Abstract
Patients with Parkinson’s disease (PD) may experience impulse control disorders (ICDs) when on dopamine agonist therapy for their motor symptoms. In the last few years, there has been a rapid growth of interest for the recognition of these aberrant behaviors and their neurobiological correlates. Recent advances in neuroimaging are helping to identify the neuroanatomical networks responsible for these ICDs, and together with psychopharmacological assessments are providing new insights into the brain status of impulsive behavior. The genetic associations that may be unique to ICDs in PD are also being identified. Complementing human studies, electrophysiological and biochemical studies in animal models are providing insights into neuropathological mechanisms associated with these disorders. New animal models of ICDs in PD patients are being implemented that should provide critical means to identify efficacious therapies for PD-related motor deficits while avoiding ICD side effects. Here, we provide an overview of these recent advances, with a particular emphasis on the neurobiological correlates reported in animal models and patients along with their genetic underpinnings.
Keywords: Dopamine agonists, pramipexole, L-DOPA, 6-OHDA, imaging, PET, fMRI, basal ganglia, prefrontal cortex
Introduction
Impulse control disorders (ICDs) are diagnosed in otherwise healthy people, and untreated patients with de novo Parkinson’s disease (PD) show a similar prevalence of ICDs as do healthy controls1. However, ICD prevalence is significantly higher in PD patients who are on dopamine agonist therapy2. ICDs are diverse, and include pathological gambling, hypersexuality, paraphilias, binge eating and excessive shopping. Although milder impulsivity is observed even in the absence of ICDs in PD, the emergence of these disorders can have an exceedingly grave impact on the quality of life for the affected PD patient, as well as their families and care takers. Some PD patients undergoing L-DOPA therapy show a related disorder, referred to as dopamine dysregulation syndrome (DDS). DDS has a different profile from ICDs, and includes compulsive drug-related seeking and procurement (akin to drug addiction) and stereotypic behaviors. The focus of this discussion will be on ICDs, and their particular association with dopamine agonists. Currently, the main therapeutic approaches for reducing ICDs in PD is dose-reduction, discontinuation of the offending agent, or switching to a different dopamine replacement protocol, all of which can undermine the motor benefits afforded by the agonist. Identifying means to avoid or manage agonist-associated ICDs is essential. Advances in clinical research are detailing the ICD profile in PD, and these descriptions provide the basis for studies on the neurobiology of the disorders, and for discovery of viable new targets for therapeutic interventions. Here, we overview recent advances in ICD identification and assessments, neurobiological and genetic underpinnings defined by both clinical and preclinical experimentation, and potential means to thwart ICDs during pharmacotherapy for PD motor symptoms.
Risk, uncertainty and impulsivity in Parkinson’s disease and rodent models
Impulsivity, often defined by the lack of behavioral inhibition, reflects abnormalities in decision making (choice) and motor control (response inhibition). Impulsive choice is characterized by a preference for immediately available rewards (even if smaller), instead of delayed rewards (even if larger), which can be quantified in delay discounting tasks. Impulsive choice can be described in PD patients with ICDs using delay discounting tasks with either hypothetical long delayed monetary rewards3,4 or real-time short delay monetary rewards3. PD patients with ICDs consistently demonstrate a strong preference for the small immediate rewards. Disrupted delay discounting with intact reward incentive performance in PD patients presenting ICDs likely reflects impairment in waiting for the delayed reward, rather than an enhanced incentive towards the small immediate reward4. While impulsive choice normally demonstrates a magnitude effect, whereby lower impulsive choices accompany increasing reward magnitude, this effect is less pronounced in PD patients with ICDs, suggesting that dopamine agonists may be associated with greater subjective devaluation of the delayed, higher, reward magnitude3. The result is greater impulsivity towards the smaller, immediate, choice. Pathological behavioral choices can be associated with either positive or negative outcomes, consistent with definitions of choice related to risk (with known or unknown probabilities)5. These can be measured in probability discounting tasks. Studies focusing on risk anticipation without outcome show that dopamine agonists increase risk-taking in PD patients with ICDs5,6. This risk-taking bias appears to be unrelated to loss aversion6. It is noteworthy that greater reflection impulsivity (or decisions under uncertainty without adequate information sampling)5, delay discounting2,5, and novelty seeking in the context of uncertainty7 may reflect underlying uncertainty about mapping future actions into rewards8. Motor response inhibition is also impaired by PD, with for example increased stop-signal reaction times and more frequent NoGo commission errors 26,43, although there is a limited role for dopamine in modulating these motor inhibition tasks. Finally, while impulsive PD patients do not perform differently from non-impulsive PD patients on the Stroop color word test (that probes inhibition of prepotent responses), dopamine agonists in PD patients with ICDs do enhance the rapidity of decision-making (also known as reflection impulsivity), suggesting that the long term negative consequences may not be as carefully considered as they otherwise would be9.
Many aspects of human ICDs and the identifying tasks can be recapitulated in (or deconstructed for) testing in laboratory animals. This is a critical step toward providing relief for those who suffer from ICDs, as animal models expand our capacity to identify neurobiological constructs that contribute to these disorders and thus, therapeutic targets. Unfortunately, the wealth of species-related tasks on ICDs have not been widely applied to animal models of PD. One study in MPTP-treated Macaca mulatta monkeys using a motor readiness (impulse control) task reported increases in reaction time at delays of 1, 2 and 3 seconds suggesting a possible increase in impulsivity in these animals10. In rats with 6-OHDA-induced lesions of the dorsolateral striatum, delayed discounting tasks using delays of 3–15 seconds and intracranial self-stimulation as the reward, reveal a greater intolerance to the longer delay than that seen in controls11. However, these outcomes do not parallel reports for the ‘normal’ incidence of ICDs in de novo PD patients1. As the delays tested in animal studies were very short, disrupted discounting may have reflected, at least in part, temporal processing errors, for interval timing within the seconds to minutes range is dysregulated in striatal neuropathologies12. Probability discounting has also been tested in rats with 6-OHDA-induced lesions of the striatum using intracranial self-stimulation13 and with this task, discounting is not altered by striatal lesions13, in keeping with normal incidence of ICDs reported for de novo PD patients1. Probability discounting with self-stimulation rewards also emulating the association of dopamine agonist treatment and ICDs in PD, for chronic treatments with pramipexole increase the preference for the risky choice in the lesioned rats (as well as in unlesioned controls), and this effect is reversed upon terminating the pramipexole, and reinstated when the agonist treatment is reintroduced13. These studies are helping to clarify the relationship between the parkinsonian brain state, and the presence of a dopamine agonist on ICD-like profiles.
Neuroanatomical substrates associated with impulse control disorders in Parkinson’s disease
Imaging studies have been used to identify the neural networks and receptor abnormalities underlying impulsivity and ICDs in PD. In the general population, impulsive subjects show larger amphetamine-induced release of dopamine in the striatum14. Similar abnormalities have also been reported in PD patients with pathological gambling. For example, following presentation of a reward, PET studies show increase dopamine release and reduction in dopamine transporter in the ventral striatum of PD patients with pathological gambling15,16. Recently, radiotracers with high-affinity for extrastriatal D2/D3 receptors (e.g., [18F] Fallypride, [11C] FLB-457) have provided evidence of the role of extrastriatal regions in the pathogenesis of ICDs in PD patients14,17. A [11C] FLB-457 PET study revealed differences in midbrain and medial prefrontal dopaminergic activity between PD patients with and without pathological gambling17. Thus dopamine receptor abnormalities15,17, including reduction in transporter proteins 16,18 support the hypothesis that PD itself may predispose patients to impulsivity. However, the contribution of the effects of chronic dopamine agonist therapy requires careful consideration19–21. This implies that dopamine agonists in general may predispose PD patients to risky behavior that is responsible for the aberrant decision making process.
Recently, it has been shown that in PD patients different aspects of inhibition control/impulsivity rely on different neural networks22. These PET activation studies showed that impulsive choices acted mainly on the decision-making neural network with reduced activation of the medial prefrontal cortex and posterior cingulate. These changes appeared quite different from what has been reported with impulsive actions associated with stimulation of the subthalamic nucleus, which largely affect the activation of the cortical areas underlying reactive and proactive response inhibition of motor response inhibition23.
Studies focusing on risk anticipation without outcome show that dopamine agonists increase risk-taking in PD patients with ICDs6,24 that is accompanied by lower ventral striatal, orbitofrontal and anterior cingulate activity6. Reductions in ventral striatal activity are consistent with an fMRI study of PD patients with ICDs using the balloon analogue risk task (BART) that examines uncertainty with feedback25. Impairments in ‘executive’ function and working memory have also been demonstrated, and are linked to changes in the dorsolateral prefrontal cortex. For example, visuospatial working memory ‘on’ medication is impaired in medicated PD patients with ICD compared with those without9. Similarly, PD patients with ICD when ‘on’ or ‘off’ medications have a significantly reduced digit span compared with PD and control groups24. These results suggest that dorsolateral cortex and ventral striatal circuitry in PD with ICD might be adversely affected by an imbalance in dopaminergic systems. This could arise from a relative ‘overdose’ from exogenous dopaminergic agonists when ‘on’ medication, and possibly even from endogenous dopamine (as compared to levels in the motor cortex to dorsal striatum) when ‘off’ medication.
The incidence of ICDs in untreated PD patients is not much greater than that seen in the normal population1. However, impulsivity can present in PD even in the absence of ICDs, and this occurs across a wide range of behavioral, symptomatic and neuropsychological measures26,42,43. It is a multifaceted construct, with choice impulsivity (as above), reductions in the analysis of available evidence for decision making (‘reflection impulsivity’ 27) and impairments on motor inhibition (e.g., Stop-signal tasks or NoGo paradigms). These dimensions of impulsivity may be synergistic and multifactorial in PD. For example, in addition to the well know deficits of dopamine, PD also depletes noradrenaline 28 and serotonin29 and changes white matter tracts that connect prefrontal regions for inhibitory control to the striatum30.
Preclinical evidence shows that serotonin regulates action restraint in terms of both behavior and activation of the critical right inferior frontal gyrus31–37. Noradrenaline reuptake inhibition also improves inhibition and activation of the right inferior frontal gyrus34,38–41. Accordingly, Ye et al. 44,45 investigated the potential for serotonergic reuptake inhibition by citalopram and noradrenergic reuptake inhibition by atomoxetine to improve response inhibition in patients with PD. They studied both changes in behavior (for clinical relevance) and fMRI (for translation between PD and systems neuroscience studies of inhibition). Atomoxetine enhanced activation of the right inferior frontal gyrus during a Stop-Signal task, in proportion to disease severity (UPDRS)45. Behavioral improvements were associated with increased activation of the right inferior frontal gyrus; higher structural frontostriatal connectivity; and functional connectivity between cortex and striatum. Citalopram similarly improved response inhibition performance, and enhanced inferior frontal activation in patients with more severe disease (UPDRS)44. A simple machine learning approach to predict a meaningful behavioral response to atomoxetine and citalopram (e.g., 30% reductions in the effect of PD on performance)46 reveals that common demographic and clinical metrics (age, UPDRS, levodopa dose) and baseline structural imaging (diffusion weighted imaging of the frontrostriatal tract) enable prediction accuracy 80%, which could support stratification into clinical trials. Together, these reports illustrate the insights gained from combining multimodal brain imaging with psychopharmacological studies. It is especially relevant to note that these potential therapies for impulsivity in PD are adjunctive to continuing dopaminergic medication.
Complementing human imaging studies are electrophysiological and biochemical assessments in awake-behaving laboratory rodents. Such studies have revealed that the prefrontal cortex, ventral striatum and their dopaminergic innervation play critical roles in directing behavior towards rewards and reinforcement learning47–50. These neural elements are implicated in PD, and as over viewed above, their role in impulsive behavior in humans, is beginning to be resolved51–53. The association with dopamine agonist treatment and ICDs in PD54, suggests an involvement of dopamine signaling in these behaviors.
The ventral striatum (including the nucleus accumbens) interfaces cortical and limbic inputs, and its outputs to downstream structures that regulate motor and reward-related behaviors. Striatal neuronal ensembles are critical for approach behaviors, providing an energizing signal for behavior55, although the exact role remains unclear. Some studies suggest that the striatum is more active during behavioral inhibition than approach,56 while others support separate neural circuits within the nucleus accumbens that govern ‘Go and ‘NoGo’ processes57. To measure how the nucleus accumbens encodes reward expectation, approach (‘Go’), and inhibition of behavior (‘NoGo’), Roitman and colleagues recorded the pattern of firing of individual nucleus accumbens neurons in rodents performing an impulsivity task58. In this task, rats were trained to press a lever that is presented unexpectedly at random intervals for a palatable, sucrose pellet reward. Rats quickly learn to engage in this reward-directed behavior. On a minority of trials (25%), lever presentation is accompanied by a ‘NoGo’ cue that instructs the rat to withhold the lever press and successful inhibition is reinforced with reward as in Go trials. The magnitude of the neural response to the onset of each trial depended whether the animal initiated or inhibited behavior. Higher levels of nucleus accumbens activity at the time of lever presentation preceded behavioral inhibition of the lever press, whether correctly for NoGo trials, or in error on Go failures58. Two populations emerged to contribute to the overall elevation in activity that preceded behavioral restraint. One population responded with increases in firing rate at the onset of each trial, with larger increases preceding the inhibition of lever presses. The second population responded with a reduction in firing rate at trial onset, with smaller reductions preceding the inhibition of the prepotent behavioral response. It is intriguing to speculate that these two populations of neurons might constitute different pathways of dopaminergic communication through the nucleus accumbens (D1/substance P/dynorphin-direct pathway versus D2/enkephalin-indirect pathway). It is also possible that the two types of responses are intermixed in both pathways, and that both contribute to the precise regulation of behavior59. Reductions in activity due to larger decreases and smaller increases would respectively bias the output towards a release of inhibition over motor initiation in such downstream structures as the ventral pallidum60. These single neuron recordings from rats are complementary to, and enhance the resolution of, human imaging studies showing that this circuitry may contribute to the risky decision making reported for PD patients who exhibit ICDs6,22 (Figure 1).
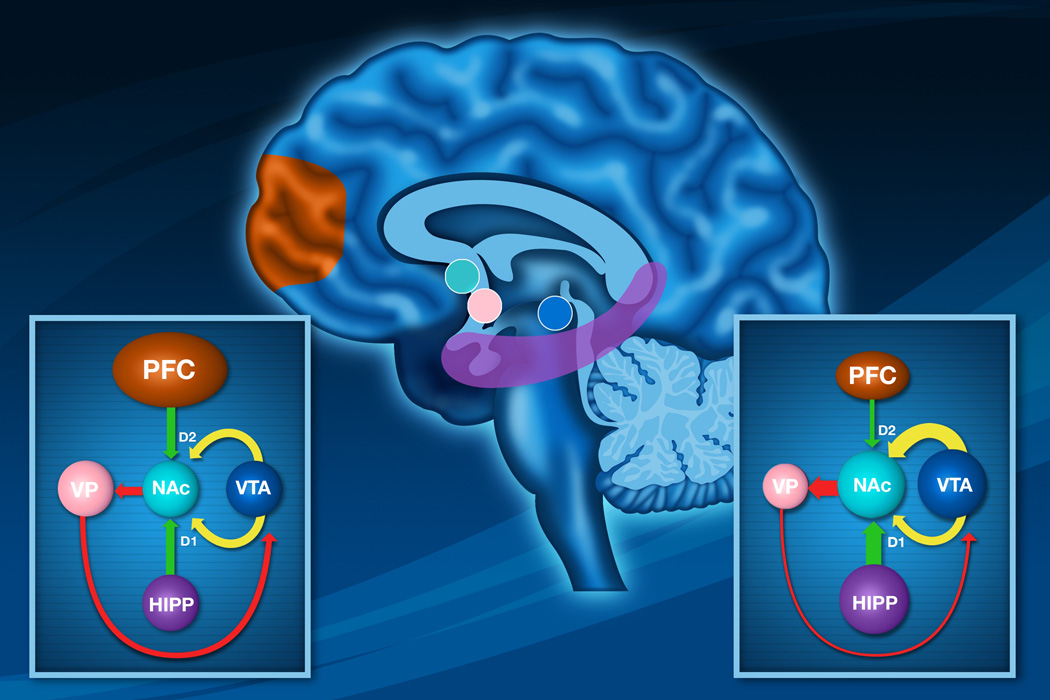
Figure 1.
A diagram showing the primary excitatory drives of the ventral striatum and its modulation by dopamine. The ventral hippocampal input arising from the hippocampal subiculum is believed to be involved in context dependency. As such, this drive should function to maintain focus on the context of the current task to the exclusion of competing stimuli. In contrast, evidence indicates that one function of the medial prefrontal cortex is to facilitate behavioral flexibility, or the propensity to deviate from a task that is no longer rewarding. The dopamine system exerts differential inputs on these pathways, with increased dopamine input facilitating the hippocampal input via a D1-dependent process, whereas D2 stimulation attenuates prefrontal cortical drive. A model of functioning of this system (Sesack & Grace, 2010) suggests that when a task is rewarding, there is an increase in dopamine input, facilitating the hippocampal drive to maintain focus on the currently-rewarded task while preventing the medial prefrontal cortex from deviating from this task.
Lower Left. A diagram showing that the hippocampal drive maintains this dopamine input via disinhibition of the VTA via striatal-ventral pallidal circuits. However, if a behavior fails to produce a reward, there would be an attenuation of dopamine neuron activity (negative reward prediction error, decreasing hippocampal drive and disinhibiting the prefrontal cortex. This would enable the prefrontal cortex to shift focus to a different responses. When a response is encountered that produces reward, the resultant increase in dopamine drive would lock the system into the new state by facilitating focus on the new task by the hippocampus while disabling prefrontal behavioral flexibility.
Lower Right. A diagram showing disruption of normal ventral striatal function in the event of overactive dopaminergic drive mediated by dopamine agonists, as proposed to occur during ICDs. These agonists have a high affinity for the D2 family of dopamine receptors which reduce excitatory influences from the prefrontal cortex. Thus, an abnormally high and persistent activation of D2 receptors is proposed to circumvent the normal efficient functioning of this gated system, and the balance of influences by inputs from the prefrontal cortex and hippocampus is disrupted. In such conditions, there would be a continued potentiation of hippocampal focus independent of the rewarding nature of the stimuli, causing the organism to perseverate an impulsive task. Because of the high levels of D2 receptor activation, the prefrontal cortex would not be capable of shifting behaviors toward a more goal-oriented condition, thereby locking the system in this behaviorally ineffective state.
The assessment of the maladaptive processes associated with PD pathology is aiding the translation from single neuron studies to the clinic. It is well documented that severe dopaminergic lesions are associated with PD motor pathology and these are associated with compensatory mechanisms within the dopamine system, including increased tyrosine hydroxylase activity, decreased reuptake, and increased D2 receptor number that occurs during the course of dopaminergic deafferentation61,62. However, one factor that doesn’t change with these large lesions, is the activity state of the dopamine neurons, i.e., the proportion of dopamine neurons active, their average firing rate and pattern are unchanged. This is thought to preserve the dynamic range of the response, i.e., the changes at the terminal enable the dopamine neuron electrophysiological activity to exhibit the same magnitude of increase to a stimulus63. One process that undermines the preservation of dynamic range is repeated treatment with dopaminergic drugs. Moreover, the type of dopaminergic drug administered can have very different consequences with regard to its impact on the dopamine system. Thus, after repeated L-DOPA, there is an increase in the proportion of dopamine neurons firing64. This maybe a double-edged sword, for while L-DOPA increases dopamine neuron activity and hence dopamine release, it also limits the ability of the system to respond with increases to a stimulus. Indeed, this is proposed to underlie the “on-off” effects observed with L-DOPA treatment. While this process reflects the compensatory changes that take place in response to a lesion and dopamine replacement therapy in the motor system, it is proposed that a similar condition can exist in the limbic system as well. Thus, repeated administration of an indirect dopamine agonist, such as amphetamine, followed by withdrawal increases the proportion of dopamine neurons firing in the ventral tegmental area65 in a manner analogous to what occurs in the substantia nigra with repeated L-DOPA administration. The consequence is that, with increased proportion of dopamine neurons firing, the system would be rendered hyper-responsive to stimuli.
Dopamine neurons fire in two states: at baseline, they fire in a single-spiking, irregular pattern66. When exposed to a salient stimulus, dopamine neurons fire in bursts67,68. To burst fire, however, a dopamine neuron has to already be firing. Thus, while burst firing may represent the dopamine “signal,” the number of neurons firing represents the amplification factor, with more neurons firing enabling a larger dopamine signal69,70. The number of firing neurons is thought to be controlled by environmental contingencies and repeated dopaminergic drug administration thwarts this process. In the case of repeated L-DOPA or amphetamine, there would be an abnormally large dopamine system activation to stimuli. The impact of such an over activation relates to the modulatory effects of dopamine in the limbic system. The ventral striatum receives two prominent excitatory inputs: the prefrontal cortex and the ventral hippocampus subiculum. The prefrontal cortex input is enables behavioral flexibility, or the ability to shift behavioral focus as task contingencies change71,72. The subiculum is a context-dependent structure73,74 that is believed to keep an organism focused on a task. These two processes are regulated in opposite manners by the dopamine system. Stimulation of D2 dopamine receptors will inhibit prefrontal cortex input, whereas D1 receptor stimulation will potentiate hippocampal input71,72. Thus, reward-related activation of the dopamine system would keep the individual focused on the task via D1-mediated potentiation of the subiculum, and D2-mediated inhibition of prefrontal cortex75. If a behavioral response fails to produce a reward, there is a resultant dip in dopamine neuron activity68 will remove subiculum potentiation and prefrontal cortex inhibition, enabling the prefrontal cortex to shift task focus. If the system is disrupted, such as after repeated dopamine agonist administration, then there would be overstimulation of the dopamine system and a persistent focus on a single task to the exclusion of prefrontal cortex-drive goal-directed behavior. Such a condition could relate to DDS seen with L-DOPA administration 76. As both repeated L-DOPA and repeated amphetamine/withdrawal65 increase dopamine neuron population activity and lead to addictive behavior, this parameter may indeed be related to addiction-like dopamine dysregulation syndrome. A very different effect has been observed with direct-acting dopamine agonists, for example, repeated administration of quinpirole decreases burst firing77; an event that could also lead to increased reward-seeking behavior. In the case of both repeated L-DOPA and repeated quinpirole, there is a down-regulation of dopamine neuron autoreceptor function64,78, which could increase the responsivity of the dopamine neurons to stimuli. It is possible that these pathophysiological processes underpin impulsive, non-adaptive behavioral processes that are associated with chronic dopamine therapy in PD patients.
Genetic vulnerabilities associated with impulse control disorders in Parkinson’s disease
The fact that virtually all PD patients are treated with dopaminergic drugs, but only a minority of them will develop ICDs suggests a predisposing and/or protecting factors, potentially of genetic origin. Indeed, vulnerability to ICD is a complex trait with substantial genetic influences that were documented by data from family, adoption and twin studies79. There are high rates (45 to 63%) of alcohol dependence and other substance use disorders among pathological gamblers80, which suggest a common underlying vulnerability81. Twin studies estimated that genetic factors account for 33–54% of the overall variance in the risk of development of pathological gambling behavior82,83.
Candidate genes encoding receptors or metabolic enzymes of neurotransmitter pathways, particularly monoamines, have been found associated with ICD susceptibility or impulsivity traits in the general population (Table 1). Dopamine, serotonin, and norepinephrine genes have been shown to contribute approximately equally to the risk of pathological gambling84,85. However, these genetic factors only explained 15–21% of the inheritance and there are still a large number of unknown genes to be discovered84. Only one genome wide association study (GWAS) has been performed on pathological gambling86. In this study, although 1,312 individuals from 894 families were analyzed, no SNP achieved genome-wide significance. Interestingly, none of the previously validated candidate genes were part of the top gene list suggesting that monoamine pathways account for only a small part of ICD susceptibility.
Table 1.
Genes associated with ICD in PD and in the general population.
| Transmitter system |
Protein | Gene/allele | General population |
References | PD | References |
|---|---|---|---|---|---|---|
| Dopamine | DAT | SLCA3/VNTR | + | Muramatsu and Higuchi 1995; Shinohara et al. 2004; Kreek et al. 2005; Guo et al. 2007; Forbes et al. 2009; Hahn et al. 2010 |
− |
Vallelunga et al., 2012 |
| DRD1 | 800 T/C | + | Comings et al. 1997; da Silva et al. 2007 |
ND | ||
| DRD2 | Taq1A | + | Blum et al. 1995; Comings et al. 1996; Neville et al. 2004; Gelernter et al. 2006; |
− |
Lee et al., 2012; Vallelunga et al., 2012 |
|
| Yang et al. 2007; Haile et al. 2007 |
||||||
| DRD3 | P-S9G | + | Kreek et al. 2005 | + | ||
| DRD4 | Exon3 | + | Perez de Castro et al. 1997; Gelernter et al. 1997; Comings et al. 1999; Levitan et al. 2004; Rogers et al. 2004; Eisenegger 2010 |
ND | ||
|
Dopamine metabolism |
COMT | Val158Met | + | Kreek et al. 2005; Hersrud et al. 2009; Lohoff et al. 2008; Dreher et al. 2009 |
− |
Vallelunga et al., 2012 |
| MAO-A | Promoter | + | Ibanez et al. 2000; Petez de Castro et al. 2002 |
ND | ||
| Serotonin | Transporter | SLC6A4 | + | Perez de Castro et al. 1999 and 2002; Devor et al. 1999; Hemmings et al. 2006 |
− | |
| Tryptophan hydroxylase |
TPH1 | + | Comings et al. 2001; Kreek et al. 2005; |
ND | ||
| Nielsen et al. 2008 | ||||||
| 5HT2A receptor |
HTR2A | + (impulsivity) | +/− (trend) |
Lee et al., 2009 |
||
| Glutamate | NMDA receptor |
GRIN2B | + | Kim et al. 2006 | + |
Lee et al., 2012 |
“+”significant association with ICD; “−“, no significant association with ICD was found; “ND”, no data.
In PD, some inheritance has also been suggested by the association of ICD with familial history of ICD, alcoholism, drug addiction, or mood disorders87. In a study comparing 58 PD patients with ICD to 346 PD patients without ICD, a significant association was found with the D3 dopamine receptor (DRD3) and the NMDA glutamate receptor 2B subunit (GRIN2B)88. Subsequent analyses in the same cohort identified a trend toward a dose-dependent association with the serotonin 2A receptor gene (HTR2A)89. By contrast to the general population, no association was found with the dopamine D2 receptor (DRD2), the C-O-methyltransferase (COMT), or the serotonin transporter (SLC64A), a result confirmed in an independent study performed in 41 PD patients with ICD with 48 matched controls (Table 1)90. However, the small number of patients in these studies and the lack of replication does not allow for definitive conclusions.
In overview, several genes from the monoamine pathways have been associated with ICD in the general population, whereas in PD only the DRD3 and the GRIN2B genes were found associated. This apparent discrepancy may be related to the exposure to dopamine agonists in PD which may trigger the association toward the drug response rather than ICD genetic susceptibility.
Summary and Conclusions
The phenomenon of ICDs in PD continues to be an untoward side effect of dopaminergic therapy with potentially devastating consequences to a significant number of patients 91. The past decade has witnessed impressive advances not only in the recognition of ICDs, but also in understanding the neurobiological and genetic associations: Human imaging has aided in mapping the neuroanatomical substrates that are engaged during active phases of ICDs, and in providing insights in those regions that are altered during PD with ICDs. These substrates map onto those that are described at the cellular and circuit levels in studies with laboratory animals. New animal models that recapitulate critical features of PD with ICDs are being developed. Genetic constructs that may be unique to ICDs in PD are being identified. These all provide exciting new venues in which the causes of ICD side effects of dopamine therapy in PD can be identified, and ultimately provide new therapies that can improve the motor pathology of PD but are devoid of ICD side effects. To accomplish this goal, the new animal models could be exploited to help identify the ICD potential of putative therapies. Future work also needs to include large case-control studies on genetic susceptibility to confirm these current results and ultimately identify genes that may be predictive for ICD development.
Acknowledgments
Funding Sources for Study:
T. Celeste Napier, Ph.D. Recent studies reported here were supported by USPHSGs NS074014 and DA0331231, the Michael J. Fox Foundation, and the National Center for Responsible Gaming.
Jean-Christophe Corvol M.D., Ph.D. This work was supported by the program “Investissements d’avenir” ANR-10-IAIHU-06.
Anthony A. Grace, Ph.D. Studies reported here were funded by USPHSGs MH57440, DA036328, and NS19608.
Jamie D. Roitman, Ph.D. Studies reported here were supported by National Institutes of Health DA027127 and the Brain Research Foundation.
James B. Rowe Ph.D., FRCP. Research was supported by the Wellcome Trust, the Medical Research Council, the National Institute for Health Research, the James S McDonnell Foundation, the Newton Trust and Evelyn Trust. The studies described here were primarily supported by the Wellcome Trust (088324), with additional funding from Parkinson's UK, the National Institute for Health Research Cambridge Biomedical Research Centre and the Medical Research Council (MC_US_A060_0016).
Valerie Voon M.D., Ph.D. is a Wellcome Trust Fellow.
Antonio P. Strafella M.D., Ph.D., FRCPC. Studies reported here were funded by Canadian Institute of Health Research, Parkinson Society Canada, Parkinson Disease Foundation, Ontario Problem Gambling Research Centre.
Footnotes
Financial Disclosures:
T. Celeste Napier, Ph.D. Dr. Napier has no financial conflict of interest with respect to the content of this manuscript. Dr. Napier has received research support from the National Institutes of Health, the Michael J. Fox Foundation and the National Center for Responsible Gaming. Dr. Napier has received compensation for the following: consulting for a not-for-profit health education center and for law offices on issues related to addictions and impulse control disorders; speaking on addictions at community town hall meetings, public high schools, community-based not-for-profits, and professional meetings of drug courts; providing grant reviews for the National Institutes of Health and other agencies; and academic lectures and grand rounds. Dr. Napier is a member of the Illinois Alliance on Problem Gambling, and she provides expert advice to Cures Within Research Foundation, LLC.
Jean-Christophe Corvol M.D., Ph.D. has no financial conflict of interest with respect to the content of this manuscript. Dr. Corvol has received research support from the French Ministry of Health, INSERM, the French Parkinson’s disease association, and the Michael J. Fox Foundation. Dr. Corvol has received honoraria for his participation to clinical trials and travel grants for scientific meetings from Novartis, UCB, Biogen, Sanofi-Aventis.
Anthony A. Grace, Ph.D. Dr. Grace has no financial conflict of interest with respect to the content of this manuscript. Dr. Grace has received research support from the National Institutes of Health, Lundbeck, and Lilly. Dr. Grace has received compensation for consulting/honoraria from Johnson & Johnson, Lundbeck, Pfizer, GSK, Puretech Ventures, Merck, Takeda, Dainippon Sumitomo, Otsuka, Lilly, Roche, Asubio, Abbott
Jamie D. Roitman PhD. Dr. Roitman has no financial conflict of interest with respect to the content of this manuscript. Dr. Roitman has received research support from the National Institutes of Health and the Brain Research Foundation.
James B. Rowe B.M., Ph.D., FRCP. Dr. Rowe has no financial conflict of interest with respect to this manuscript. Dr Rowe has received research support from the Wellcome Trust, the Medical Research Council, the National Institute for Health Research, the James S McDonnell Foundation, the Newton Trust and Evelyn Trust.
Valerie Voon M.D., Ph.D., FRCPC. Dr. Voon has no financial conflict of interest with respect to the content of this manuscript. Dr. Voon has received support from the Wellcome Trust.
Antonio P. Strafella M.D., Ph.D., FRCPC. Dr. Strafella has no financial conflict of interest with respect to the content of this manuscript. Dr. Strafella has received research support from Canadian Institute of Health Research, Parkinson Society Canada, Parkinson Disease Foundation, National Parkinson Society, Ontario Problem Gambling Research Centre.
References
- 1.Weintraub D, Papey K, Siderowf A. Screening for impulse control symptoms in patients with de novo Parkinson disease. Neurology. 2013;80(2):176–180. doi: 10.1212/WNL.0b013e31827b915c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67:589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- 3.Voon V, Sohr M, Lang AE, Potenza MN, Siderowf AD, Whetteckey J, et al. Impulse control disorders in Parkinson disease: a multicenter case--control study. Ann Neurol. 2011;69:986–996. doi: 10.1002/ana.22356. [DOI] [PubMed] [Google Scholar]
- 4.Housden CR, O'Sullivan SS, Joyce EM, Lees AJ, Roiser JP. Intact reward learning but elevated delay discounting in Parkinson's disease patients with impulsive-compulsive spectrum behaviors. Neuropsychopharmacology. 2010;35:2155–2164. doi: 10.1038/npp.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Djamshidian A, Sanotsky Y, Matviyenko Y, O'Sullivan SS, Sharman S, Selikhova M, et al. Increased reflection impulsivity in patients with ephedrone-induced Parkinsonism. Addiction. 2013;108:771–779. doi: 10.1111/add.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voon V, Gao J, Brezing C, Symmonds M, Ekanayake V, Fernandez H, et al. Dopamine agonists and risk: impulse control disorders in Parkinson's disease. Brain. 2011;134:1438–1446. doi: 10.1093/brain/awr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Djamshidian A, O'Sullivan SS, Wittmann BC, Lees AJ, Averbeck BB. Novelty seeking behaviour in Parkinson's disease. Neuropsychologia. 2011;49:2483–2488. doi: 10.1016/j.neuropsychologia.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Averbeck BB, Djamshidian A, O'Sullivan SS, Housden CR, Roiser JP, Lees AJ. Uncertainty about mapping future actions into rewards may underlie performance on multiple measures of impulsivity in behavioral addiction: evidence from Parkinson's disease. Behavioral neuroscience. 2013;127:245–255. doi: 10.1037/a0032079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voon V, Reynolds B, Brezing C, Gallea C, Skaljic M, Ekanayake V, et al. Impulsive choice and response in dopamine agonist-related impulse control behaviors. Psychopharmacology (Berl) 2010;207:645–659. doi: 10.1007/s00213-009-1697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Decamp E, Schneider JS. Effect of nicotinic therapies on attention and executive functions in chronic low-dose MPTP-treated monkeys. European Journal of Neuroscience. 2006;24(7):2098–2104. doi: 10.1111/j.1460-9568.2006.05077.x. [DOI] [PubMed] [Google Scholar]
- 11.Tedford SE, Persons AL, Napier TC. Lesions of the dorsolateral striatum increase delay discounting in a rat model of impulsive choice using intracranial self-stimulation. 2014 doi: 10.1371/journal.pone.0122063. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meck WH, Benson AM. Dissecting the brain's internal clock: How frontal-striatal circuitry keeps time and shifts attention. Brain and Cognition. 2002;48(1):195–211. doi: 10.1006/brcg.2001.1313. [DOI] [PubMed] [Google Scholar]
- 13.Rokosik SL, Napier TC. Pramipexole-induced increased probabilistic discounting: comparison between a rodent model of Parkinson's disease and controls. Neuropsychopharmacology. 2012;37:1397–1408. doi: 10.1038/npp.2011.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckholtz JW, Treadway MT, Cowan RL, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steeves TDL, Miyasaki J, Zurowski M, et al. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: a [11C] raclopride PET study. Brain. 2009;132:1376–1385. doi: 10.1093/brain/awp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cilia R, Ko JH, Cho SS, van Eimeren T, Marotta G, Pellecchia G, et al. Reduced dopamine transporter density in the ventral striatum of patients with Parkinson's disease and pathological gambling. Neurobiol Dis. 2010;39:98–104. doi: 10.1016/j.nbd.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Ray N, Miyasaki JM, Zurowski M, Ko JH, Cho SS, Pellecchia G, et al. Extrastriatal dopaminergic abnormalities of DA homeostasis in Parkinson’s patients with medication-induced pathological gambling: A [11C] FLB-457 and PET study. Neurobiol Dis. 2012;48:519–525. doi: 10.1016/j.nbd.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vriend C, Nordbeck AH, booji J, van der Werf YD, Pattij T, Voorn P, Raijmakers P, Foncke EMJ, van de Giessen E, Berendse HW, van de Heuvel OA. Reduced dopamine transporter binding predates impulse control disorders in Parkinson's disease. Movement Disorders. 2014;29:904–911. doi: 10.1002/mds.25886. [DOI] [PubMed] [Google Scholar]
- 19.van Eimeren T, Pellecchia G, Cilia R, Ballanger B, Steeves TD, Houle S, et al. Drug-induced deactivation of inhibitory networks predicts pathological gambling in PD. Neurology. 2010;5:1711–1716. doi: 10.1212/WNL.0b013e3181fc27fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cilia R, Cho SS, van Eimeren T, Marotta G, Siri C, Ko JH, et al. Pathological gambling in patients with Parkinson's disease is associated with fronto-striatal disconnection: A path modeling analysis. Mov Disord. 2011;26(2):225–233. doi: 10.1002/mds.23480. [DOI] [PubMed] [Google Scholar]
- 21.Ray NJ, Strafella AP. Imaging impulse control disorders in Parkinson's disease and their relationship to addiction. J Neural Transm. 2013;120(4):659–664. doi: 10.1007/s00702-012-0933-5. [DOI] [PubMed] [Google Scholar]
- 22.Antonelli F, Ko JH, Miyasaki J, Lang AE, Houle S, Valzania F, et al. Dopamine-Agonists and Impulsivity in Parkinson’s Disease: Impulsive Choices vs. Impulsive Actions Human Brain Mapping. 2014;35(6):2499–2506. doi: 10.1002/hbm.22344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballanger B, van Eimeren T, Moro E, Lozano AM, Hamani C, Boulinguez P, et al. Stimulation of the subthalamic nucleus and impulsivity: Release your horses. Ann Neurol. 2009;66:817–824. doi: 10.1002/ana.21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Djamshidian A, Jha A, O'Sullivan SS, Silveira-Moriyama L, Jacobson C, Brown P, et al. Risk and learning in impulsive and nonimpulsive patients with Parkinson's disease. Mov Disord. 2010;25:2203–2210. doi: 10.1002/mds.23247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao H, Mamikonyan E, Detre JA, Siderowf AD, Stern MB, Potenza MN, et al. Decreased ventral striatal activity with impulse control disorders in Parkinson's disease. Mov Disord. 2010;25:1660–1669. doi: 10.1002/mds.23147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nombela C, Rittman T, Robbins TW, Rowe JB. Multiple modes of impulsivity in Parkinson's disease. PLoS ONE. 2014;9:e85747. doi: 10.1371/journal.pone.0085747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furl N, Averbeck BB. Parietal cortex and insula relate to evidence seeking relevant to reward-related decisions. J Neurosci. 2011;31:17572–17582. doi: 10.1523/JNEUROSCI.4236-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstein DS, Sullivan P, Holmes C, Kopin IJ, Basile MJ, Mash DC. Catechols in post-mortem brain of patients with Parkinson disease. Eur J Neurol. 2011;18:703–710. doi: 10.1111/j.1468-1331.2010.03246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Politis M, Wu K, Loane C, et al. Staging of serotonergic dysfunction in Parkinson's disease: an in vivo 11C-DASB PET study. Neurobiol Dis. 2010;40:216–221. doi: 10.1016/j.nbd.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 30.Rae CL, Correia MM, Altena E, Hughes LE, Barker RA, Rowe JB. White matter pathology in Parkinson's disease: the effect of imaging protocol differences and relevance to executive function. Neuroimage. 2012;62:1675–1684. doi: 10.1016/j.neuroimage.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Homberg JR, Pattij T, Janssen MC, et al. Serotonin transporter deficiency in rats improves inhibitory control but not behavioural flexibility. Eur J Neurosci. 2007;26:2066–2073. doi: 10.1111/j.1460-9568.2007.05839.x. [DOI] [PubMed] [Google Scholar]
- 32.Del-Ben CM, Deakin JF, McKie S, et al. The effect of citalopram pretreatment on neuronal responses to neuropsychological tasks in normal volunteers: an FMRI study. Neuropsychopharmacology. 2005;30:1724–1734. doi: 10.1038/sj.npp.1300728. [DOI] [PubMed] [Google Scholar]
- 33.Macoveanu J, Hornboll B, Elliott R, et al. Serotonin 2A receptors, citalopram and tryptophan-depletion: a multimodal imaging study of their interactions during response inhibition. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2012.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson ES, Dalley JW, Theobald DE, et al. Opposing roles for 5-HT2A and 5-HT2C receptors in the nucleus accumbens on inhibitory response control in the 5-choice serial reaction time task. Neuropsychopharmacology. 2008;33:2398–2406. doi: 10.1038/sj.npp.1301636. [DOI] [PubMed] [Google Scholar]
- 35.Rubia K, Lee F, Cleare AJ, et al. Tryptophan depletion reduces right inferior prefrontal activation during response inhibition in fast, event-related fMRI. Psychopharmacology (Berl) 2005;179:791–803. doi: 10.1007/s00213-004-2116-z. [DOI] [PubMed] [Google Scholar]
- 36.Harrison AA, Everitt BJ, Robbins TW. Central serotonin depletion impairs both the acquisition and performance of a symmetrically reinforced go/no-go conditional visual discrimination. Behav Brain Res. 1999;100:99–112. doi: 10.1016/s0166-4328(98)00117-x. [DOI] [PubMed] [Google Scholar]
- 37.Vollm B, Richardson P, McKie S, Elliott R, Deakin JF, Anderson IM. Serotonergic modulation of neuronal responses to behavioural inhibition and reinforcing stimuli: an fMRI study in healthy volunteers. Eur J Neurosci. 2006;23:552–560. doi: 10.1111/j.1460-9568.2005.04571.x. [DOI] [PubMed] [Google Scholar]
- 38.Bari A, Eagle DM, Mar AC, Robinson ES, Robbins TW. Dissociable effects of noradrenaline, dopamine, and serotonin uptake blockade on stop task performance in rats. Psychopharmacology (Berl) 2009;205:273–283. doi: 10.1007/s00213-009-1537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bari A, Mar AC, Theobald DE, et al. Prefrontal and monoaminergic contributions to stop-signal task performance in rats. J Neurosci. 2011;31:9254–9263. doi: 10.1523/JNEUROSCI.1543-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chamberlain SR, Del Campo N, Dowson J, et al. Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol Psychiatry. 2007;62:977–984. doi: 10.1016/j.biopsych.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311:861–863. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gauggel S, Rieger M, Feghoff TA. Inhibition of ongoing responses in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 2004;75:539–544. doi: 10.1136/jnnp.2003.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Obeso I, Wilkinson L, Casabona E, et al. Deficits in inhibitory control and conflict resolution on cognitive and motor tasks in Parkinson's disease. Exp Brain Res. 2011;212:371–384. doi: 10.1007/s00221-011-2736-6. [DOI] [PubMed] [Google Scholar]
- 44.Ye Z, Altena E, Nombela C, et al. Selective serotonin reuptake inhibition modulates response inhibition in Parkinson's disease. Brain. 2014 Apr;137(Pt 4):1145–1155. doi: 10.1093/brain/awu032. Epub 2014 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye Z, Altena E, Nombela C, et al. Improving impulsivity in Parkinson’s disease with atomoxetine. Biological Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye Z, Nombela C, Ham T, et al. Predictive modelling of Atomoxetine and Citalopram’s effects on response inhibition in Parkinson’s disease in Organization of Human Brain Mapping annual meeting, 2014: Hamburg. Poster #445 [Google Scholar]
- 47.Carelli RM. Nucleus accumbens cell firing and rapid dopamine signaling during goal-directed behaviors in rats. Neuropharmacology. 2004;47(Suppl 1):180–189. doi: 10.1016/j.neuropharm.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 48.Carelli RM, Wightman RM. Functional microcircuitry in the accumbens underlying drug addiction: insights from real-time signaling during behavior. Curr Opin Neurobiol. 2004;14(6):763–768. doi: 10.1016/j.conb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 49.Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30(5):203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 50.Goto Y, Grace AA. Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci. 2008;31(11):552–558. doi: 10.1016/j.tins.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghahremani DG, Lee B, Robertson CL, Tabibnia G, Morgan AT, De Shetler N, et al. Striatal dopamine D(2)/D(3) receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. J Neurosci. 2012;32(21):7316–7324. doi: 10.1523/JNEUROSCI.4284-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rao H, Korczykowski M, Pluta J, Hoang A, Detre JA. Neural correlates of voluntary and involuntary risk taking in the human brain: an fMRI Study of the Balloon Analog Risk Task (BART) Neuroimage. 2008;42(2):902–910. doi: 10.1016/j.neuroimage.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rao H, Mamikonyan E, Detre JA, Siderowf AD, Stern MB, Potenza MN, et al. Decreased ventral striatal activity with impulse control disorders in Parkinson's disease. Mov Disord. 2010;25(11):1660–1669. doi: 10.1002/mds.23147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Claassen DO, van den Wildenberg WP, Ridderinkhof KR, Jessup CK, Harrison MB, Wooten GF, et al. The risky business of dopamine agonists in Parkinson disease and impulse control disorders. Behavioral neuroscience. 2011;125(4):492–500. doi: 10.1037/a0023795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26(9):2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li CS, Yan P, Sinha R, Lee TW. Subcortical processes of motor response inhibition during a stop signal task. Neuroimage. 2008;41(4):1352–1363. doi: 10.1016/j.neuroimage.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moustafa AA, Cohen MX, Sherman SJ, Frank MJ. A role for dopamine in temporal decision making and reward maximization in parkinsonism. J Neurosci. 2008;28(47):12294–12304. doi: 10.1523/JNEUROSCI.3116-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roitman JD, Loriaux AL. Nucleus accumbens responses differentiate execution and restraint in reward-directed behavior. J Neurophysiol. 2014;111(2):350–360. doi: 10.1152/jn.00350.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494(7436):238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Napier TC. Contribution of the amygdala and nucleus accumbens to ventral pallidal responses to dopamine agonists. Synapse. 1992;10:110–119. doi: 10.1002/syn.890100205. [DOI] [PubMed] [Google Scholar]
- 61.Zigmond MJ, Berger TW, Grace AA, Stricker EM. Compensatory responses to nigrostriatal bundle injury. Studies with 6-hydroxydopamine in an animal model of parkinsonism. Mol Chem Neuropathol. 1989 Jun;10(3):185–200. doi: 10.1007/BF03159728. [DOI] [PubMed] [Google Scholar]
- 62.Zigmond MJ, Abercrombie ED, Berger TW, Grace AA, Stricker EM. Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci. 1990 Jul;13(7):290–296. doi: 10.1016/0166-2236(90)90112-n. [DOI] [PubMed] [Google Scholar]
- 63.Hollerman JR, Grace AA. The effects of dopamine-depleting brain lesions on the electrophysiological activity of rat substantia nigra dopamine neurons. Brain Research. 1990;533(2):203–212. doi: 10.1016/0006-8993(90)91341-d. [DOI] [PubMed] [Google Scholar]
- 64.Harden DG, Grace AA. Activation of dopamine cell firing by repeated L-DOPA administration to dopamine-depleted rats: its potential role in mediating the therapeutic response to L-DOPA treatment. Journal of Neuroscience. 1995;15(9):6157–6166. doi: 10.1523/JNEUROSCI.15-09-06157.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lodge DJ, Grace AA. Amphetamine activation of hippocampal drive of mesolimbic dopamine neurons: a mechanism of behavioral sensitization. J Neurosci. 2008;28(31):7876–7882. doi: 10.1523/JNEUROSCI.1582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. Journal of Neuroscience. 1984;4(11):2866–2876. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. Journal of Neuroscience. 1984;4(11):2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schultz W. The phasic reward signal of primate dopamine neurons. Advances in Pharmacology. 1998;42:686–690. doi: 10.1016/s1054-3589(08)60841-8. [DOI] [PubMed] [Google Scholar]
- 69.Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31(7):1356–1361. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- 70.Grace AA, Floresco SB, et al. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30(5):220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 71.Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nature-Neuroscience. 2005;8:805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- 72.Goto Y, Grace AA. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron. 2005;47(2):255–266. doi: 10.1016/j.neuron.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 73.Maren S. Neurotoxic or electrolytic lesions of the ventral subiculum produce deficits in the acquisition and expression of Pavlovian fear conditioning in rats. Behav Neurosci. 1999;113(2):283–290. doi: 10.1037//0735-7044.113.2.283. [DOI] [PubMed] [Google Scholar]
- 74.Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110(1–2):73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- 75.Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35(1):27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Evans AH, 1, Katzenschlager R, Paviour D, O'Sullivan JD, Appel S, Lawrence AD, Lees AJ. Punding in Parkinson's disease: its relation to the dopamine dysregulation syndrome. Mov Disord. 2004 Apr;19(4):397–405. doi: 10.1002/mds.20045. [DOI] [PubMed] [Google Scholar]
- 77.Sesia T, Bizup B, Grace AA. Evaluation of animal models of obsessive-compulsive disorder: Correlation with phasic dopamine neuron activity. International Journal of Neuropsychopharmacology. 2013;16:1295–1307. doi: 10.1017/S146114571200154X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Henry DJ, Hu XT, White FJ. Adaptations in the mesoaccumbens dopamine system resulting from repeated administration of dopamine D1 and D2 receptor-selective agonists: relevance to cocaine sensitization. Psychopharmacology (Berl) 1998 Nov;140(2):233–242. doi: 10.1007/s002130050762. [DOI] [PubMed] [Google Scholar]
- 79.Karkowski LM, Prescott CA, Kendler KS. Multivariate assessment of factors influencing illicit substance use in twins from female-female pairs. Am J Med Genet. 2000;96(5):665–670. [PubMed] [Google Scholar]
- 80.Black DW, Moyer T. Clinical features and psychiatric comorbidity of subjects with pathological gambling behavior. Psychiatr Serv. 1998;49(11):1434–1439. doi: 10.1176/ps.49.11.1434. [DOI] [PubMed] [Google Scholar]
- 81.Slutske WS, Eisen S, True WR, Lyons MJ, Goldberg J, Tsuang M. Common genetic vulnerability for pathological gambling and alcohol dependence in men. Arch Gen Psychiatry. 2000;57(7):666–673. doi: 10.1001/archpsyc.57.7.666. [DOI] [PubMed] [Google Scholar]
- 82.Eisen SA, Lin N, Lyons MJ, et al. Familial influences on gambling behavior: an analysis of 3359 twin pairs. Addiction. 1998;93(9):1375–1384. doi: 10.1046/j.1360-0443.1998.93913758.x. [DOI] [PubMed] [Google Scholar]
- 83.Slutske WS, Zhu G, Meier MH, Martin NG. Genetic and environmental influences on disordered gambling in men and women. Arch Gen Psychiatry. 2010;67(6):624–630. doi: 10.1001/archgenpsychiatry.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Comings DE, Gade-Andavolu R, Gonzalez N, et al. The additive effect of neurotransmitter genes in pathological gambling. Clin Genet. 2001;60(2):107–116. doi: 10.1034/j.1399-0004.2001.600204.x. [DOI] [PubMed] [Google Scholar]
- 85.Cormier F, Mueliner J, Corvol J-C. Genetics of impusle control disorders in Parkinson’s disease. J Neural Transm. 2013;120:665–671. doi: 10.1007/s00702-012-0934-4. [DOI] [PubMed] [Google Scholar]
- 86.Lind PA, Zhu G, Montgomery GW, et al. Genome-wide association study of a quantitative disordered gambling trait. Addict Biol. 2013;18(3):511–522. doi: 10.1111/j.1369-1600.2012.00463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- 88.Lee JY, Lee EK, Park SS, et al. Association of DRD3 and GRIN2B with impulse control and related behaviors in Parkinson's disease. Mov Disord. 2009;24(12):1803–1810. doi: 10.1002/mds.22678. [DOI] [PubMed] [Google Scholar]
- 89.Lee JY, Jeon BS, Kim HJ, Park SS. Genetic variant of HTR2A associates with risk of impulse control and repetitive behaviors in Parkinson's disease. Parkinsonism Relat Disord. 2012;18(1):76–78. doi: 10.1016/j.parkreldis.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 90.Vallelunga A, Flaibani R, Formento-Dojot P, Biundo R, Facchini S, Antonini A. Role of genetic polymorphisms of the dopaminergic system in Parkinson's disease patients with impulse control disorders. Parkinsonism Relat Disord. 2012;18(4):397–399. doi: 10.1016/j.parkreldis.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 91.Santangelo G, 1, Barone P, Trojano L, Vitale C. Pathological gambling in Parkinson's disease. A comprehensive review. Parkinsonism Relat Disord. 2013;19(7):645–653. doi: 10.1016/j.parkreldis.2013.02.007. [DOI] [PubMed] [Google Scholar]