Abstract
Objective
Epidemiological data have suggested maternal infection and fever to be associated with increased risk of ASD. Animal studies show that gestational infections perturb fetal brain development and result in offspring with the core features of autism and have demonstrated that behavioral effects of maternal immune activation (MIA) are dependent on genetic susceptibility. The goal of this study was to explore the impact of ASD-associated CNVs and prenatal maternal infection on clinical severity of ASD within a dataset of prenatal history and complete genetic and phenotypic findings.
Method
We analyzed data from the Simons Simplex Collection sample including 1971 children with a diagnosis of ASD aged 4 to 18 years who underwent array CGH screening. Information on infection and febrile episodes during pregnancy was collected through parent interview. ASD severity was clinically measured through parent-report interview and questionnaires.
Results
We found significant interactive effects between presence of CNVs and maternal infection during pregnancy on autistic symptomatology, such that individuals with CNVs and history of maternal infection demonstrated increased rates of social communicative impairments and repetitive/restricted behaviors. In contrast, no significant interactions were found between presence of CNVs and prenatal infections on cognitive and adaptive functioning of individuals with ASD.
Conclusion
Our findings support a gene-environment interaction model of autism impairment, in that individuals with ASD-associated CNVs are more susceptible to the effects of maternal infection and febrile episodes in pregnancy on behavioral outcomes, and suggest that these effects are specific to ASD rather than to global neurodevelopment.
Key Terms: autism, autism spectrum disorders, fever, gene-environment, infection, pregnancy
Introduction
Autism spectrum disorder (ASD) has a strong genetic basis and converging lines of evidence implicate genetic factors as a predominant cause of ASD.1 DNA microarrays enabled the discovery of rare and recurrent copy number variants (CNVs) as important contributors to ASD and led to rapid gains in the understanding of autism genetics and the identification of individuals genetically susceptible to autism.2,3 Hotspots of recurrent CNVs, including those at 16p11.2, 22q11.2, 1q21.1, 7q11.23, and 15q11-q13, have been shown to be strongly associated with ASDs.3-5
A number of risk factors related to early fetal environment have been found in epidemiological research to be associated with increased rates of ASD.6 Taken together, these environmental insults share in common the activation of the maternal immune system. Maternal immune activation (MIA) as a risk factor in ASD has been supported by a number of population-based cohort studies, which found associations between autism risk and maternal viral infection in the first trimester, bacterial infection in the second trimester, influenza, and febrile episodes during pregnancy.7,8 These epidemiological findings are supported by evidence from rodent and non-human primate model studies of autism-like behaviors in offspring following MIA induction via systemic administration of synthetic double-stranded RNA (dsRNA) or lipopolysaccharide (LPS) to mimic infection in the pregnant mother. Studies of offspring behavior after MIA have shown that gestational infections trigger a maternal immune response that can perturb fetal brain development, by acting indirectly on the fetal brain chiefly through interleukin (IL) 6, and result in offspring with the core features of autism.9,10 Although mounting evidence supports the connection between a strong immune response during gestation with risk of development of autistic traits, most mothers who develop an infection during pregnancy do not give birth to autistic children. The hypothesis that genetic susceptibility combined with a strong immune reaction during gestation results in increased autism severity and a greater likelihood of a diagnosis of ASD is supported by rodent model studies showing that offspring of mothers from an inbred strain predisposed to exhibit ASD-like behaviors were more severely affected following MIA than pups of a species-typical strain, demonstrating that behavioral and immunological effects are strain-dependent.11 These findings also demonstrated evidence for genetic subtypes in ASD due to differential behavioral profiles associated with gestational age and the onset of the environmental insult (eg: MIA).11 Furthermore, the combination of MIA and genetic susceptibility (CNVs) produces more severe behavioral profiles than either one of these factors alone, suggesting the heterogeneity of behavioral phenotypes associated with ASD may have three distinct etiological contributions: genetic, environmental, and a combination of these factors.11 In the current study we investigate this gene-environment interaction hypothesis by evaluating the interactive effects of maternal infection in pregnancy and the presence of CNVs considered likely to play a contributing role in symptoms of ASD (ASD-associated CNVs) using a dataset of parent-reported prenatal history and genetic and phenotypic findings.
Methods and Materials
Study Population
Participants included 1971 children (1711 males, 260 females, 79% Caucasian, 89% non-Hispanic) with ASD between the ages of 4 and 18 years (Mean = 9.0, SD = 3.6) from the Simons Simplex Collection (SSC; see sfari.org). Individuals were selected for this analysis from the total sample in Distribution 14 of 2761 children with ASD if complete genetic, maternal pregnancy history, and phenotypic information was available.
The SSC is a project funded by the Simons Foundation Autism Research Initiative (SFARI) to identify de novo genetic variants related to ASD. Data was collected across 12 sites in North America. Inclusion criteria for the SSC required that the child meet ASD clinical cutoffs on the Autism Diagnostic Observation Schedule12 (ADOS) and on the Autism Diagnostic Interview- Revised13 (ADI-R) as well as meet diagnostic criteria for ASD using the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR).14 Probands were excluded for conditions that might jeopardize the validity of diagnostic instruments, and for family history that might decrease likelihood of discovery of de novo events; a complete listing of inclusion and exclusion criteria can be found on www.sfari.org.15
This study was approved by the University of Washington institutional review board, and appropriate informed consent was obtained from all participants across all sites.
Genetic Analysis
CNV data were extracted from published array CGH experiments on 2,478 individuals from the SSC collection. These arrays were designed to identify recurrent CNVs or hotspots flanked by high identity repeat sequences including segmental duplications and non-recurrent CNVs in the genomic backbone.5 A dichotomous variable was used to indicate presence of any rare recurrent CNV previously identified to be enriched in individuals with autism. 153 individuals in our sample had at least one recurrent large hotspot event identified by Girirajan and colleagues (see Table, Supplemental Digital Content 1, for a complete list of previously identified CNVs).5
Maternal Infection and Febrile Episodes
Information on maternal infections and febrile episodes during pregnancy with the proband were collected as part of a larger medical history interview conducted with one or both parents (primarily mothers) during enrollment into the SSC. The specific infectious illnesses reported and the numbers of mothers who endorsed these infections during varying points of the pregnancy are outlined in Table 1. A dichotomous variable was created for history of maternal infection during the entire pregnancy as there was no significant difference between onset of infection during pregnancy and a similar pattern of results was seen regardless of trimester when the infection occurred.
Table 1. Reported infectious illnesses and observed copy number variants (CNVs) in probands (N=1971).
| # of exposed probands with CNVs | # of exposed probands without CNVs | # of non-exposed probands with CNVs | # of non-exposed probands without CNVs | |
|---|---|---|---|---|
| Any infection or fever | 29 | 366 | 124 | 1452 |
| Other viral infection | 10 | 154 | 141 | 1640 |
| Maternal fever over 101F | 10 | 63 | 141 | 1738 |
| Influenza | 7 | 94 | 144 | 1708 |
| Herpes type 2 (HSV-2) | 2 | 31 | 148 | 1770 |
| Herpes type 1 (HSV-1) | 1 | 37 | 150 | 1763 |
| Chicken pox | 1 | 3 | 150 | 1799 |
| Shingles | 1 | 1 | 150 | 1801 |
| Viral hepatitis | 0 | 3 | 151 | 1799 |
| Infectious mononucleosis | 0 | 2 | 151 | 1799 |
| Measles | 0 | 0 | 151 | 1802 |
| Mumps | 0 | 0 | 151 | 1802 |
| Rubella | 0 | 0 | 151 | 1802 |
Autism Presentation
Degree of autistic impairment for the probands for whom array CGH results were available was determined through parent-report measures of symptoms related to ASD: ADI-R, the Repetitive Behavior Scale-Revised (RBS-R), and the Social Responsiveness Scale (SRS). The ADI-R is a standardized semi-structured parent interview aligned with DSM-IV criteria used to aid in ASD diagnosis.13 Three domains of the ADI-R were used as metrics of autistic symptomatology: Reciprocal Social Interaction (RSI), Communication (COM), and Restricted, Repetitive, and Stereotyped Patterns of Behavior (RRSB). The RBS-R is a parent report questionnaire regarding the presence and significance of restricted and repetitive behaviors that yields a total raw score summing 6 subscales: Stereotyped, Self-Injurious, Compulsive, Routine, Sameness, and Restricted Behaviors.16,17 The Social Responsiveness Scale (SRS) is a 65-item rating scale assessing social awareness, social information processing, capacity for reciprocal social communication, social anxiety/avoidance, and autistic preoccupations and traits that yields a total T score using for quantitative measurement of ASD related traits.18 Higher scores across all measures suggest greater number of ASD-related behaviors, suggesting greater impairment in ASD symptoms.
In addition to parent-reported measures of the degree of autism-related symptoms, we also investigated the relationship between genetic susceptibility, maternal infections, and cognitive and adaptive functioning. Verbal and nonverbal IQ was measured using age appropriate standardized assessment of cognitive functioning, including the Differential Abilities Scale – 2nd Edition (DAS-II)19, the Wechsler Intelligence Scale for Children20, or the Mullen Scales of Early Learning.21 Adaptive functioning was assessed via the Vineland Adaptive Behavior Scales, 2nd Edition – Survey Interview Form, a standardized parent interview that provides a general assessment of functioning across communication, daily living skills, and socialization domains.22 The composite standard score was used as a measure of adaptive functioning.
Results
Analytical Strategy
To investigate the main and interactive effects of the presence of any ASD-associated CNV and self-reported infection or fever episode during pregnancy on ASD severity, we conducted a series of univariate two-way analyses of variance to maximize sample size for each dependent variable. Due to the low frequency of each individual infection (see Table 1), we did not investigate the effects of any single infection on autism severity, and instead used a dichotomous variable to indicate whether the mother reported experiencing any infectious illness or febrile episode during her pregnancy. Finally, we separately examined the interactions between maternal infection or fever in pregnancy and presence of CNV on severity of symptoms on the ADI-R, SRS, and RBS-R for individuals with deletions and individuals with duplications.
Several potential confounding factors were examined to determine whether they should be entered as covariates into statistical analyses. It is feasible that earlier onset of ASD symptoms may relate to better recall of events on the medical history interview during pregnancy since events may have been more salient to parents at the time. However, the age that the first developmental abnormality manifested did not differ between probands exposed to infection during pregnancy versus those who were not, t(1946) = 0.39, p = .70, suggesting that parental recall of events during pregnancy was not confounded with earlier symptom presentation. Additionally, proband age at the time of medical history interview did not differ between probands exposed to infection during pregnancy versus those who were not, t(1989) = 0.11, p = .92; consequently, recall bias based on length of time since proband pregnancy is unlikely. Therefore, proband age and age of symptom presentation were not entered as covariates in analyses.
We also attempted to address the potential impact of several external factors on the presence of CNV status and maternal infection. There were no differences between probands with/without CNVs and with/without maternal prenatal infections in maternal age at proband birth (F(3, 1955) = 1.85, p = .14), paternal age at proband birth (F(3, 1955) = 1.24, p = .29), maternal education (F(3, 1915) = 0.38, p = .77), and annual household income (F(3, 1829) = 0.75, p = .52). As a result, these demographic variables were not entered as controls into interaction analyses.
Interaction between CNVs and Maternal Infection
As summarized in Table 2 and illustrated in Figure 1, we observed a statistically significant interactive effect of presence of CNV and maternal infection on all domains of the ADI-R, including Reciprocal Social Interaction (RSI; F(1, 1967) = 7.56, p = .006), Communication (COM; F(1, 1737) = 7.54, p = .006), and Restrictive, Repetitive, and Stereotyped Patterns of Behavior (RRSB; F(1, 1967) = 5.74, p = .017). Similarly significant interactive effects were observed on impairment on the Repetitive Behavior Scale – Revised (RBS-R; F(1, 1966) = 6.36, p = .012) and the Social Responsiveness Scale (SRS; F(1, 1958) = 6.00, p = .014). Individuals with ASD-associated CNVs and maternal infection had increased impairment across all measures relative to individuals with CNVs but no maternal infection, individuals with maternal infection but no CNVs, and individuals with neither risk factor. No significant interactive effects of presence of a CNV and maternal infection or fever episode during pregnancy on cognitive or adaptive functioning were observed.
Table 2.
Clinical phenotypes of offspring diagnosed with ASD after self-reported infection or fever during pregnancy.
| Autism Diagnostic Interview - Revised | Repetitive Behavior Scale M (95% CI) | Social Responsiveness Scale M (95% CI) | Verbal IQ M (95% CI) | Nonverbal IQ M (95% CI) | Vineland Adaptive Behavior Scales M (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Reciprocal Social Interaction M (95% CI) | Communi-cation M (95% CI) | Restrictive, Repetitive, Stereotyped Behaviors M (95% CI) | |||||||
| No CNV | No maternal infection | 20.46 (20.16-20.75) | 16.48 (16.25-16.72) | 6.49 (6.36-6.62) | 27.47 (26.59-28.36) | 79.77 (79.24-80.30) | 77.13 (75.53-78.73) | 84.56 (83.22-85.91) | 72.97 (72.35-73.59) |
| Maternal infection reported | 19.62 (19.04-20.20) | 16.35 (15.89-16.81) | 6.76 (6.50-7.01) | 27.84 (26.08-29.61) | 79.93 (78.87-80.99) | 80.39 (77.20-83.57) | 87.07 (84.39-89.76) | 74.25 (73.02-75.48) | |
| CNV Present | No maternal infection | 20.14 (19.14-21.14) | 16.28 (15.47-17.08) | 6.49 (6.05-6.93) | 24.50 (21.46-27.54) | 77.82 (75.99-79.65) | 76.46 (70.99-81.93) | 80.02 (75.41-84.62) | 71.61 (69.50-73.73) |
| Maternal infection reported | 22.66 (20.58-24.73) | 18.69 (17.14-20.24) | 8.03 (7.13-8.94) | 34.21 (27.92-40.49) | 83.41 (79.65-87.18) | 76.72 (65.41-88.04) | 79.31 (69.78-88.84) | 69.59 (65.22-73.96) | |
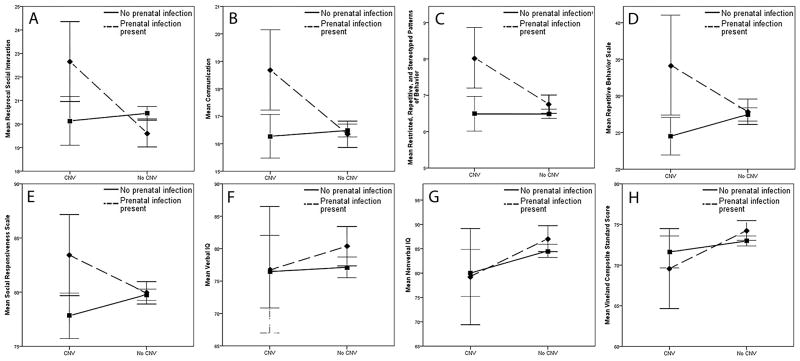
Figure 1.
Autism symptomatology and cognitive and adaptive functioning of children with ASD-associated copy number variants (CNVs) and history of maternal infection or fever during pregnancy. N=1971. Error bars = 95% Confidence Interval. As shown in graph C (p = .010), a main effect for presence of infections is demonstrated. As shown in graphs G (p =.019) and H (p = .049), main effects for presence of CNV are demonstrated. As shown in graphs A-E: (A) p = .006; (B) p = .006; (C) p = .017; (D) p = .012; (E) p = .014, significant interactions are observed. No significant interactions are observed in graphs F-H.
Deletions vs. Duplications
We observed a significant interaction between presence of a deletion of a region implicated in ASD and maternal self-reported infection or fever in pregnancy on ASD severity as measured by the ADI-R (RSI domain: F(1, 1900) = 8.78, p = .003; COM domain: F(1, 1676) = 7.37, p = .007; RRSB domain: F(1, 1900) = 9.84, p = .002). The interactive effect was observed when only individuals with duplications were compared to those without CNVs on the RBS-R: F(1, 1880) = 6.88, p = .009. No other significant interactive effects were observed (see Figure, Supplemental Digital Content 2, which shows autism symptomatology and cognitive and adaptive functioning of children with ASD-associated CNVs and history of maternal infection or fever during pregnancy by type of copy number event, N=1971, error bars = 95% Confidence Interval).
Main Effects of Presence of CNVs
There was a main effect of CNV presence on nonverbal IQ (F(1, 1968) = 5.49, p =.019) but not on verbal IQ (F(1, 1968) = .225, p = .635), and on adaptive functioning, (F(1, 1968) = 3.87, p = .049), such that individuals with a CNV showed more impaired nonverbal IQ and poorer adaptive functioning. No main effects of the presence of a CNV were found on the ASD-related measures (ADI RSI domain (F(1, 1968) = .442, p = .506), ADI COM domain (F(1, 1738) = .746, p = .388), and ADI RRSB domain (F(1, 1968) = 1.36), p = .243)). No effects were found on social responsiveness via the SRS (F(1, 1959) = 1.08, p = .299) nor on restrictive/repetitive behaviors via the RBS-R (F(1, 1967) = .675, p = .411).
CNVs in our sample were evenly distributed in regards to the inheritance pattern, with 23% (40 of 173) CNVs paternally inherited, 27% (47 of 173) maternally inherited, 25% de novo CNVs (43 of 173), and the rest (43 of 173) with unknown inheritance. A possible confounding interaction is that maternally inherited CNVs increase maternal susceptibility to infection as well as offspring ASD symptomatology. However, we found no difference in prevalence of infections for individuals with maternally inherited CNVs (χ2 = .526).
Main Effect of Maternal Infection
We saw a significant main effect of self-reported maternal infection or fever episode during pregnancy on the ADI-R in the RRSB domain (F(1, 1968) = 6.67, p = .010), a trend toward significance in the RSI domain (F(1, 1968) = 3.35, p = .068) and no effect in the COM domain (F(1, 1738) = .074, p = .785). No effects were observed on the SRS (F(1, 1959) = .935, p = .334) nor on the RBS-R (F(1, 1967) = 1.20, p = .273). No main effects of maternal infection or fever during pregnancy on cognitive (Verbal IQ (F(1, 1968) = 3.013, p = .083); Nonverbal IQ (F (1, 1968) = 2.38, p = .123)) or adaptive functioning (F(1, 1968) = 2.36, p = .124) were observed.
Discussion
We found strong evidence supporting the interaction between genetic susceptibility as defined by the presence of ASD-associated CNVs and environmental insults in the form of maternal infection or fever during pregnancy in the etiology of increased severity of autism symptomatology in children with ASD. Individuals with a CNV and prenatal history of maternal infection/illness presented with greater autistic impairment, but showed no difference in cognitive or adaptive functioning, than children with either risk factor alone or neither risk factor. This pattern was reflected across all domains of ASD impairment and resulted in clinically relevant changes in behavioral outcomes. For instance, in the case of social responsiveness on the SRS, mean T scores bordered on the moderate/severe impairment cut-off for all groups except those individuals with both risk factors, whose mean score fell solidly in the severe category, using descriptive categories provided by the SRS (see Table 2 and Figure 1). The observed interactive effect between ASD-associated CNVs and maternal infection or fever in pregnancy on autism symptomatology as measured by the ADI-R was driven primarily by deletions.
In our large sample of well-characterized individuals with ASD, we extend previous work that identifies genetic susceptibility2-5 and environmental insults6-8 as independent risk factors in ASD by illuminating the relationship between these risk factors and ASD symptom presentation. Our findings support previous research on hotspots of recurrent CNVs associated with ASDs in which individuals with an identified CNV are more likely to demonstrate clinically related impairments.3-5 Replicating results of previous work on CNV hotpots associated with cognitive and adaptive functioning, we found increased impairments in non-verbal IQ, but not verbal IQ, and poorer adaptive functioning associated with presence of any ASD-associated CNV.5
Our findings also support epidemiological and rodent model experiments that identify MIA in pregnancy as associated with an increased risk of ASD. We observed significantly more impairment in repetitive and restrictive behaviors among children with ASD whose mothers reported prenatal infection or fever over 101F during pregnancy with the proband. These findings mirror recent large epidemiological studies indicating approximately a 30% increase of risk in ASD when maternal infections result in hospitalization, suggesting immune-mediated mechanisms play a role in the etiology of ASD.23 Our findings also reflect work on rodent models which suggests that gestational infections, activating a maternal immune response, perturb fetal brain development mainly via IL-6 and result in offspring with the core autism characteristics.9,10,24
Most importantly, our finding of the significant interactive effect of ASD-associated CNVs and maternal infection or fever in pregnancy parallels recent rodent model work in characterizing the relationship between genetic susceptibility and environmental insult. Schwartzer et al11 compared the effects of maternal immune activation on pups of mothers from an inbred strain predisposed to exhibit ASD-like behaviors to pups of a species typical strain and found greater behavioral impairment in pups with both genetic susceptibility and exposure to maternal immune activation than in pups with either risk factor alone, concluding that behavioral and immunological effects are strain-dependent. Our similar finding that individuals genetically predisposed to ASD in the form of CNVs with exposure to maternal infection and fever had more severe behavioral phenotypes than those with the genetic risk factor or environmental insult alone further supports the hypothesis that the effect of maternal infection or immune activation may be dependent on genetic predisposition.
Interestingly, maternal infection alone and in interaction with ASD-associated CNVs impacted ASD symptom severity, but not cognitive or adaptive severity. Previous research suggests a link between elevated cytokine levels present in altered immune profiles and severity of behavioral outcomes, particularly with deficits in social interaction and communication.25 Mouse models support the role of cytokines as putative in core behavioral features of autism: administration of IL-6 during pregnancy alone is enough to induce autism-like traits in the offspring,9 and it has been proposed that cytokines affect behavior by altering levels of specific hormones, namely oxytocin and serotonin.26 Further, gene-ontology analysis of all mouse and human CNV genes has shown overrepresentation of immune system function and enrichment for neurodevelopment, specifically, for those within genomic hotspots.27,28 Postmortem studies also found upregulated immune system genes in autism brains with environmental rather than genetic etiology; these dysregulated immune related genes seemed to also play a role in synaptic development and function.29 A potential mechanism underlying the effects of maternal infection and fever on neural development that results in ASD-like behaviors is the major histocompatibility complex (MHC) I – myocyte enhancer factor (MEF) 2 pathway which is altered in MIA offspring resulting in lower synapse density, and represents a common molecular pathway downstream of both genetic mutations and environmental factors that contribute to ASD.30 Therefore, it is possible that CNVs that alter the immune system as well as pathways in neural synapse development, when combined with a severe activation of the maternal immune system early during gestation, lead to alteration in a common molecular pathway and result in the core behavioral features of ASD.
This study has several strengths: the SSC is the largest collection of well characterized children with ASD with complete phenotypic and genotypic information available to date, gathered as part of a collaborative, multi-site study. To our knowledge, this is the first study to investigate the interactive effects of genetic susceptibility and environmental insults on the degree of autistic impairment in humans. A consideration in the interpretation of this study is that we relied on parent self-report of infection during pregnancy. However, previous studies which have compared patient reports of illness in pregnancy against available medical records found fairly good agreement between the two sources, suggesting that maternal-reported data on infection is a valuable, albeit imperfect, source of medical history data.7 In the current study, we focused on copy number variants in general, but future work should examine closer the role of other structural changes. In addition, we did not investigate the contributions of individual infections or specific recurrent CNVs on autism severity because of the rarity of specific events; a larger cohort study in the future may be useful to elucidate the effects of particular infections on maternal immune activation and resultant autism impairment. Finally, all the children considered in this study have a diagnosis of ASD. As such our conclusions extend previous epidemiological, genetic, and animal model work identifying risk factors for ASD by examining the specific contribution to ASD symptom presentation rather than autism etiology per se. Also, since ascertainment was limited to participants with ASD, our conclusions pertaining to cognitive and adaptive severity were restricted to some extent. Differential patterns of cognitive and adaptive severity in a sample ascertained on the diagnosis of intellectual disability would further elucidate the relationship between behavioral phenotypes associated with maternal infection and CNVs. Future population-based studies are needed to further explore and characterize the relationship between these risk factors.
In conclusion, our results suggest that children with de novo or inherited ASD-associated CNVs may be more susceptible to environmental insults due to maternal immune activation during fetal development, as demonstrated by observed significantly stronger impairments across all domains of autism severity among our large sample of children with ASD. While the discovery of CNVs associated with autism has advanced ASD understanding, further examination of the interactive factors that link genotype with the complex autistic presentation is still needed.
Supplementary Material
Acknowledgments
Funding for this study was provided by the Medical Student Research Training Program (MSRTP), University of Washington School of Medicine, Seattle, WA. The funding source did not participate in any part of the performance of the study. This work was supported by two grants from the Simons Foundation (SFARI #89368 to R.B. and SFARI #137578 to E.E.E.) and a grant from the National Institute of Mental Health (#1R01MH101221 to E.E.E.). E.E.E. is an investigator of the Howard Hughes Medical Institute.
We are grateful to all of the families at the participating Simons Simplex Collection (SSC) sites, as well as the principal investigators (A. Beaudet, R. Bernier, J. Constantino, E. Cook, E. Fombonne, D. Geschwind, R. Goin-Kochel, E. Hanson, D. Grice, A. Klin, D. Ledbetter, C. Lord, C. Martin, D. Martin, R. Maxim, J. Miles, O. Ousley, K. Pelphrey, B. Peterson, J. Piggot, C. Saulnier, M. State, W. Stone, J. Sutcliffe, C. Walsh, Z. Warren, E. Wijsman). We appreciate obtaining access to phenotypic data on SFARI Base. Approved researchers can obtain the SSC population dataset described in this study by applying at https://base.sfari.org.
Source of Funding: Funding for this study was provided by the Medical Student Research Training Program (MSRTP), University of Washington School of Medicine, Seattle, WA. The funding source did not participate in any part of the performance of the study. E.E.E. is on the scientific advisory board (SAB) of DNAnexus, Inc. and was an SAB member of Pacific Biosciences, Inc. (2009-2013) and SynapDx Corp. (2011-2013).
Footnotes
Conflicts of Interest: For all other authors, no biomedical financial interests or potential conflicts of interest were reported.
References
- 1.Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9(5):341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sebat J, Lakshmi B, Malhotra D, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316(5823):445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo R, Sanders Stephan J, Tian Y, et al. Genome-wide Transcriptome Profiling Reveals the Functional Impact of Rare De Novo and Recurrent CNVs in Autism Spectrum Disorders. The American Journal of Human Genetics. 2012;91(1):38–55. doi: 10.1016/j.ajhg.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanders SJ, Ercan-Sencicek AG, Hus V, et al. Multiple recurrent de novo CNVs, including duplications of the 7q11. 23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70(5):863–885. doi: 10.1016/j.neuron.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girirajan S, Dennis MY, Baker C, et al. Refinement and discovery of new hotspots of copy-number variation associated with autism spectrum disorder. American journal of human genetics. 2013;92(2):221–237. doi: 10.1016/j.ajhg.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: comprehensive meta-analysis. The British Journal of Psychiatry. 2009;195(1):7–14. doi: 10.1192/bjp.bp.108.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atladóttir HÓ, Henriksen TB, Schendel DE, et al. Autism After Infection, Febrile Episodes, and Antibiotic Use During Pregnancy: An Exploratory Study. Pediatrics. 2012 doi: 10.1542/peds.2012-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zerbo O, Iosif AM, Walker C, et al. Is Maternal Influenza or Fever During Pregnancy Associated with Autism or Developmental Delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) Study. Journal of autism and developmental disorders. 2013;43(1):25–33. doi: 10.1007/s10803-012-1540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith SE, Li J, Garbett K, et al. Maternal immune activation alters fetal brain development through interleukin-6. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(40):10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malkova NV, Yu CZ, Hsiao EY, et al. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain, Behavior, and Immunity. 2012;26(4):607–616. doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartzer JJ, Careaga M, Onore CE, et al. Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Transl Psychiatry. 2013;3:e240. doi: 10.1038/tp.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lord C, Risi S, Lambrecht L, et al. The Autism Diagnostic Observation Schedule—Generic: A Standard Measure of Social and Communication Deficits Associated with the Spectrum of Autism. Journal of autism and developmental disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 13.Lord C, Rutter M, Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of autism and developmental disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 14.American Psychiatric Association. Diagnostic criteria from DSM-IV-TR. xii. Washington, D.C.: American Psychiatric Association; 2000. p. 370. [Google Scholar]
- 15.Fischbach GD, Lord C. The Simons Simplex Collection: A Resource for Identification of Autism Genetic Risk Factors. Neuron. 2010;68(2):192–195. doi: 10.1016/j.neuron.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Lam KL, Aman M. The Repetitive Behavior Scale-Revised: Independent Validation in Individuals with Autism Spectrum Disorders. Journal of autism and developmental disorders. 2007;37(5):855–866. doi: 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- 17.Mirenda P, Smith I, Vaillancourt T, et al. Validating the Repetitive Behavior Scale-Revised in Young Children with Autism Spectrum Disorder. Journal of autism and developmental disorders. 2010;40(12):1521–1530. doi: 10.1007/s10803-010-1012-0. [DOI] [PubMed] [Google Scholar]
- 18.Constantino J, Davis S, Todd R, et al. Validation of a Brief Quantitative Measure of Autistic Traits: Comparison of the Social Responsiveness Scale with the Autism Diagnostic Interview-Revised. Journal of autism and developmental disorders. 2003;33(4):427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- 19.Elliott CD. Differential Ability Scales-ll. San Antonio, TX: Pearson; 2007. [Google Scholar]
- 20.Wechsler D. Wechsler Intelligence Scale for Children. 1949 [Google Scholar]
- 21.Mullen E. Mullen scales of early learning. Circle Pines, MN: American Guidance Service; 1995. Inc(21) [Google Scholar]
- 22.Sparrow SS, Cicchetti DV. Major psychological assessment instruments, Vol 2. Needham Heights, MA, US: Allyn & Bacon; 1989. The Vineland Adaptive Behavior Scales; pp. 199–231. [Google Scholar]
- 23.Lee BK, Magnusson C, Gardner RM, et al. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.09.001. published online ahead of print September 16 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsiao EY, McBride SW, Chow J, et al. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proceedings of the National Academy of Sciences. 2012;109(31):12776–12781. doi: 10.1073/pnas.1202556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain, Behavior, and Immunity. 2012;26(3):383–392. doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller VM, Zhu Y, Bucher C, et al. Gestational flu exposure induces changes in neurochemicals, affiliative hormones and brainstem inflammation, in addition to autism-like behaviors in mice. Brain, Behavior, and Immunity. 2013 doi: 10.1016/j.bbi.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen DQ, Webber C, Ponting CP. Bias of selection on human copy-number variants. PLoS genetics. 2006;2(2):e20. doi: 10.1371/journal.pgen.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saxena V, Ramdas S, Ochoa CR, et al. Structural, genetic, and functional signatures of disordered neuro-immunological development in autism spectrum disorder. PloS one. 2012;7(12):e48835. doi: 10.1371/journal.pone.0048835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voineagu I, Wang X, Johnston P, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474(7351):380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elmer BM, Estes ML, Barrow SL, et al. MHCI Requires MEF2 Transcription Factors to Negatively Regulate Synapse Density during Development and in Disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(34):13791–13804. doi: 10.1523/JNEUROSCI.2366-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.