54.0 | 54.6 | 9.6 | − 0.6 | − 0.226 | 67 | 0.822 | − 0.05 |
| Plan/Organize | 54.3 | 54.3 | 53.3 | 9.9 | 1.0 | 0.339 | 67 | 0.736 | 0.09 |
| ISCI | 51.7 | 51.7 | 54.5 | 11.1 | − 2.8 | − 1.015 | 67 | 0.314 | − 0.25 |
| FI | 52.3 | 52.3 | 51.6 | 9.4 | 0.7 | 0.304 | 67 | 0.762 | 0.07 |
| EMI | 54.3 | 54.3 | 54.5 | 9.7 | − 0.2 | − 0.05 | 67 | 0.960 | − 0.02 |
| GEC | 53.1 | 53.1 | 54.5 | 10.1 | − 1.4 | − 0.516 | 67 | 0.608 | − 0.13 |
But the teachersʼ assessments also clearly showed that there were more suspicious findings in the primary scale Inhibit among the children of mothers who had smoked than for the children of non-smoking mothers. The difference in means between both groups is half a standard deviation and is thus within similar ranges as the difference in means in the parental assessment (difference in parental assessment = 4.6 vs. difference in teachersʼ assessment = 4.2).
Parental and teacher ratings of executive functions show medium to high agreement
As executive functioning was assessed by teachers for part of the study population, it is possible to comment on the agreement between parental and teacher assessments. The differences in correlation coefficients between parental and teachersʼ assessment, which were initially calculated separately for both groups (children of smoking mothers vs. children of non-smoking mothers), were not statistically significant, meaning that the coefficient which was calculated for the total study population (n = 142) can be used.
With the exception of the primary scale Emotional Control there was a medium to high linear correlation between groups (cf. Table 6), which points to a good correlation between parental and teacher assessment of most aspects of executive functioning. But these findings should be interpreted with care, as ratings by both parents and teachers were not available for all children in the study population.
Table 6 Correlation between parental rating and teachersʼ rating of executive functioning (BRIEF-P).
| Children of mothers who smoked (n = 42) | Children of mothers who did not smoke (n = 27) | ||||||
|---|---|---|---|---|---|---|---|
| r1 | p | r2 | p | pa | r12 b | rn | |
| Note: r = correlation coefficient, a = calculated p-values to test the null hypothesis for both correlation coefficients, b = pooled correlation coefficients using Fisher z-transformation, rn = correlation coefficient of the standardization sample population, ISCI = Inhibitory Self-Control Index, FI = Flexibility Index, EMI = Emergent Metacognition Index, GEC = General Executive Composite. | |||||||
| Inhibit | 0.51 | 0.007 | 0.54 | 0.000 | 0.879 | 0.53 | 0.55 |
| Shift | 0.34 | 0.079 | 0.48 | 0.002 | 0.518 | 0.43 | 0.45 |
| Emotional Control | 0.25 | 0.203 | 0.23 | 0.152 | 0.929 | 0.24 | 0.35 |
| Working Memory | 0.78 | 0.000 | 0.59 | 0.000 | 0.162 | 0.67 | 0.60 |
| Plan/Organize | 0.62 | 0.001 | 0.35 | 0.029 | 0.167 | 0.47 | 0.49 |
| ISCI | 0.59 | 0.001 | 0.55 | 0.000 | 0.844 | 0.56 | 0.52 |
| FI | 0.39 | 0.046 | 0.36 | 0.022 | 0.908 | 0.37 | 0.43 |
| EMI | 0.74 | 0.000 | 0.54 | 0.000 | 0.202 | 0.63 | 0.58 |
| GEC | 0.68 | 0.000 | 0.53 | 0.000 | 0.346 | 0.59 | 0.56 |
Discussion
The results of this study into the cognitive and behavioral development of children of mothers who smoked indicate that maternal nicotine use during pregnancy has a negative impact on various aspects of the childʼs executive functions. According to the parental assessment, the children of mothers who smoked had higher mean t-values in all scales of the BRIEF-P. The difference to the group of children of non-smoking mothers was up to half a standard deviation (particularly for the primary scale Inhibit). The questions listed in the scale Inhibit describe the childʼs ability to regulate his/her own behavior, i.e., the childʼs ability to inhibit its own impulses and stop its own behavior. Limited ability to self-regulate is considered to be one of the core deficits associated with attention deficit/hyperactivity disorder 24. It can therefore be assumed that there is a relationship between nicotine use during pregnancy, deficits in executive functioning and ADHD, as not all aspects of executive functioning are equally impaired in children with ADHD 25. The most obvious relationship between executive functioning and ADHD is with regard to impulse control, i.e., inhibition. The relationship between executive function and ADHD is already well documented for school-age children; however there is far less research available for preschool-aged children. In their study of 160 preschool children, Sonuga-Barke et al. 26 showed that there was an association with ADHD, particularly for tasks measuring impulse control. No significant associations were found between ADHD and other aspects of executive function such as working memory or planning. The findings of our study which were based on parental and teacher assessments obtained with BRIEF-P indicate a similar scenario. As both parental and teacher assessments came to similar results with regard to the childʼs level of impulse control/inhibition, it must be concluded that the reduced impulse control was a general behavioral problem which occurred in a number of different settings. This satisfies a further criterion for the diagnosis of ADHD: symptoms must occur in different situations; thus, deficits such as a lack of inhibition/impulse control must be observed both at home and elsewhere (in this case, in kindergarten). Another diagnostic criterion of ADHD is that the symptoms must be present prior to the age of 7 years.
In addition, Sonuga-Barke and colleagues 26 were able to show a positive correlation between deficits in inhibition and the severity of ADHD. Another recent study also found a clear association between ADHD and deficits in central areas of executive functioning, once again in the area of inhibition/impulse control 27. Skogan and colleagues conjectured that there could be a connection between deficits in executive functions and various behavioral disorders, whereby the behavioral problems reported in school-age children developed from executive function deficits already identifiable at preschool age. BRIEF-P could be used in this context as a way to detect these preschool indications for subsequent behavioral problems.
Roberts, Martel and Nigg 28 showed that executive function deficits can be used to differentiate and describe subtypes of ADHD. The authors were able to identify three groups who could be differentiated from one another based on either (1) lower ability to shift attention flexibly, (2) poor inhibitory control, or (3) unremarkable executive functioning.
If it can be assumed that deficits in inhibitory control or impulse control are predictors for ADHD, then the conclusion for our study is that children of mothers who smoked during pregnancy are at higher risk of ADHD compared to children of non-smokers. This applies above all to the subtype of ADHD associated with deficits in inhibitory control.
However, it is not possible to assert a causal relationship between prenatal factors such as maternal nicotine use during pregnancy and the postnatal development of children in various areas (here: inhibitory deficits as a sub-area of executive function) based on the results presented in this study. The problem is that mothers who smoke during pregnancy may also have a number of additional risk factors which can negatively affect the childʼs development. These can include nutritional behavior or health behavior 11. However, there does appear to be an association between maternal nicotine use and deficits in the childʼs inhibitory control. But whether nicotine use itself is responsible for the deficits or whether the deficits are due to genetic predisposition 29, other environmental toxins such as PCB or lead 30 or are facilitated by characteristics such as the familyʼs socio-economic status is still being discussed. Millenet et al. 31 also assumed that interactions between genetic and various environmental factors could contribute to ADHD. Various studies have reported an association between prenatal exposure to nicotine through the mother and important neurobiological changes in prenatal brain maturation 32, 33, 34, which could create a basis for cognitive and behavioral problems. Dwyer and colleagues 35 have described in detail the changes in prenatal brain development caused by nicotine. Because of its similarity to the neurotransmitter acetylcholine, nicotine stimulates the nicotinergic acetylcholine receptors, which in turn affect heart beat and blood pressure. Nicotine also has an indirect influence on the number of nerve cells in the brain, as nicotine prematurely terminates the proliferation phase of nerve cells 36. Nicotine also negatively influences the development of dopaminergic neurons 37, which are responsible for motion control and behavioral control. Dopamine is considered to be an important factor in the etiology of ADHD; the hypothesis that patients with ADHD have lower dopamine concentrations in the synaptic gap is considered proven (for a summary of this point, cf. 38).
The results of our study are in accordance with the findings of the studies discussed above. Assessments by both parents and preschool teachers reported deficits in inhibitory control already in preschool-aged children, i.e. at an early stage before reaching the age of seven, for children of mothers who had smoked during pregnancy. This early point in time indicates that the causes are more likely to be primarily biological rather than due to postnatal factors.
It can safely be assumed that the deficits in impulse control as a consequence of maternal nicotine use during pregnancy will have a negative impact on the childʼs further development. Studies have shown that executive function deficits in school-age children lead to poorer performance in other areas of cognitive functioning (for a review, cf. 39).
Medical secondary findings for our study population showed that the children of smoking mothers had both a lower birth weight and a higher risk of developing respiratory disease compared to the children of non-smoking mothers. The difference between groups did not achieve statistical significance, but this tendency was in agreement with the results of other studies 8, 9, 10.
The small size of the study population is a limitation for the interpretation of data, as it did not permit the inclusion of further variables which could have been used for multivariate analysis. Another limitation was the interview method used: data on nicotine use was obtained only from a parental questionnaire; the reliability and accuracy of the information provided on smoking behavior during pregnancy must therefore be scrutinized carefully. In addition, detailed information is lacking on whether during pregnancy the mother was exposed to secondhand smoke and whether the child was exposed postnatally to secondhand smoke and what the further conditions of development were like.
Conclusions for Clinical Practice
The findings provide a starting point for prevention and intervention. Preventive measures would primarily focus on nicotine use during pregnancy. In this context it would be necessary to gather data on nicotine use already during the prenatal care of pregnant women, not simply as a risk factor but differentiated according to daily nicotine dose, to provide suitable advice to the mother. The German Federal Center for Health Education (www.bzga.de) has issued a number of brochures for pregnant women, mothers and their partners, available (in German) from their website (www.bzga.de) under the heading Förderung des Nichtrauchens (Promoting Non-smoking) to inform mothers and help them find a way to stop smoking. The German National Center for Early Support (Nationales Zentrum Frühe Hilfen, NZFH) also provides various support systems and coordinated offers of assistance (such as health visitors) for parent and children (www.fruehehilfen.de). A number of projects aimed at pregnant women have been launched in different federal states in Germany; one example of this is the project PATERAS (Promotion of Smoking Cessation during Pregnancy and in the First Year after Birth) in Hamburg 40. It would be useful if the prevention strategies to promote non-smoking tailored to the needs of the individual pregnant woman and to protect the mother from inhaling secondhand smoke during pregnancy were addressed across all of Germany and the necessary measures implemented 41. Similarly, it would also be useful if the information on prevention and the support for stopping smoking not only targeted women when they were pregnant but also included the time after giving birth. It would be worth investigating whether and in which form information on nicotine use by pregnant women (and of her partner, if necessary) could be passed on to the childʼs pediatrician in a case form or doctorʼs letter. The law on Cooperation and Information in Child Protection, passed on December 22, 2011 (BGBl. I S. 2975), could serve as the basis for this cooperation. Intervention measures targeting pregnant women have been shown to have at least a short-term effect 42 but clearly these measures still do not reach enough women. Moreover, despite all the information, women are given different, often contradictory information on smoking behavior. In the conclusion to their study, Rasenack and Jähne 43 described various measures to provide information to pregnant women, including improving the understanding of addiction by medical staff, particularly gynecologists.
When considering the behavioral problems of the children, several studies have confirmed that behavior therapy had a positive impact on executive functioning, particularly inhibitory control 44. Improving self-regulation, specifically cognitive control, played an important role. Cognitive control is a subsystem of self-regulation and is significantly defined by executive function. Gawrilow et al. 45 reported that training sessions using so-called if-then planning as a strategy for self-regulation and to inhibit reactions resulted in improved inhibitory performance in children with ADHD. Early intervention could help prevent secondary impairment during the childʼs subsequent development, particularly during school lessons 46.
Footnotes
Conflict of Interest None.
Supporting Information
German supporting information for this article
References
- 1.Lampert T, von der Lippe E, Müters S. Verbreitung des Rauchens in der Erwachsenenbevölkerung in Deutschland. Bundesgesundheitsblatt. 2013;56:802–808. doi: 10.1007/s00103-013-1698-1. [DOI] [PubMed] [Google Scholar]
- 2.Kröger C, Mons U, Klärs G. et al. Evaluation des Gesundheitsziels „Tabakkonsum reduzieren“. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2010;53:91–102. doi: 10.1007/s00103-009-1016-0. [DOI] [PubMed] [Google Scholar]
- 3.Schneider S, Maul H, Freerksen N. et al. Who smokes during pregnancy? An analysis of the German Perinatal Quality Survey 2005. Public Health. 2008;122:1210–1216. doi: 10.1016/j.puhe.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Fleitmann S, Dohnke B, Balke K. et al. Frauen und Rauchen. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2010;53:117–124. doi: 10.1007/s00103-009-1005-3. [DOI] [PubMed] [Google Scholar]
- 5.Scholz R, Voigt M, Schneider K. et al. Analysis of the German Perinatal Survey of the years 2007–2011 and comparison with data from 1995–1997: Maternal characteristics. Geburtsh Frauenheilk. 2013;73:1247. doi: 10.1055/s-0033-1350830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Julvez J, Ribas-Fito N, Torrent M. et al. Maternal smoking habits and cognitive development of children at age 4 years in a population-based birth cohort. Int J Epidemiol. 2007;36:825–832. doi: 10.1093/ije/dym107. [DOI] [PubMed] [Google Scholar]
- 7.Danielsson J, de Boer M, Petermann F. et al. Nikotinexposition in der Schwangerschaft – Auswirkungen auf die kognitive Entwicklung im Kindergartenalter. Geburtsh Frauenheilk. 2009;69:692–697. [Google Scholar]
- 8.Mund M, Louwen F, Klingelhoefer D. et al. Smoking and pregnancy – a review on the first major environmental risk factor of the unborn. Int J Environ Res Public Health. 2013;10:6485–6499. doi: 10.3390/ijerph10126485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koch S, Vilser C, Groß W. et al. Rauchen während der Schwangerschaft – Risiko für intrauterine Wachstumsrestriktion und bleibende Kleinwüchsigkeit. Z Geburtshilfe Neonatol. 2012;216:77–81. doi: 10.1055/s-0032-1308958. [DOI] [PubMed] [Google Scholar]
- 10.Murphy D J, Dunney C, Mullally A. et al. Population-based study of smoking behaviour throughout pregnancy and adverse perinatal outcomes. Int J Environ Res Public Health. 2013;10:3855–3867. doi: 10.3390/ijerph10093855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knopik V S. Maternal smoking during pregnancy and child outcomes: Real or spurious effect? Dev Neuropsychol. 2009;34:1–36. doi: 10.1080/87565640802564366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thakur G A, Sengupta S M, Grizenko N. et al. Maternal smoking during pregnancy and ADHD: a comprehensive clinical and neurocognitive characterization. Nicotine Tob Res. 2013;15:149–157. doi: 10.1093/ntr/nts102. [DOI] [PubMed] [Google Scholar]
- 13.Huijbregts S CJ, Séguin J R, Zoccolillo M. et al. Associations of maternal prenatal smoking with early childhood physical aggression, hyperactivity-impulsivity, and their co-occurrence. J Abnorm Child Psychol. 2007;35:203–215. doi: 10.1007/s10802-006-9073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piper B J, Corbett S M. Executive function profile in the offspring of women that smoked during pregnancy. Nicotine Tob Res. 2012;14:191–199. doi: 10.1093/ntr/ntr181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huijbregts S CJ, Warren A J, de Sonneville L MJ. et al. Hot and cool forms of inhibitory control and externalizing behavior in children of mothers who smoked during pregnancy: an exploratory study. J Abnorm Child Psychol. 2008;36:323–333. doi: 10.1007/s10802-007-9180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clifford A, Lang L, Chen R. Effects of maternal cigarette smoking during pregnancy on cognitive parameters of children and young adults: a literature review. Neurotoxicol Teratol. 2012;34:560–570. doi: 10.1016/j.ntt.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Carlson S M. Developmentally sensitive measures of executive function in preschool children. Dev Neuropsychol. 2005;28:595–616. doi: 10.1207/s15326942dn2802_3. [DOI] [PubMed] [Google Scholar]
- 18.Sarsour K, Sheridan M, Jutte D. et al. Family socioeconomic status and child executive functions: the roles of language, home environment, and single parenthood. J Int Neuropsychol Soc. 2010;17:120–132. doi: 10.1017/S1355617710001335. [DOI] [PubMed] [Google Scholar]
- 19.Röthlisberger M, Neuenschwander R, Michel E. et al. Exekutive Funktionen: Zugrundeliegende kognitive Prozesse und deren Korrelate bei Kindern im späten Vorschulalter. Z Entwicklungspsychol Padagog Psychol. 2010;42:99–110. [Google Scholar]
- 20.Neuenschwander R, Röthlisberger M, Cimeli P. et al. How do different aspects of self-regulation predict successful adaptation to school? J Exp Child Psychol. 2012;113:353–371. doi: 10.1016/j.jecp.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Jednoróg K, Altarelli I, Monzalvo K. et al. The influence of socioeconomic status on childrenʼs brain structure. PLoS One. 2012;7:e42486. doi: 10.1371/journal.pone.0042486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daseking M, Petermann F. Bern: Huber; 2013. Verhaltensinventar zur Beurteilung exekutiver Funktionen für das Kindergartenalter (BRIEF-P). Deutschsprachige Adaptation des Behavior Rating Inventory of Executive function – Preschool Version (BRIEF-P) von G. A. Gioia, K. A. Espy & P. K. Isquith. [Google Scholar]
- 23.Gioia G A, Espy K A, Isquith P K. Odessa, FL: PAR; 2003. Behavior Rating Inventory of Executive Function – Preschool version (BRIEF-P) [Google Scholar]
- 24.Barkley R A. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 25.Willcutt E G, Doyle A E, Nigg J T. et al. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Sonuga-Barke E J, Dalen L, Daley D. et al. Are planning, working memory, and inhibition associated with individual differences in preschool ADHD symptoms? Dev Neuropsychol. 2002;21:255–272. doi: 10.1207/S15326942DN2103_3. [DOI] [PubMed] [Google Scholar]
- 27.Skogan A H, Zeiner P, Egeland J. et al. Inhibition and working memory in young preschool children with symptoms of ADHD and/or oppositional-defiant disorder. Child Neuropsychol. 2014;20:607–624. doi: 10.1080/09297049.2013.838213. [DOI] [PubMed] [Google Scholar]
- 28.Roberts B A, Martel M M, Nigg J T. Are there executive dysfunction subtypes within ADHD? J Atten Disord. 2013 doi: 10.1177/1087054713510349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thapar A, Rice F, Hay D. et al. Prenatal smoking might not cause attention-deficit/hyperactivity disorder: evidence from a novel design. Biol Psychiatry. 2009;66:722–727. doi: 10.1016/j.biopsych.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nigg J T, Nikolas M, Mark Knottnerus G. et al. Confirmation and extension of association of blood lead with attention-deficit/hyperactivity disorder (ADHD) and ADHD symptom domains at population-typical exposure levels. J Child Psychol Psychiatry. 2010;51:58–65. doi: 10.1111/j.1469-7610.2009.02135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millenet S, Hohmann S, Poustka L. et al. Risikofaktoren und frühe Vorläufersymptome der Aufmerksamkeitsdefizit-/Hyperaktivitätsstörung (ADHS) Kindh Entwickl. 2013;22:201–208. [Google Scholar]
- 32.Slotkin T A, Card J, Stadler A. et al. Effects of tobacco smoke on PC12 cell neurodifferentiation are distinct from those of nicotine or benzo[a]pyrene. Neurotoxicol Teratol. 2014;43:19–24. doi: 10.1016/j.ntt.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rauh V A, Horton M K, Miller R L. et al. Neonatology and the environment: early exposure to airborne environmental toxicants. Neoreviews. 2010;11:e363–e369. doi: 10.1542/neo.11-7-e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wessels C, Winterer G. Nikotin und Gehirnentwicklung. Nervenarzt. 2008;79:7–16. doi: 10.1007/s00115-007-2392-z. [DOI] [PubMed] [Google Scholar]
- 35.Dwyer J B, McQuown S C, Leslie F M. The dynamic effects of nicotine on the developing brain. Pharmacol Ther. 2009;122:125–139. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dani J A. Overview of nicotinic receptors and their roles in the central nervous system. Biol Psychiatry. 2001;49:166–174. doi: 10.1016/s0006-3223(00)01011-8. [DOI] [PubMed] [Google Scholar]
- 37.Azam L, Chen Y, Leslie F M. Developmental regulation of nicotinic acetylcholine receptors within midbrain dopamine neurons. Neurosci. 2007;144:1347–1360. doi: 10.1016/j.neuroscience.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinmann E, Siniatchkin M, Petermann F. et al. ADHS im Kindesalter: ätiologische und therapeutische Ansätze mit dem Schwerpunkt der Bildgebung. Z Neuropsychol. 2012;23:193–203. [Google Scholar]
- 39.Chen R, Clifford A, Lang L. et al. Is exposure to secondhand smoke associated with cognitive parameters of children and adolescents?–a systematic literature review. Ann Epidemiol. 2013;23:652–661. doi: 10.1016/j.annepidem.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Krug B, Haasen C, Schlankardt M. PATERAS – ein Hamburger Praxisprojekt zur Förderung des Nichtrauchens in Schwangerschaft und Säuglingszeit. Suchttherapie. 2008;9:22–25. [Google Scholar]
- 41.Fleitmann S, Dohnke B, Balke K. et al. Frauen und Rauchen. Bundesgesundheitsblatt. 2010;53:117–124. doi: 10.1007/s00103-009-1005-3. [DOI] [PubMed] [Google Scholar]
- 42.Nuesslein T G, Struwe A, Maiwald N. et al. Mütterlicher Tabakkonsum lässt sich durch einfache Intervention des Kinderarztes reduzieren. Klin Padiatr. 2006;218:283–286. doi: 10.1055/s-2005-872459. [DOI] [PubMed] [Google Scholar]
- 43.Rasenack R, Jähne A. Tabakkonsum und Tabakentwöhnung in der Schwangerschaft. Sucht. 2010;56:183–196. [Google Scholar]
- 44.Toussaint A, Petermann F, Schmidt S. et al. Wirksamkeit verhaltenstherapeutischer Maßnahmen auf die Aufmerksamkeits- und Exekutivfunktionen bei Kindern und Jugendlichen mit ADHS. Z Klin Psychol Psychiatr Psychother. 2011;59:25–36. [Google Scholar]
- 45.Gawrilow C, Schmitt K, Rauch W. Kognitive Kontrolle und Selbstregulation bei Kindern mit ADHS. Kindh Entwickl. 2011;20:41–48. [Google Scholar]
- 46.Gawrilow C, Petermann F, Schuchardt K. ADHS im Vorschulalter. Kindh Entwickl. 2013;22:189–192. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
German supporting information for this article


