Abstract
The clinical benefits of the glutamate receptor antagonists memantine and ketamine have helped sustain optimism that glutamate receptors represent viable targets for development of therapeutic drugs. Both memantine and ketamine antagonize N-methyl-d-aspartate receptors (NMDARs), a glutamate receptor subfamily, by blocking the receptor-associated ion channel. Although many of the basic characteristics of NMDAR inhibition by memantine and ketamine appear similar, their effects on humans and to a lesser extent on rodents are strongly divergent. Some recent research suggests that preferential inhibition by memantine and ketamine of distinct NMDAR subpopulations may contribute to the drugs' differential clinical effects. Here we review studies that shed light on possible explanations for differences between the effects of memantine and ketamine.
Introduction
The strikingly broad involvement of N-methyl-d-aspartate receptors (NMDARs) in nervous system disorders has led to persistent hope that pharmacological NMDAR modulators will provide a rich source of pharmaceuticals. However, many NMDAR-focused drug development efforts have ended with failed clinical trials. Although the failures resulted in part from weaknesses in trial design [1-3], an important implication is that nonspecific NMDAR inhibition is unlikely to yield successful treatments, probably because NMDARs play many fundamental physiological roles. Optimism endures that NMDARs may be a fruitful pharmaceutical target using drugs that select for receptor subpopulations based on NMDAR subtype, location, and/or mechanism of activation. The encouraging but divergent clinical effects of the NMDAR antagonists memantine and ketamine have helped motivate continuing efforts to develop new drugs based on NMDAR modulation. Understanding the mechanistic bases of the beneficial effects of these drugs may help guide development of more effective therapies based on NMDAR modulation. Here we review research that sheds light on the similarities and differences in memantine and ketamine actions, focusing where possible on research that compares memantine and ketamine directly.
NMDARs and their inhibition by memantine and ketamine
NMDARs are tetrameric ionotropic glutamate receptors found at nearly all vertebrate excitatory synapses. NMDARs are centrally involved in fundamental nervous system functions including learning and memory [3,4]. NMDAR dysfunction has been implicated in nervous system disorders including Alzheimer's disease, Huntington's disease, depression, schizophrenia, chronic and neuropathic pain, epilepsy, and neuron death following stroke [5-7]. NMDARs are obligate heterotetramers composed of GluN1 subunits in combination with GluN2 and/or GluN3 subunits [3,4,8]. The GluN1 subunit is encoded by a single gene; four genes encode the GluN2 subunits (GluN2A, GluN2B, GluN2C, and GluN2D); two genes encode the GluN3 subunits (GluN3A and GluN3B). Most NMDARs are composed of two GluN1 subunits and two GluN2 subunits, and their activation requires binding of agonists to all four subunits. The principal endogenous agonists that bind to the GluN1 subunit are glycine and d-serine, whereas the principal endogenous agonist that binds to GluN2 subunits is glutamate. The open channel of NMDARs mediates permeation predominantly of Na+, K+, and Ca2+; the influx of Ca2+ ions through NMDAR channels is critical to both the physiological and the pathological effects of receptor activation. Many endogenous substances modulate NMDAR activity, including Mg2+, Zn2+, H+, polyamines, neurosteroids, and fatty acids [3]. Mg2+ is a physiologically crucial modulator that blocks the channel of NMDARs, conferring strong voltage dependence to NMDAR-mediated conductance.
Both memantine and ketamine inhibit NMDARs by occupying the NMDAR's ion channel and occluding current flow. Both drugs are open channel blockers: when the channel is closed, the drugs have little or no ability to enter an unblocked channel or to unbind after blocking the channel. Both drugs exhibit voltage dependence, entering the channel more quickly, leaving the channel more slowly, and inhibiting more effectively as a cell's membrane potential is hyperpolarized. The basic characteristics of NMDAR inhibition by memantine and ketamine, including IC50, kinetics, and voltage dependence, do not differ strongly [9-12]. Many studies report that ketamine inhibits NMDAR channels with slightly lower IC50 and slower kinetics than memantine; however, the differences are small (generally less than a factor of 2). However, ketamine is used in most experiments as a racemic mixture of two enantiomers, S- and R-ketamine; each enantiomer has somewhat different pharmacological properties [13,14]. Voltage dependence of memantine and ketamine are similar, although memantine's has been reported to be slightly greater [15]. Because both memantine's and ketamine's binding site in the NMDAR channel (Figure 1) overlaps with the Mg2+ binding site, Mg2+ competes with both drugs for binding to NMDAR channels. As a result, physiological concentrations of Mg2+ (∼1 mM) substantially increase the IC50, modify the voltage dependence, and alter the NMDAR subtype-selectivity of both memantine and ketamine [16-18].
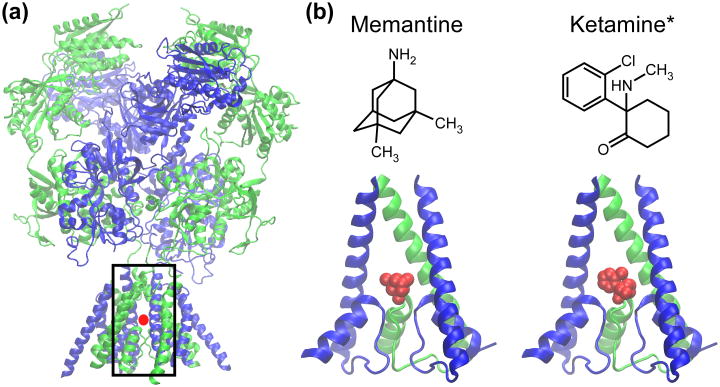
Figure 1.
Images of NMDAR channel blocked by memantine and ketamine. (a) Two nearly complete X-ray crystal structures of NMDARs composed of GluN1 and GluN2B subunits recently were published [130,131]. Here, one of the structures (Protein Data Bank (PDB) code 4TLM [131]) is shown with a red dot at the likely approximate location of memantine and ketamine binding sites. The black box indicates the area of the receptor blown up in (b). (b) Top, the structure of memantine (left) and ketamine (right). *, ketamine, which has two enantiomers ((S)- and (R)-ketamine), is depicted without chirality in this planar representation. Bottom, a view of the channel region of an NMDAR composed of GluN1 and GluN2A subunits with memantine (left) and with (R)-ketamine (right) blocking the channel. The structure of the NMDAR channel region is based on the homology model of [132]; the memantine structure is from www.edinformatics.com; the (R)-ketamine structure is from PDB code 4F8H [133]. There are no structures of NMDARs with a resolved channel blocker; memantine and ketamine are placed with the charged nitrogen close to the critical NMDAR channel asparagines [121,134,135]. GluN1 subunits are shown in green and GluN2 subunits in blue. Structural images were prepared using the molecular visualization program VMD [136].
Despite their many similarities, the clinical effects of memantine and ketamine, and to a lesser extent the behavioral effects in rodents, are surprisingly distinct.
Clinical, behavioral, and circuit effects of memantine and ketamine
Human studies
There are clear differences between the clinical effects of memantine and ketamine. Memantine is the only glutamate receptor ligand that is approved for treatment of Alzheimer's disease (AD). The clinical benefits of memantine in AD patients are modest but broad, and include positive effects on communication, comprehension, memory, and activities of daily living. Memantine is very well tolerated and appears to have no abuse potential [19-21]. Ketamine, in contrast, is a drug of abuse that produces schizophrenia-like symptoms in healthy adults and exacerbates symptoms in schizophrenics [11,22,23].
Ketamine also has demonstrated impressive beneficial effects in clinical studies. Along with its well-established utility as a general anesthetic, ketamine has been found useful in the treatment of several disorders, including depression and pain. A single ketamine infusion has been found to alleviate rapidly and for an extended period the symptoms of major depressive disorder [6,24]. Ketamine also is effective in pain management [25,26]. Memantine, however, does not appear to be effective in treating either depression [27,28] or pain [29]. Thus, the differences between the effects of memantine and ketamine in humans appear robust and consistent.
Rodent studies
Based in part on the ability of ketamine to produce schizophrenia-like symptoms in humans, ketamine administration has been widely used to produce rodent models of schizophrenia [30,31]. Although memantine generally is not used to model schizophrenia, comparisons of the behavioral effects of memantine and ketamine in rodents reveal similarities as well as differences, with differences weaker than in human studies. Especially at lower doses (very approximately, and depending on route of administration, below 20 mg/kg), memantine and ketamine have broadly similar effects on locomotor and exploratory activity, stereotypic behavior, impulsive choice, and attention [32-37]. Several of those studies also found similar tendencies for memantine and ketamine to impair memory function, although low doses of memantine can improve memory [38-40], an observation not reported for ketamine. Both memantine and ketamine decrease ethanol ingestion by alcohol-preferring rats, but only the effect of ketamine is blocked by mTOR (mammalian target of rapamycin) inhibition [41]. Differences at low doses between the effects of memantine and ketamine were reported for aggressive behavior when combined with alcohol ingestion [42], and striking differences in antidepressant-like effects were observed [43]. At higher doses, a wide variety of differences between the locomotor and cognitive effects of memantine and ketamine emerged [33,36]. When memantine and ketamine were compared in drug discrimination studies, ketamine displayed complete substitution for PCP or MK-801, and memantine displayed complete [44] or partial [11,45] substitution.
Many NMDAR channel blockers have been found to exhibit properties thought to be associated with activation of brain circuits. Ketamine powerfully increases gamma (∼30 – 90 Hz) oscillations in cortex (for review, see [46]) and delta (∼0.5 – 4 Hz) oscillations in multiple brain regions [47]. Although the effects of memantine on oscillations have been less extensively studied, a recent article showed that memantine as well as ketamine increased gamma oscillations in rat cortex, whereas ketamine but not memantine increased delta oscillations [48]. Both memantine and ketamine increased 2-deoxyglucose (2-DG) uptake, a marker of neuronal activation [49]. Ketamine has been hypothesized to disinhibit cortical circuits [12,50,51], a process that may underlie increases in gamma oscillations and 2-DG uptake. Similarly, memantine inhibition of NMDARs was proposed to produce cognitive improvements in AD patients through disinhibition [52], although memantine's ability to mediate disinhibition has not been directly assessed. Ketamine reduces expression of the important GABAergic interneuron markers parvalbumin (a Ca2+ binding protein) and GAD67 (a GABA synthetic enzyme) in rodents, thereby compromising inhibitory neuron function [53-55]. The relation of decreased interneuron function to increased oscillations, however, has been questioned [56,57]. Memantine and ketamine also have been proposed to inhibit a subpopulation of interneurons (but see [58]) as a result of the drugs' selectivity in physiological Mg2+ for GluN2C and GluN2D subunit-containing NMDARs [16]. Because GluN2D subunits are expressed predominantly by inhibitory neurons in mature cortex and hippocampus [59,60], preferential inhibition of GluN2D-containing receptors could mediate disinhibition.
Both memantine and ketamine have been shown to be neuroprotective using many in vivo and in vitro paradigms, and their neuroprotective actions are thought to contribute to their clinical benefits (for reviews, see [10,61,62]). There has been very limited comparison of the neuroprotective properties of memantine and ketamine. In one direct comparison of their ability to reduce the effects of oxygen-glucose deprivation in cultured hippocampal slices at equal concentrations, ketamine was found to be slightly more effective than memantine [63].
Thus, the effects of memantine and ketamine in rodent studies demonstrate both strong similarities and clear differences; in human studies, the drugs' effects differ conspicuously.
Mechanistic bases for differential effects of memantine and ketamine
Pharmacological differences between memantine and ketamine
We will consider several possible explanations for the differential effects of memantine and ketamine noted above.
Drugs with the same site of action can differ in their clinical and behavioral effects because of pharmacokinetic differences. The increase in serum and brain concentration, and subsequent elimination, is much faster for ketamine than memantine in both humans and rodents, a difference that could be responsible for the drugs' differential effects (see [9,10,36,64,65]). Several lines of evidence argue against the hypothesis that pharmacokinetic differences between memantine and ketamine are the principal explanation for their differential clinical and behavioral effects. First, numerous studies of ketamine action in humans have involved drug infusion protocols (e.g., [66,67]), some of which have been demonstrated to maintain a steady serum concentration [68]. Nevertheless, the effects of infused ketamine differed strongly from the effects of memantine, which is maintained at stable levels in patients treated by oral administration due to its slow pharmacokinetics [10]. Second, phencyclidine, an analog of ketamine, has much slower pharmacokinetics than ketamine [69], but greater psychotomimetic effects [70]. Third, a recent study compared in rats the behavioral effects of memantine and ketamine at two time points: 15 min after i.p. injection, when ketamine concentration should be near peak but memantine concentration rising, and 45 min after i.p. injection, when ketamine but not memantine concentration should have substantially decreased. The behavioral effects of memantine and ketamine at low doses were similar at both time points, and differences in the drugs' effects at higher doses were similar at both time points [36]. The results suggested that the pharmacokinetic differences between the drugs do not make a major contribution to their differential behavioral effects in rodents. It appears likely that some of the observed differences between the effects of memantine and ketamine, for example sensitivity to transient inhibition of downstream effectors [41], could result from pharmacokinetic differences. However, it appears unlikely that the clinical and behavioral effects of memantine and ketamine differ predominantly because of the faster pharmacokinetics of ketamine.
A second possibility is that the differential clinical and behavioral effects of memantine and ketamine result from differences in their action at sites other than NMDARs. Multiple other sites of action have been reported for each drug (e.g., [26,71,72]). For example, memantine inhibits multiple acetylcholine receptors subtypes [73-76] and 5-HT3 serotonin receptors [10,77], whereas ketamine binds to dopamine D2 and 5-HT2 serotonin receptors [12,78] and to HCN1 channels [79]. Although multiple lines of evidence support the hypothesis that the actions of memantine and ketamine depend predominantly on NMDAR binding [10,70,80,81], there also is strong evidence supporting the importance of other sites of action [79]. It seems likely that some of the differences in the drugs' effects, especially at higher doses, depend on action at targets other than NMDARs.
A third possibility is that the effects of drug metabolites contribute to the differential pharmacological effects of memantine and ketamine. The (S)- and (R)-enantiomers of norketamine are major metabolites of ketamine, and inhibit NMDARs, although with lower potency than (S)- and (R)-ketamine [14,82,83]. Similar to ketamine, (R,S)-norketamine and (2S,6S)-hydroxynorketamine, another ketamine metabolite [83], can increase mTOR function [84]. Several ketamine metabolites potently inhibit α7-nicotinic acetylcholine receptor-mediated currents [85]. Although, to our knowledge, no active memantine metabolites have been reported, differences in the activity of metabolites of ketamine and potentially memantine at NMDARs or at non-NMDAR sites could underlie some of their differential clinical and behavioral effects.
A fourth possibility is that memantine and ketamine block overlapping but distinct populations of NMDARs. NMDARs play diverse roles in nervous system function, and differential inhibition of receptors involved in distinct functions could lead to divergent clinical and behavioral effects. Although memantine and ketamine bind to overlapping sites on NMDARs, there are multiple mechanisms by which they might inhibit distinct receptor subpopulations. In the next section we will focus on studies that address the hypothesis that memantine and ketamine inhibit distinct subpopulations of NMDARs.
Differential inhibition of NMDAR subpopulations by memantine and ketamine
Current understanding of the mechanisms of action of memantine and ketamine do not permit a confident determination of whether, and if so how, they inhibit distinct subpopulations of NMDARs. However, data pointing to an important dichotomy in the NMDAR subpopulations inhibited by memantine and ketamine have emerged.
Many recent studies suggest that the important NMDARs inhibited by memantine are predominantly extrasynaptic, whereas the important NMDARs inhibited by ketamine are synaptic. The significance of differential relative inhibition of synaptic and extrasynaptic NMDARs derives from a hypothesis particularly relevant to neurodegenerative diseases: that synaptic NMDAR stimulation activates cell survival pathways, whereas extrasynaptic NMDAR stimulation activates cell death pathways [86-88]. Activation of extrasynaptic NMDARs by ambient glutamate mediates tonic NMDAR current [89-91], and augmented extrasynaptic receptor activation has been hypothesized to compromise neuron health in nervous system disorders [86-88]. However, it is important to note that there is no consensus on the differential implications of synaptic and extrasynaptic NMDAR activation [6,24,92-95].
Memantine has been found to inhibit extrasynaptic NMDARs more potently than synaptic NMDARs ([96-102]; but see [63,93,94]). However, memantine inhibition of synaptic NMDARs can increase with increasing intensity of synaptic stimulation [93,103]. Memantine can restore long term potentiation impaired by tonic NMDAR activation following reduction of Mg2+ in hippocampal slices [104]; since tonic NMDAR current depends mainly on extrasynaptic NMDARs [89], these data are generally consistent with the idea that memantine preferentially inhibits extrasynaptic NMDARs. In Huntington's disease model mice, memantine reduced functional extrasynaptic NMDAR expression, reversed aberrant activation of cell death pathways by suppressing p38 MAPK activation and increasing nuclear CREB signaling, and reversed disease-associated deficits [98,100,102].
In contrast, the NMDAR subpopulation of central importance to the rapid anti-depressant effects of ketamine was proposed to be synaptic, and possibly a subgroup of NMDARs predominantly activated by spontaneous synaptic vesicle release [6,43,105,106]. Acute inhibition of synaptic NMDARs by ketamine at doses sufficient to produce antidepressant behavioral effects in rodents deactivated eukaryotic elongation factor 2 (eEF2) kinase, reducing eEF2 phosphorylation, relieved block of BDNF translation, and increased surface expression of AMPARs [105,106]. A recent study found that in the presence of physiological Mg2+, ketamine inhibited synaptic NMDARs in hippocampal pyramidal neurons much more effectively than memantine [43]. The same study showed that in the absence of Mg2+, inhibition of synaptic NMDARs by memantine and ketamine was indistinguishable, consistent with previous findings [63]. These results suggest that Mg2+, which has been excluded in many basic studies of memantine and ketamine action on NMDARs, could play a key role by influencing relative inhibition of NMDAR subpopulations by memantine and ketamine.
Potential mechanisms of differential inhibition
We next will consider mechanisms by which a channel blocker could differentiate NMDAR subpopulations. There are at least three ways inhibitors could distinguish synaptic from extrasynaptic NMDARs: (1) by differential inhibition of NMDAR subtypes expressed synaptically versus extrasynaptically; (2) by differential inhibition based on the concentration of glutamate that activates receptors; (3) by differential inhibition based on the time course of receptor activation.
There is evidence for differential expression of NMDAR subunits by subcellular location. GluN2B-containing NMDARs have been reported to be preferentially localized extrasynaptically, and GluN2A-containing NMDARs to be preferentially localized synaptically in cortical and hippocampal neurons ([107-109]; but see [110,111]). However, neither memantine nor ketamine distinguish strongly between GluN2A- and GluN2B-containing NMDARs [14,16]. A caveat is that memantine and ketamine inhibition of triheteromeric receptors, which are highly expressed in the brain (see [4]), has not been characterized. Newly developed approaches for study of isolated triheteromeric receptors will facilitate determination of possible differential drug selectivity [112]. There also is evidence for preferential extrasynaptic expression of GluN2D-containing NMDARs in multiple brain regions [113,114], including hippocampus [115,116]. Because memantine and ketamine preferentially inhibit GluN2C- and GluN2D-containing NMDARs in physiological Mg2+ [16], extrasynaptic localization of GluN2D-containing NMDARs could underlie the drugs' enhanced inhibition of extrasynaptic receptors.
There also is evidence that memantine inhibits NMDARs more effectively at higher agonist concentrations ([117], but see [15,118]). However, this observation would not explain preferential inhibition of extrasynaptic receptors, since extrasynaptic NMDARs are activated by much lower glutamate concentrations than synaptic receptors.
Whether NMDAR inhibition by memantine and/or ketamine depends on the duration of agonist exposure has not been directly investigated. If memantine but not ketamine were to preferentially inhibit NMDARs tonically activated by the extracellular glutamate to which extrasynaptic receptors are exposed, then only memantine would preferentially inhibit extrasynaptic NMDARs. As described above, there are conflicting data on whether memantine distinguishes synaptic and extrasynaptic receptors in 0 Mg2+, but evidence that differential actions of memantine and ketamine appear in the presence of physiological Mg2+ [43]. Although initially the powerful effect of Mg2+ on inhibition by channel blockers was suggested to affect memantine and ketamine similarly [16], subsequent data suggest that the effect of Mg2+ may differ among channel blockers [18]. Further characterization of memantine and ketamine inhibition of NMDAR responses in the presence of physiological Mg2+ is warranted.
If memantine and ketamine do inhibit distinct populations of NMDARs, then there must be an underlying difference in the drugs' mechanism of interaction with NMDARs. One difference that has been described is memantine's ability to bind to a superficial site on NMDARs to which ketamine does not bind. Memantine binding to the superficial site contributes to partial trapping of memantine, a phenomenon that has been proposed to reduce inhibition of synaptic receptors [15,119-122]. The impact of the superficial memantine binding site on inhibition in the presence of Mg2+ is unexplored. Another possibility is that occupation of the channel by memantine or ketamine may differentially affect transition rates between NMDAR states (e.g., between open and closed, agonist-bound and agonist-unbound, and/or desensitized and undesensitized states) of blocked receptors [123-126]. The presence of a blocker in a channel can powerfully influence gating transitions, as suggested by Figure 1(b); the M3 α-helices, which surround the blockers, are centrally involved in channel gating [3]. “Foot-in-the-door” blockers, which do not permit channel closure when bound [127], provide an extreme example of how channel blockers can affect channel gating. Some NMDAR channel blockers act as foot-in-the-door blockers [124,128], but others accelerate channel closure [125] and agonist unbinding [126]. The effect of a channel blocker on transitions between blocked states influences many characteristics of inhibition, including dependence of inhibition on agonist concentration [129] and on duration of agonist presentation (NG Glasgow and JW Johnson, abstract in Soc Neurosci Abstr 2014, 501.08). Thus, there are biophysically plausible explanations for why, despite their similarities, memantine and ketamine could inhibit distinct populations of NMDARs.
Conclusions
The divergent clinical and behavioral effects of memantine and ketamine could be a consequence of multiple differences between the drugs. Their very different pharmacokinetics along with differences in their actions at binding sites other than NMDARs are likely to make some contribution to differences in the drugs' clinical and behavioral effects. There is considerable evidence, however, that the important NMDAR subpopulations inhibited by memantine and ketamine differ: many recent studies have attributed the beneficial effects of memantine to preferential inhibition of extrasynaptic NMDARs, whereas the rapid antidepressant effects of ketamine have been attributed to inhibition of synaptic NMDARs. Although the validity of this dichotomy has been questioned and a mechanistic basis for differential NMDAR inhibition by memantine and ketamine is not established, there are plausible biophysical explanations that remain to be tested. More extensive direct comparison of the effects of memantine and ketamine at multiple experimental levels will provide critical insight into the important mechanisms responsible for the clinical benefits of these NMDAR antagonists.
Highlights.
Memantine and ketamine block open NMDAR channels via apparently similar Mechanisms
Memantine is a very well-tolerated drug approved for treatment of Alzheimer's disease
Ketamine has rapid antidepressant effects, but replicates symptoms of schizophrenia
The drugs' differential effects may require inhibition of distinct NMDAR populations
Acknowledgments
The authors were supported by National Institutes of Health grants R01MH045817 and T32NS073548
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jon W. Johnson, Email: jjohnson@pitt.edu.
Nathan G. Glasgow, Email: ngglasgow@gmail.com.
Nadezhda V. Povysheva, Email: nvp1@pitt.edu, povysheva@gmail.com.
References and recommended reading
* of special interest
** of outstanding interest
- 1.Gladstone DJ, Black SE, Hakim AM Heart, Stroke Foundation of Ontario Centre of Excellence in Stroke R. Toward wisdom from failure: Lessons from neuroprotective stroke trials and new therapeutic directions. Stroke; a journal of cerebral circulation. 2002;33(8):2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- 2.Muir KW. Glutamate-based therapeutic approaches: Clinical trials with nmda antagonists. Curr Opin Pharmacol. 2006;6(1):53–60. doi: 10.1016/j.coph.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacological reviews. 2010;62(3):405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14(6):383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- 5.Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Archiv : European journal of physiology. 2010;460(2):525–542. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- 6.Kavalali ET, Monteggia LM. Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry. 2012;169(11):1150–1156. doi: 10.1176/appi.ajp.2012.12040531. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Q, Sheng M. NMDA receptors in nervous system diseases. Neuropharmacology. 2013;74:69–75. doi: 10.1016/j.neuropharm.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 8.Glasgow NG, Retchless BS, Johnson JW. Molecular bases of nmda receptor subtype-dependent properties. J Physiol. 2014 doi: 10.1113/jphysiol.2014.273763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson JW, Kotermanski SE. Mechanism of action of memantine. Curr Opin Pharmacol. 2006;6(1):61–67. doi: 10.1016/j.coph.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Parsons CG, Stoffler A, Danysz W. Memantine: A NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system - too little activation is bad, too much is even worse. Neuropharmacology. 2007;53(6):699–723. doi: 10.1016/j.neuropharm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Rammes G, Danysz W, Parsons CG. Pharmacodynamics of memantine: An update. Curr Neuropharmacol. 2008;6(1):55–78. doi: 10.2174/157015908783769671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frohlich J, Van Horn JD. Reviewing the ketamine model for schizophrenia. J Psychopharmacol. 2014;28(4):287–302. doi: 10.1177/0269881113512909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamakura T, Sakimura K, Shimoji K. The stereoselective effects of ketamine isomers on heteromeric N-methyl-d-aspartate receptor channels. Anesth Analg. 2000;91(1):225–229. doi: 10.1097/00000539-200007000-00042. [DOI] [PubMed] [Google Scholar]
- 14.Dravid SM, Erreger K, Yuan H, Nicholson K, Le P, Lyuboslavsky P, Almonte A, Murray E, Mosely C, Barber J, French A, et al. Subunit-specific mechanisms and proton sensitivity of NMDA receptor channel block. J Physiol. 2007;581(Pt 1):107–128. doi: 10.1113/jphysiol.2006.124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilling KE, Jatzke C, Hechenberger M, Parsons CG. Potency, voltage-dependency, agonist concentration-dependency, blocking kinetics and partial untrapping of the uncompetitive N-methyl-d-aspartate (NMDA) channel blocker memantine at human nmda (GluN1/GluN2a) receptors. Neuropharmacology. 2009;56(5):866–875. doi: 10.1016/j.neuropharm.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Kotermanski SE, Johnson JW. Mg2+ imparts nmda receptor subtype selectivity to the alzheimer's drug memantine. J Neurosci. 2009;29(9):2774–2779. doi: 10.1523/JNEUROSCI.3703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otton HJ, Lawson McLean A, Pannozzo MA, Davies CH, Wyllie DJ. Quantification of the Mg2+-induced potency shift of amantadine and memantine voltage-dependent block in human recombinant GluN1/GluN2a NMDARs. Neuropharmacology. 2011;60(2-3):388–396. doi: 10.1016/j.neuropharm.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 18*.Nikolaev MV, Magazanik LG, Tikhonov DB. Influence of external magnesium ions on the NMDA receptor channel block by different types of organic cations. Neuropharmacology. 2012;62(5-6):2078–2085. doi: 10.1016/j.neuropharm.2011.12.029. The powerful effect of Mg2+ on NMDAR inhibition by organic channel blockers was found to differ among channel blockers. [DOI] [PubMed] [Google Scholar]
- 19.Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochemical pharmacology. 2008;75(1):218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winblad B, Gauthier S, Astrom D, Stender K. Memantine benefits functional abilities in moderate to severe alzheimer's disease. J Nutr Health Aging. 2010;14(9):770–774. doi: 10.1007/s12603-010-0122-x. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson D. A review of the effects of memantine on clinical progression in alzheimer's disease. Int J Geriatr Psychiatry. 2012;27(8):769–776. doi: 10.1002/gps.2788. [DOI] [PubMed] [Google Scholar]
- 22.Krystal JH, D'Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: Toward a paradigm shift in medication development. Psychopharmacology (Berl) 2003;169(3-4):215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- 23.Corazza O, Assi S, Schifano F. From “special k” to “special m”: The evolution of the recreational use of ketamine and methoxetamine. CNS Neurosci Ther. 2013;19(6):454–460. doi: 10.1111/cns.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamatergic antidepressants: The path to ketamine and beyond. Biol Psychiatry. 2013;73(12):1133–1141. doi: 10.1016/j.biopsych.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prommer EE. Ketamine for pain: An update of uses in palliative care. J Palliat Med. 2012;15(4):474–483. doi: 10.1089/jpm.2011.0244. [DOI] [PubMed] [Google Scholar]
- 26.Persson J. Ketamine in pain management. CNS Neurosci Ther. 2013;19(6):396–402. doi: 10.1111/cns.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pringle A, Parsons E, Cowen LG, McTavish SF, Cowen PJ, Harmer CJ. Using an experimental medicine model to understand the antidepressant potential of the n-methyl-d-aspartic acid (nmda) receptor antagonist memantine. J Psychopharmacol. 2012;26(11):1417–1423. doi: 10.1177/0269881112446535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sani G, Serra G, Kotzalidis GD, Romano S, Tamorri SM, Manfredi G, Caloro M, Telesforo CL, Caltagirone SS, Panaccione I, Simonetti A, et al. The role of memantine in the treatment of psychiatric disorders other than the dementias: A review of current preclinical and clinical evidence. CNS drugs. 2012;26(8):663–690. doi: 10.2165/11634390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29.Alviar MJ, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane database of systematic reviews. 2011;(12):CD006380. doi: 10.1002/14651858.CD006380.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Bubenikova-Valesova V, Horacek J, Vrajova M, Hoschl C. Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. Neurosci Biobehav Rev. 2008;32(5):1014–1023. doi: 10.1016/j.neubiorev.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Moghaddam B, Krystal JH. Capturing the angel in “angel dust”: Twenty years of translational neuroscience studies of NMDA receptor antagonists in animals and humans. Schizophrenia bulletin. 2012;38(5):942–949. doi: 10.1093/schbul/sbs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Creeley C, Wozniak DF, Labruyere J, Taylor GT, Olney JW. Low doses of memantine disrupt memory in adult rats. J Neurosci. 2006;26(15):3923–3932. doi: 10.1523/JNEUROSCI.4883-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koros E, Rosenbrock H, Birk G, Weiss C, Sams-Dodd F. The selective mGlu5 receptor antagonist mtep, similar to NMDA receptor antagonists, induces social isolation in rats. Neuropsychopharmacology. 2007;32(3):562–576. doi: 10.1038/sj.npp.1301133. [DOI] [PubMed] [Google Scholar]
- 34.More L, Gravius A, Nagel J, Valastro B, Greco S, Danysz W. Therapeutically relevant plasma concentrations of memantine produce significant l-N-methyl-d-aspartate receptor occupation and do not impair learning in rats. Behavioural pharmacology. 2008;19(7):724–734. doi: 10.1097/FBP.0b013e3283123cad. [DOI] [PubMed] [Google Scholar]
- 35.Smith JW, Gastambide F, Gilmour G, Dix S, Foss J, Lloyd K, Malik N, Tricklebank M. A comparison of the effects of ketamine and phencyclidine with other antagonists of the NMDA receptor in rodent assays of attention and working memory. Psychopharmacology. 2011;217(2):255–269. doi: 10.1007/s00213-011-2277-5. [DOI] [PubMed] [Google Scholar]
- 36*.Kotermanski SE, Johnson JW, Thiels E. Comparison of behavioral effects of the NMDA receptor channel blockers memantine and ketamine in rats. Pharmacology, biochemistry, and behavior. 2013;109:67–76. doi: 10.1016/j.pbb.2013.05.005. The behavioral effects of memantine and ketamine were compared at two time points after administration; the weak time dependence observed suggested that pharmacokinetics play a minor role in the drugs' behavioral effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Cottone P, Iemolo A, Narayan AR, Kwak J, Momaney D, Sabino V. The uncompetitive NMDA receptor antagonists ketamine and memantine preferentially increase the choice for a small, immediate reward in low-impulsive rats. Psychopharmacology (Berl) 2013;226(1):127–138. doi: 10.1007/s00213-012-2898-3. The effects of memantine and ketamine on impulsive choice in rats were directly compared, and found to be remarkably similar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wise LE, Lichtman AH. The uncompetitive N-methyl-d-aspartate (NMDA) receptor antagonist memantine prolongs spatial memory in a rat delayed radial-arm maze memory task. European journal of pharmacology. 2007;575(1-3):98–102. doi: 10.1016/j.ejphar.2007.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuede CM, Dong H, Csernansky JG. Anti-dementia drugs and hippocampal-dependent memory in rodents. Behav Pharmacol. 2007;18(5-6):347–363. doi: 10.1097/FBP.0b013e3282da278d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zoladz PR, Campbell AM, Park CR, Schaefer D, Danysz W, Diamond DM. Enhancement of long-term spatial memory in adult rats by the noncompetitive NMDA receptor antagonists, memantine and neramexane. Pharmacology, biochemistry, and behavior. 2006;85(2):298–306. doi: 10.1016/j.pbb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 41*.Sabino V, Narayan AR, Zeric T, Steardo L, Cottone P. mTOR activation is required for the anti-alcohol effect of ketamine, but not memantine, in alcohol-preferring rats. Behav Brain Res. 2013;247:9–16. doi: 10.1016/j.bbr.2013.02.030. Both memantine and ketamine reduced ethanol consumption in alcohol-preferring rats. However, inhibition of mTOR reversed the effects of ketamine but not of memantine, suggesting that the drugs' effects depend on divergent pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Newman EL, Chu A, Bahamon B, Takahashi A, Debold JF, Miczek KA. NMDA receptor antagonism: Escalation of aggressive behavior in alcohol-drinking mice. Psychopharmacology (Berl) 2012;224(1):167–177. doi: 10.1007/s00213-012-2734-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43**.Gideons ES, Kavalali ET, Monteggia LM. Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proc Natl Acad Sci U S A. 2014;111(23):8649–8654. doi: 10.1073/pnas.1323920111. Comparison of memantine and ketamine revealed that only ketamine demonstrated antidepressant-like effects in mice; ketamine inhibited synaptic NMDAR responses more effectively than memantine in the presence of Mg2+; ketamine but not memantine affected downstream intracellular cascades associated with antidepressant action. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grant KA, Colombo G, Grant J, Rogawski MA. Dizocilpine-like discriminative stimulus effects of low-affinity uncompetitive NMDA antagonists. Neuropharmacology. 1996;35(12):1709–1719. doi: 10.1016/s0028-3908(96)00147-5. [DOI] [PubMed] [Google Scholar]
- 45.Swedberg MD, Ellgren M, Raboisson P. mGluR5 antagonist-induced psychoactive properties: MTEP drug discrimination, a pharmacologically selective non-NMDA effect with apparent lack of reinforcing properties. J Pharmacol Exp Ther. 2014;349(1):155–164. doi: 10.1124/jpet.113.211185. [DOI] [PubMed] [Google Scholar]
- 46.Kocsis B, Brown RE, McCarley RW, Hajos M. Impact of ketamine on neuronal network dynamics: Translational modeling of schizophrenia-relevant deficits. CNS Neurosci Ther. 2013;19(6):437–447. doi: 10.1111/cns.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Yoshida T, Katz DB, Lisman JE. NMDAR antagonist action in thalamus imposes delta oscillations on the hippocampus. Journal of neurophysiology. 2012;107(11):3181–3189. doi: 10.1152/jn.00072.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48*.Hiyoshi T, Kambe D, Karasawa J, Chaki S. Differential effects of NMDA receptor antagonists at lower and higher doses on basal gamma band oscillation power in rat cortical electroencephalograms. Neuropharmacology. 2014;85:384–396. doi: 10.1016/j.neuropharm.2014.05.037. Both memantine and ketamine were found to increase gamma oscillation power in rat cortical electroencephalogram recording, but only ketamine increased delta oscillations. [DOI] [PubMed] [Google Scholar]
- 49.Dedeurwaerdere S, Wintmolders C, Straetemans R, Pemberton D, Langlois X. Memantine-induced brain activation as a model for the rapid screening of potential novel antipsychotic compounds: Exemplified by activity of an mGlu2/3 receptor agonist. Psychopharmacology. 2011;214(2):505–514. doi: 10.1007/s00213-010-2052-z. [DOI] [PubMed] [Google Scholar]
- 50.Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31(5):234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anticevic A, Cole MW, Repovs G, Savic A, Driesen NR, Yang G, Cho YT, Murray JD, Glahn DC, Wang XJ, Krystal JH. Connectivity, pharmacology, and computation: Toward a mechanistic understanding of neural system dysfunction in schizophrenia. Frontiers in psychiatry. 2013;4:169. doi: 10.3389/fpsyt.2013.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmitt HP. On the paradox of ion channel blockade and its benefits in the treatment of alzheimer disease. Med Hypotheses. 2005;65(2):259–265. doi: 10.1016/j.mehy.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 53.Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing nmda receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci. 2006;26(5):1604–1615. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318(5856):1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 55.Zhou Z, Zhang G, Li X, Liu X, Wang N, Qiu L, Liu W, Zuo Z, Yang J. Loss of phenotype of parvalbumin interneurons in rat prefrontal cortex is involved in antidepressant- and propsychotic-like behaviors following acute and repeated ketamine administration. Mol Neurobiol. 2014 doi: 10.1007/s12035-014-8798-2. [DOI] [PubMed] [Google Scholar]
- 56.Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29(8):2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Volman V, Behrens MM, Sejnowski TJ. Downregulation of parvalbumin at cortical GABA synapses reduces network gamma oscillatory activity. J Neurosci. 2011;31(49):18137–18148. doi: 10.1523/JNEUROSCI.3041-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martina M, Comas T, Mealing GA. Selective pharmacological modulation of pyramidal neurons and interneurons in the CA1 region of the rat hippocampus. Front Pharmacol. 2013;4:24. doi: 10.3389/fphar.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12(3):529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 60.Standaert DG, Landwehrmeyer GB, Kerner JA, Penney JB, Jr, Young AB. Expression of NMDAR2D glutamate receptor subunit mRNA in neurochemically identified interneurons in the rat neostriatum, neocortex and hippocampus. Brain Res Mol Brain Res. 1996;42(1):89–102. doi: 10.1016/s0169-328x(96)00117-9. [DOI] [PubMed] [Google Scholar]
- 61.Volbracht C, van Beek J, Zhu C, Blomgren K, Leist M. Neuroprotective properties of memantine in different in vitro and in vivo models of excitotoxicity. The European journal of neuroscience. 2006;23(10):2611–2622. doi: 10.1111/j.1460-9568.2006.04787.x. [DOI] [PubMed] [Google Scholar]
- 62.Wang C, Liu F, Patterson TA, Paule MG, Slikker W., Jr Preclinical assessment of ketamine. CNS Neurosci Ther. 2013;19(6):448–453. doi: 10.1111/cns.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63**.Emnett CM, Eisenman LN, Taylor AM, Izumi Y, Zorumski CF, Mennerick S. Indistinguishable synaptic pharmacodynamics of the N-methyl-d-aspartate receptor channel blockers memantine and ketamine. Mol Pharmacol. 2013;84(6):935–947. doi: 10.1124/mol.113.089334. In contrast to popular hypotheses, memantine and ketamine were found to affect synaptic NMDAR responses almost identically in the absence of Mg2+; both drugs were neuroprotective in oxygen glucose deprivation experiments, although ketamine was slightly more effective. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gilmour G, Pioli EY, Dix SL, Smith JW, Conway MW, Jones WT, Loomis S, Mason R, Shahabi S, Tricklebank MD. Diverse and often opposite behavioural effects of NMDA receptor antagonists in rats: Implications for “NMDA antagonist modelling” of schizophrenia. Psychopharmacology (Berl) 2009;205(2):203–216. doi: 10.1007/s00213-009-1530-7. [DOI] [PubMed] [Google Scholar]
- 65.Lord B, Wintmolders C, Langlois X, Nguyen L, Lovenberg T, Bonaventure P. Comparison of the ex vivo receptor occupancy profile of ketamine to several NMDA receptor antagonists in mouse hippocampus. Eur J Pharmacol. 2013;715(1-3):21–25. doi: 10.1016/j.ejphar.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 66.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51(3):199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 67.Nagels A, Kirner-Veselinovic A, Wiese R, Paulus FM, Kircher T, Krach S. Effects of ketamine-induced psychopathological symptoms on continuous overt rhyme fluency. Eur Arch Psychiatry Clin Neurosci. 2012;262(5):403–414. doi: 10.1007/s00406-011-0281-8. [DOI] [PubMed] [Google Scholar]
- 68.Absalom AR, Lee M, Menon DK, Sharar SR, De Smet T, Halliday J, Ogden M, Corlett P, Honey GD, Fletcher PC. Predictive performance of the domino, hijazi, and clements models during low-dose target-controlled ketamine infusions in healthy volunteers. Br J Anaesth. 2007;98(5):615–623. doi: 10.1093/bja/aem063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hendrickson HP, Whaley EC, Owens SM. A validated liquid chromatographic/tandem mass spectrometric method for the determination of phencyclidine in microliter samples of rat serum. J Mass Spectrom. 2005;40(1):19–24. doi: 10.1002/jms.766. [DOI] [PubMed] [Google Scholar]
- 70.Rogawski MA, Wenk GL. The neuropharmacological basis for the use of memantine in the treatment of alzheimer's disease. CNS Drug Reviews. 2003;9(3):275–308. doi: 10.1111/j.1527-3458.2003.tb00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu CW, Lin TY, Wang SJ. Memantine depresses glutamate release through inhibition of voltage-dependent Ca2+ entry and protein kinase C in rat cerebral cortex nerve terminals: An NMDA receptor-independent mechanism. Neurochem Int. 2010;57(2):168–176. doi: 10.1016/j.neuint.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 72.McAllister J, Ghosh S, Berry D, Park M, Sadeghi S, Wang KX, Parker WD, Swerdlow RH. Effects of memantine on mitochondrial function. Biochemical pharmacology. 2008;75(4):956–964. doi: 10.1016/j.bcp.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buisson B, Bertrand D. Open-channel blockers at the human alpha4beta2 neuronal nicotinic acetylcholine receptor. Molecular Pharmacology. 1998;53(3):555–563. doi: 10.1124/mol.53.3.555. [DOI] [PubMed] [Google Scholar]
- 74.Maskell PD, Speder P, Newberry NR, Bermudez I. Inhibition of human alpha 7 nicotinic acetylcholine receptors by open channel blockers of N-methyl-d-aspartate receptors. British Journal of Pharmacology. 2003;140(7):1313–1319. doi: 10.1038/sj.bjp.0705559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aracava Y, Pereira EF, Maelicke A, Albuquerque EX. Memantine blocks alpha7* nicotinic acetylcholine receptors more potently than N-methyl-d-aspartate receptors in rat hippocampal neurons. Journal of Pharmacology & Experimental Therapeutics. 2005;312(3):1195–1205. doi: 10.1124/jpet.104.077172. [DOI] [PubMed] [Google Scholar]
- 76.Lee RH, Tseng TY, Wu CY, Chen PY, Chen MF, Kuo JS, Lee TJ. Memantine inhibits alpha3beta2-nachrs-mediated nitrergic neurogenic vasodilation in porcine basilar arteries. PLoS One. 2012;7(7):e40326. doi: 10.1371/journal.pone.0040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rammes G, Rupprecht R, Ferrari U, Zieglgansberger W, Parsons CG. The N-methyl-d-aspartate receptor channel blockers memantine, mrz 2/579 and other amino-alkyl-cyclohexanes antagonise 5-ht(3) receptor currents in cultured hek-293 and n1e-115 cell systems in a non-competitive manner. Neurosci Lett. 2001;306(1-2):81–84. doi: 10.1016/s0304-3940(01)01872-9. [DOI] [PubMed] [Google Scholar]
- 78.Kapur S, Seeman P. NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implications for models of schizophrenia. Molecular psychiatry. 2002;7(8):837–844. doi: 10.1038/sj.mp.4001093. [DOI] [PubMed] [Google Scholar]
- 79.Chen X, Shu S, Bayliss DA. HCN1 channel subunits are a molecular substrate for hypnotic actions of ketamine. J Neurosci. 2009;29(3):600–609. doi: 10.1523/JNEUROSCI.3481-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: Memantine and beyond. Nat Rev Drug Discov. 2006;5(2):160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- 81.Nisijima K, Kuboshima K, Shioda K, Yoshino T, Iwamura T, Kato S. Memantine attenuates 3,4-methylenedioxymethamphetamine-induced hyperthermia in rats. Neurosci Lett. 2012;531(2):198–203. doi: 10.1016/j.neulet.2012.10.059. [DOI] [PubMed] [Google Scholar]
- 82.Ebert B, Mikkelsen S, Thorkildsen C, Borgbjerg FM. Norketamine, the main metabolite of ketamine, is a non-competitive NMDA receptor antagonist in the rat cortex and spinal cord. Eur J Pharmacol. 1997;333(1):99–104. doi: 10.1016/s0014-2999(97)01116-3. [DOI] [PubMed] [Google Scholar]
- 83.Mion G, Villevieille T. Ketamine pharmacology: An update (pharmacodynamics and molecular aspects, recent findings) CNS Neurosci Ther. 2013;19(6):370–380. doi: 10.1111/cns.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Paul RK, Singh NS, Khadeer M, Moaddel R, Sanghvi M, Green CE, O'Loughlin K, Torjman MC, Bernier M, Wainer IW. (R,S)-ketamine metabolites (R,S)-norketamine and (2S,6S)-hydroxynorketamine increase the mammalian target of rapamycin function. Anesthesiology. 2014;121(1):149–159. doi: 10.1097/ALN.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moaddel R, Abdrakhmanova G, Kozak J, Jozwiak K, Toll L, Jimenez L, Rosenberg A, Tran T, Xiao Y, Zarate CA, Wainer IW. Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in alpha7 nicotinic acetylcholine receptors. Eur J Pharmacol. 2013;698(1-3):228–234. doi: 10.1016/j.ejphar.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gladding CM, Raymond LA. Mechanisms underlying NMDA receptor synaptic/extrasynaptic distribution and function. Mol Cell Neurosci. 2011;48(4):308–320. doi: 10.1016/j.mcn.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 87.Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: Implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11(10):682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Parsons MP, Raymond LA. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron. 2014;82(2):279–293. doi: 10.1016/j.neuron.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 89.Le Meur K, Galante M, Angulo MC, Audinat E. Tonic activation of nmda receptors by ambient glutamate of non-synaptic origin in the rat hippocampus. J Physiol. 2007;580(Pt. 2):373–383. doi: 10.1113/jphysiol.2006.123570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fleming TM, Scott V, Naskar K, Joe N, Brown CH, Stern JE. State-dependent changes in astrocyte regulation of extrasynaptic NMDA receptor signalling in neurosecretory neurons. J Physiol. 2011;589(Pt 16):3929–3941. doi: 10.1113/jphysiol.2011.207340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Povysheva NV, Johnson JW. Tonic NMDA receptor-mediated current in prefrontal cortical pyramidal cells and fast-spiking interneurons. Journal of neurophysiology. 2012;107(8):2232–2243. doi: 10.1152/jn.01017.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Papouin T, Ladepeche L, Ruel J, Sacchi S, Labasque M, Hanini M, Groc L, Pollegioni L, Mothet JP, Oliet SH. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell. 2012;150(3):633–646. doi: 10.1016/j.cell.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 93*.Wroge CM, Hogins J, Eisenman L, Mennerick S. Synaptic NMDA receptors mediate hypoxic excitotoxic death. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(19):6732–6742. doi: 10.1523/JNEUROSCI.6371-11.2012. Reassessment of the roles of synaptic and extrasynaptic NMDARs in neurotoxicity and neuroprotection led to the conclusion that synaptic as well as extrasynaptic NMDAR activation mediates hypoxic excitotoxicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou X, Hollern D, Liao J, Andrechek E, Wang H. NMDA receptor-mediated excitotoxicity depends on the coactivation of synaptic and extrasynaptic receptors. Cell death & disease. 2013;4:e560. doi: 10.1038/cddis.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou X, Ding Q, Chen Z, Yun H, Wang H. Involvement of the GluN2A and GluN2B subunits in synaptic and extrasynaptic N-methyl-d-aspartate receptor function and neuronal excitotoxicity. J Biol Chem. 2013;288(33):24151–24159. doi: 10.1074/jbc.M113.482000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leveille F, El Gaamouch F, Gouix E, Lecocq M, Lobner D, Nicole O, Buisson A. Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors. Faseb J. 2008;22(12):4258–4271. doi: 10.1096/fj.08-107268. [DOI] [PubMed] [Google Scholar]
- 97.Papadia S, Soriano FX, Leveille F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V, McKenzie G, et al. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008;11(4):476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Okamoto S, Pouladi MA, Talantova M, Yao D, Xia P, Ehrnhoefer DE, Zaidi R, Clemente A, Kaul M, Graham RK, Zhang D, et al. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat Med. 2009;15(12):1407–1413. doi: 10.1038/nm.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99*.Xia P, Chen HS, Zhang D, Lipton SA. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(33):11246–11250. doi: 10.1523/JNEUROSCI.2488-10.2010. Based on measurements using hippocampal autaptic cultures, memantine was found to inhibit extrasynaptic NMDARs more effectively than synaptic NMDARs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Milnerwood AJ, Gladding CM, Pouladi MA, Kaufman AM, Hines RM, Boyd JD, Ko RW, Vasuta OC, Graham RK, Hayden MR, Murphy TH, et al. Early increase in extrasynaptic NMDA receptor signaling and expression contributes to phenotype onset in huntington's disease mice. Neuron. 2010;65(2):178–190. doi: 10.1016/j.neuron.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 101.Kaufman AM, Milnerwood AJ, Sepers MD, Coquinco A, She K, Wang L, Lee H, Craig AM, Cynader M, Raymond LA. Opposing roles of synaptic and extrasynaptic NMDA receptor signaling in cocultured striatal and cortical neurons. J Neurosci. 2012;32(12):3992–4003. doi: 10.1523/JNEUROSCI.4129-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102*.Dau A, Gladding CM, Sepers MD, Raymond LA. Chronic blockade of extrasynaptic NMDA receptors ameliorates synaptic dysfunction and pro-death signaling in huntington disease transgenic mice. Neurobiol Dis. 2014;62:533–542. doi: 10.1016/j.nbd.2013.11.013. The neuroprotective effects of memantine were found to be associated with blockade of extrasynaptic NMDARs; signaling pathways involved in excitotoxicity were explored. [DOI] [PubMed] [Google Scholar]
- 103.Wild AR, Akyol E, Brothwell SL, Kimkool P, Skepper JN, Gibb AJ, Jones S. Memantine block depends on agonist presentation at the NMDA receptor in substantia nigra pars compacta dopamine neurones. Neuropharmacology. 2013;73:138–146. doi: 10.1016/j.neuropharm.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Frankiewicz T, Parsons CG. Memantine restores long term potentiation impaired by tonic N-methyl-d-aspartate (NMDA) receptor activation following reduction of Mg2+ in hippocampal slices. Neuropharmacology. 1999;38(9):1253–1259. doi: 10.1016/s0028-3908(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 105**.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475(7354):91–95. doi: 10.1038/nature10130. The mechanisms by which synaptic NMDAR inhibition by ketamine can lead to rapid and long-lasting antidepressant effects were explored. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nosyreva E, Szabla K, Autry AE, Ryazanov AG, Monteggia LM, Kavalali ET. Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J Neurosci. 2013;33(16):6990–7002. doi: 10.1523/JNEUROSCI.4998-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19(10):4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Steigerwald F, Schulz TW, Schenker LT, Kennedy MB, Seeburg PH, Kohr G. C-terminal truncation of NR2A subunits impairs synaptic but not extrasynaptic localization of NMDA receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20(12):4573–4581. doi: 10.1523/JNEUROSCI.20-12-04573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, Choquet D. NMDA receptor surface mobility depends on NR2A-2B subunits. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(49):18769–18774. doi: 10.1073/pnas.0605238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thomas CG, Miller AJ, Westbrook GL. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. Journal of neurophysiology. 2006;95(3):1727–1734. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- 111.Harris AZ, Pettit DL. Extrasynaptic and synaptic NMDA receptors form stable and uniform pools in rat hippocampal slices. The Journal of physiology. 2007;584(Pt 2):509–519. doi: 10.1113/jphysiol.2007.137679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112*.Hansen KB, Ogden KK, Yuan H, Traynelis SF. Distinct functional and pharmacological properties of triheteromeric GluN1/GluN2A/GluN2B NMDA receptors. Neuron. 2014;81(5):1084–1096. doi: 10.1016/j.neuron.2014.01.035. An ingenious approach for study in isolation of triheteromeric NMDARs, which may be the predominant types of NMDARs in vertebrate nervous systems, is described. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Momiyama A. Distinct synaptic and extrasynaptic NMDA receptors identified in dorsal horn neurones of the adult rat spinal cord. J Physiol. 2000;523(Pt 3):621–628. doi: 10.1111/j.1469-7793.2000.t01-1-00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brickley SG, Misra C, Mok MH, Mishina M, Cull-Candy SG. NR2B and NR2D subunits coassemble in cerebellar golgi cells to form a distinct NMDA receptor subtype restricted to extrasynaptic sites. J Neurosci. 2003;23(12):4958–4966. doi: 10.1523/JNEUROSCI.23-12-04958.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lozovaya NA, Grebenyuk SE, Tsintsadze T, Feng B, Monaghan DT, Krishtal OA. Extrasynaptic NR2B and NR2D subunits of NMDA receptors shape ‘superslow’ afterburst epsc in rat hippocampus. J Physiol. 2004;558(Pt 2):451–463. doi: 10.1113/jphysiol.2004.063792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Harney SC, Jane DE, Anwyl R. Extrasynaptic NR2D-containing NMDARs are recruited to the synapse during LTP of NMDAR-EPSCs. J Neurosci. 2008;28(45):11685–11694. doi: 10.1523/JNEUROSCI.3035-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen HS, Pellegrini JW, Aggarwal SK, Lei SZ, Warach S, Jensen FE, Lipton SA. Open-channel block of N-methyl-d-aspartate (NMDA) responses by memantine: Therapeutic advantage against NMDA receptor-mediated neurotoxicity. J Neurosci. 1992;12(11):4427–4436. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gilling KE, Jatzke C, Parsons CG. Agonist concentration dependency of blocking kinetics but not equilibrium block of N-methyl-d-aspartate receptors by memantine. Neuropharmacology. 2007;53(3):415–420. doi: 10.1016/j.neuropharm.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 119.Blanpied TA, Boeckman FA, Aizenman E, Johnson JW. Trapping channel block of NMDA-activated responses by amantadine and memantine. J Neurophysiol. 1997;77(1):309–323. doi: 10.1152/jn.1997.77.1.309. [DOI] [PubMed] [Google Scholar]
- 120.Mealing GA, Lanthorn TH, Murray CL, Small DL, Morley P. Differences in degree of trapping of low-affinity uncompetitive N-methyl-d-aspartic acid receptor antagonists with similar kinetics of block. J Pharmacol Exp Ther. 1999;288(1):204–210. [PubMed] [Google Scholar]
- 121.Chen HSV, Lipton SA. Pharmacological implications of two distinct mechanisms of interaction of memantine with N-methyl-d-aspartate-gated channels. Journal of Pharmacology and Experimental Therapeutics. 2005;314(3):961–971. doi: 10.1124/jpet.105.085142. [DOI] [PubMed] [Google Scholar]
- 122.Kotermanski SE, Wood JT, Johnson JW. Memantine binding to a superficial site on NMDA receptors contributes to partial trapping. J Physiol. 2009;587(Pt 19):4589–4604. doi: 10.1113/jphysiol.2009.176297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Antonov SM, Johnson JW. Voltage-dependent interaction of open-channel blocking molecules with gating of NMDA receptors in rat cortical neurons. J Physiol (Lond) 1996;493(Pt 2):425–445. doi: 10.1113/jphysiol.1996.sp021394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sobolevsky AI, Koshelev SG, Khodorov BI. Probing of NMDA channels with fast blockers. J Neurosci. 1999;19(24):10611–10626. doi: 10.1523/JNEUROSCI.19-24-10611.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Blanpied TA, Clarke RJ, Johnson JW. Amantadine inhibits NMDA receptors by accelerating channel closure during channel block. J Neurosci. 2005;25(13):3312–3322. doi: 10.1523/JNEUROSCI.4262-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yuan H, Erreger K, Dravid SM, Traynelis SF. Conserved structural and functional control of N-methyl-d-aspartate receptor gating by transmembrane domain m3. J Biol Chem. 2005;280(33):29708–29716. doi: 10.1074/jbc.M414215200. [DOI] [PubMed] [Google Scholar]
- 127.Neher E, Steinbach JH. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol (Lond) 1978;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Benveniste M, Mayer ML. Trapping of glutamate and glycine during open channel block of rat hippocampal neuron NMDA receptors by 9-aminoacridine. J Physiol. 1995;483(Pt 2):367–384. doi: 10.1113/jphysiol.1995.sp020591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Johnson JW, Qian A. Interaction between channel blockers and channel gating of NMDA receptors. Biol Membr. 2002;19(1):17–22. [Google Scholar]
- 130*.Karakas E, Furukawa H. Crystal structure of a heterotetrameric NMDA receptor ion channel. Science. 2014;344(6187):992–997. doi: 10.1126/science.1251915. See next reference. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131*.Lee CH, Lu W, Michel JC, Goehring A, Du J, Song X, Gouaux E. NMDA receptor structures reveal subunit arrangement and pore architecture. Nature. 2014;511(7508):191–197. doi: 10.1038/nature13548. References 130 and 131 are the first nearly complete NMDAR crystal structures published to date; the availability of improved structures will lead to better understanding of the mechanism of action of NMDAR ligands. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Siegler Retchless B, Gao W, Johnson JW. A single GluN2 subunit residue controls NMDA receptor channel properties via intersubunit interaction. Nature neuroscience. 2012;15(3):406–413. S401–402. doi: 10.1038/nn.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pan J, Chen Q, Willenbring D, Mowrey D, Kong XP, Cohen A, Divito CB, Xu Y, Tang P. Structure of the pentameric ligand-gated ion channel glic bound with anesthetic ketamine. Structure. 2012;20(9):1463–1469. doi: 10.1016/j.str.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yamakura T, Mori H, Masaki H, Shimoji K, Mishina M. Different sensitivities of NMDA receptor channel subtypes to non- competitive antagonists. Neuroreport. 1993;4(6):687–690. doi: 10.1097/00001756-199306000-00021. [DOI] [PubMed] [Google Scholar]
- 135.Kashiwagi K, Masuko T, Nguyen CD, Kuno T, Tanaka I, Igarashi K, Williams K. Channel blockers acting at N-methyl-d-aspartate receptors: Differential effects of mutations in the vestibule and ion channel pore. Mol Pharmacol. 2002;61(3):533–545. doi: 10.1124/mol.61.3.533. [DOI] [PubMed] [Google Scholar]
- 136.Humphrey W, Dalke A, Schulten K. Vmd: Visual molecular dynamics. J Mol Graph. 1996;14(1):33–38. 27–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]