Abstract
Light offers unique advantages for studying and manipulating biomolecules and the cellular processes that they control. Optical control of ionotropic and metabotropic glutamate receptors has garnered significant interest, since these receptors are central to signaling at neuronal synapses and only optical approaches provide the spatial and temporal resolution required to directly probe receptor function in cells and tissue. Following the classical method of glutamate photo-uncaging, recently developed methods have added other forms of remote control, including those with high molecular specificity and genetic targeting. These tools open the door to the direct optical control of synaptic transmission and plasticity, as well as the probing of native receptor function in intact neural circuits.
Introduction
Glutamate serves as the major excitatory neurotransmitter in the central nervous system. Glutamate receptors (GluRs) mediate synaptic transmission, regulate synaptic homeostasis and confer plasticity onto synapses. Glutamate is sensed by two types of receptors: Ionotropic glutamate receptors (iGluRs), which are ligand-gated ion channels, and metabotropic glutamate receptors (mGluRs), which are G protein-coupled receptors [1,2]. Decades of research have yielded a rich repertoire of pharmacological tools to target GluRs, including agonists, antagonists, allosteric modulators and blockers. Indeed, the specificity profile of AMPA, kainate and NMDA was used to define the three main iGluR subfamilies before the receptors were cloned. The development of specific pharmacology is spurred by structural information, which first became available for isolated ligand bindings domains (LBDs) and has more recently expanded to structures of full-length and transmembrane domains of iGluRs and mGluRs, respectively. GluRs are established and sought after drug targets for clinical treatments, since they are involved in numerous pathological conditions ranging from schizophrenia to addiction.
The molecular and functional diversity of GluRs (Figure 1a) and their distribution to multiple locations in synapses poses major challenges for understanding how individual GluRs contribute to neuronal signaling – a problem, which cannot be addressed by classical pharmacological approaches alone [1,2]. Pharmacological agents with high specificity are only available for some receptor subtypes. This may bias research towards those addressable GluRs and hamper the interpretation of experiments with partially-specific compounds. Alternative splicing, RNA editing, the formation of heteromeric complexes and context-dependent coupling to different downstream signaling pathways increases their functional diversity further. Aside from their molecular properties, GluRs play roles at different cellular locations (Figure 1b). They are not restricted to the postsynaptic density, but take distinct roles in the presynaptic zone, at extrasynaptic locations, and in surrounding glial cells. Importantly, the same receptor subtype may be found in multiple locations within the same synapse, as well as at nearby inhibitory and excitatory synapses. Moreover, GluRs are ubiquitous throughout the nervous system, which can confound circuit analysis and limits the use of drugs for dissecting specific roles of receptors in certain brain regions.
Figure 1. Functional diversity of ionotropic and metabotropic glutamate receptors (iGluRs and mGluRs).
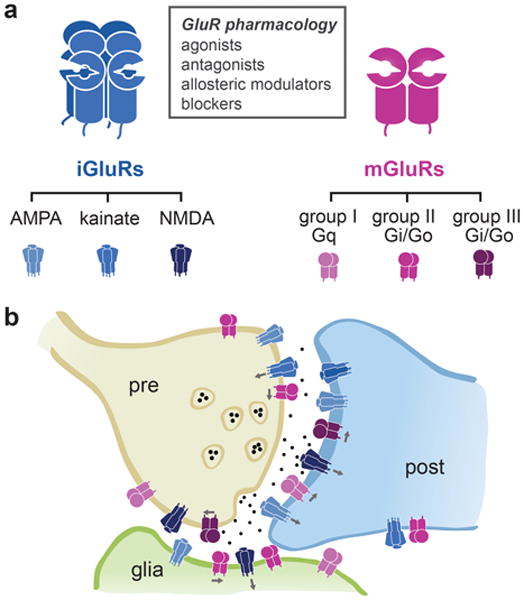
(a) GluRs and pharmacology: iGluRs are tetrameric ion channels (left) and mGluRs are dimeric G protein-coupled receptors (right). Heteromerization within different subfamilies increases the functional diversity of iGluRs and mGluRs further. (b) iGluRs and mGluRs occur pre- and postsynaptically, where they play multiple roles in signal transmission, the control of neurotransmitter release and regulation of synaptic strength. They are also found in extrasynaptic locations and glial cells, highlighting their regulatory functions.
Optical methods for control of GluRs
In recent years both genetic and optical approaches have become major tools for studying GluRs as a complement to purely pharmacological approaches. Genetic approaches offer high molecular and cellular specificity by enabling expression, alteration, or knock-out of receptor subunits in defined subsets of neurons and have proven very useful for probing the function of specific GluRs. However, genetic manipulations have inherent limitations due to their irreversibility, slow mode of action, and compensatory mechanisms. Optical techniques, on the other hand, provide spatial and temporal resolution, which is necessary to interrogate neuronal signaling. Signal transmission primarily takes place on the level of individual synapses, that is in dendritic spines and presynaptic terminals on the order of ∼500 nm in size, and on the timescale of a few to hundreds of milliseconds. These local and brief signaling events feed into regulatory mechanisms (timescale of seconds), which, in turn, can trigger long-lasting changes in synaptic strength and composition. The power of optical approaches for studying synaptic signaling has been demonstrated by numerous imaging applications, which include the localization of synaptic proteins with high resolution, the tracing of circuits in vivo and the optical measurement of membrane potential, second messengers, and neurotransmitters.
Starting with photo-uncaging of second messengers and neurotransmitters, light has been utilized for direct manipulation of signaling with high temporal and spatial resolution. Here we give a brief overview of the main approaches with a focus on recent developments to control both iGluRs and mGluRs with light. We then turn to representative applications and the enormous potential that can emerge from combining opto-pharmacological approaches with the specificity of genetic approaches.
Photo-uncaging of pharmacological agents
The need for optical techniques to study neurotransmitter-mediated signaling is highlighted by the wide use of ‘caged’ compounds, i.e. compounds that are masked with photo-labile protecting groups that can be ‘uncaged’ with pulses of light (Figure 2a). Photo-uncaging of glutamate derivatives has become a popular choice to release glutamate locally and rapidly [3], and excellent reviews addressing the chemical and technical aspects of uncaging exist [4,5]. Photo-uncaging of methoxynitroindolinyl glutamate (MNI-Glu) has been broadly applied for neuronal excitation, functional mapping and studying glutamate-mediated signaling in dendritic spines and branches, including 2-photon-mediated and in vivo uncaging [6,7]. Off-target effects of classical caged-glutamate derivatives on GABAergic transmission were addressed with RuBi-Glu, a photo-sensitive ruthenium-organic complex [8,9]. In recent years, the palette of protecting groups has been further extended, e.g. by introducing DEAC450, which enables 2-photon uncaging at 900 nm to achieve orthogonal 2-photon uncaging of different neurotransmitters within the same experiment [10]. Uncaged glutamate acts on all nearby receptors and thus does not offer specificity to which GluRs are activated. This issue can be overcome by caging of subunit-specific iGluR agonists, such as MNI-NMDA, MNI-kainate or NPEC-AMPA, or antagonists and blockers, as recently demonstrated with a caged-version of MK801 (dizocilpine), DMNB-MK801, a use-dependent blocker of NMDA receptors [11]. Recently, mGluR specific caged agonists, such as NPEC-ACPD and NPEC-DHPG, have also become available [12]. However, the specificity of caged compounds is only as good as the specificity of the pharmacological agents they are based on but they may also exhibit additional off-target effects.
Figure 2. Approaches to optically control glutamate receptor signaling.
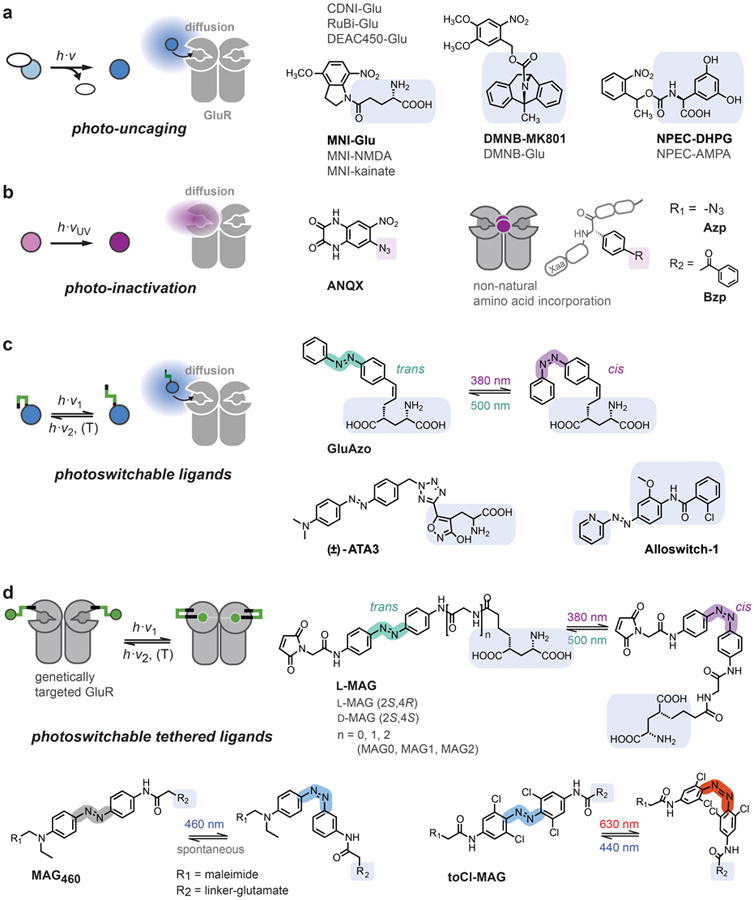
(a) Photo-uncaging of ligands that are masked with photo-labile protecting groups. (b) Photo-inactivation of receptors with photoreactive ligands (ANQX) or after incorporation of photo-reactive amino acids (Azp, Bzp). (c) Photoswitchable ligands that can be reversibly switched between high and low affinity states. Azobenzene derivatives, which can be photoisomerized between trans and cis configurations, commonly serve as photoswitches. GluAzo is a photoswitchable glutamate derivative, ATA3 an AMPAR agonist and Alloswitch-1 a mGluR5 negative allosteric modulator (NAM). (d) Photoswitchable tethered ligands (PTLs) are covalently attached to genetically engineered receptor subunits. For GluRs, MAG ligands are used, which encompass a maleimide group for covalent attachment to cysteine residues, a central azobenzene photoswitch and a 4-alkyl glutamate ligand. Details are described in the text.
Photo-uncaging can be readily implemented and acts on endogenously expressed receptor populations, but it does not allow for targeting of specific cells. This becomes a major limitation, for instance, when one seeks to delineate the contributions of pre- and postsynaptic GluRs to changes in synaptic plasticity. Another issue is that the spatio-temporal profile of glutamate release cannot be defined precisely because uncaging depends on the available concentration, the optical volume, the amount of uncaging, as well as on diffusion, which are parameters that are hard to control in biological preparations.
Photo-inactivation of endogenous or genetically targeted GluRs
In addition to its ability to uncage compounds that activate receptors, light may also be used to activate compounds that inactivate GluRs (Figure 2b). An early example of photo-inactivation is the use of ANQX, a photo-reactive ligand for AMPA receptors, which becomes reactive upon illumination with UV light [13]. A recent, conceptually interesting development is the incorporation of photo-reactive groups into the receptor itself, using non-natural amino acids with a photo-reactive side chain [14-16]. Using a stop codon suppression system, the photo-reactive amino acid is introduced at a specific position, where activation with light produces a reactive species that irreversibly alters or impedes receptor function. This approach has been used to incorporate the aromatic amino acids p-azidophenylalanine (Azp) and p-benzoylphenylalanine (Bzp) into both NMDA and AMPA receptors, allowing for their inactivation by light [14-16]. The general advantage of this approach is that it provides molecular selectivity and an acute ‘knock-out’ of receptor function, but a shortcoming is that the effect is irreversible. A significant portion of the early development for Azp and Bzp incorporation was performed in GPCRs [17], which should make the technique adaptable to mGluRs, as well. Expression of photo-inactivatable GluRs in vivo is conceivable, since non-natural amino acid incorporation has been demonstrated after in utero electroporation of DNA encoding the tRNA/synthetase and a ventricular injection of the non-natural amino acid [18]. However, native receptor expression levels may be hard to achieve. Moreover, the currently used amino acids require seconds to minutes of illumination with high intensity UV light, which likely limits their use for studying biological systems, although they are powerful tools for biophysical studies [14,16].
Soluble photoswitches (reversibly ‘caged’ ligands)
Photo-uncaging of ligands and photo-inactivation of receptors are irreversible processes. Photoswitching, in contrast, refers to the reversible manipulation of receptors using photoswitchable compounds. This can be achieved by functionalizing a conventional ligand with a photoswitchable group that changes its conformation upon illumination and thereby alters the binding affinity to its target receptor, giving rise to binding-competent and binding-incompetent conformations (Figure 2c). Azobenzenes are well suited as photoswitchable groups, since they can be photoisomerized between an extended trans configuration and a more compact cis configuration, that is some wavelengths of light favor the cis configuration and others favor the relaxed trans configuration [19,20]. Azobenzenes have excellent photostability, their photoisomerization is fast and reversible, and their photophysical properties can be tuned by chemical modification. An early application was GluAzo, a photoswitchable glutamate derivative that can be reversibly switched between a low affinity cis configuration and a high affinity trans configuration using near UV and blue light, respectively [21]. A recently obtained structure of trans GluAzo bound to GluK2 might serve as a guide for improving its efficacy as a trans-agonist [22]. The soluble photoswitch approach has also been applied to a series of AMPA receptor specific agonists, called ATAs, based on a benzyltetrazolyl-substituted AMPA derivative [23]. Most recently, Pittolo et al. described Alloswitch-1, a compound based on the mGluR4 positive allosteric modulator (PAM) VUO415374, which functions as a photoswitchable mGluR5-specific negative allosteric modulator (NAM) [24]. GluAzo, ATAs and Alloswitch-1 are functionally active in the relaxed trans state, i.e. light is required to keep them in the inactive cis state, which may hamper their applicability for studies in vivo. One should also keep in mind that the specificity and efficacy of soluble photoswitches has to be characterized for all potential targets.
Similar to photo-uncaging, the binding of soluble photoswitches is governed by diffusion, which poses limits to the spatial and temporal resolution. Furthermore, the difference in affinity of the two photoswitch conformations and their purity at any given wavelength might be relatively modest. The concentration at which the photoswitch is applied, is therefore key for its selectivity, efficacy and mode of action. Nevertheless, ‘opto-pharmacological’ approaches, which allow for targeting of native receptors, stir broad interest and are being applied to a rapidly expanding number of biologically active compounds [25,26].
Photoswitchable Tethered Ligands
In addition to conferring photosensitivity to soluble ligands, chemical photoswitches may also be used to directly control receptor function via site-specific attachment (Figure 2d). A versatile approach to control GluRs is based on the MAG (maleimide-azobenzene-glutamate) family of photoswitchable tethered ligands (PTLs): The maleimide group allows for covalent coupling of MAG to the receptor of interest via the introduction of a cysteine residue close to the binding pocket. The azobenzene group serves as a robust cis/trans photoswitch, which prevents the glutamate head-group from reaching the binding pocket in one configuration, but allows for proper binding and receptor activation in the other configuration. This approach was successfully used to engineer light-gated iGluRs, named LiGluRs [27,28], and mGluRs, named LimGluRs [29] (Figure 3). The ideal position for MAG attachment has to be determined by screening cysteine substitutions around the binding pocket [29,30]. iGluRs, such as the kainate receptor GluK2 (a.k.a. iGluR6), can be effectively controlled with MAG ligands with L-stereochemistry resembling the high efficacy agonist (2S,4R)-4-methylglutamate, SYM 2081 (“LiGluR”) [27,28,30-32] (Figure 3a,b), whereas photo-agonism of mGluR2 (“LimGluR2”) and mGluR3 (“LimGluR3”) required attachment with D-stereochemistry [29] (Figure 3c). Variation of linker lengths (MAG0, MAG1, MAG2, see Figure 2d for details) can be used to tune the efficacy of the trans and cis configuration [30]. Moreover, MAG ligands can be utilized as photo-antagonists (Figure 3d), if their photoswitched conformation at a given labeling position does not lead to activation but impedes glutamate binding and/or receptor activation. This offers an acute, specific and reversible block of receptor function, as seen with LimGluR2-block [29].
Figure 3. Optical control of LiGluR and LimGluRs with photoswitchable tethered ligands.
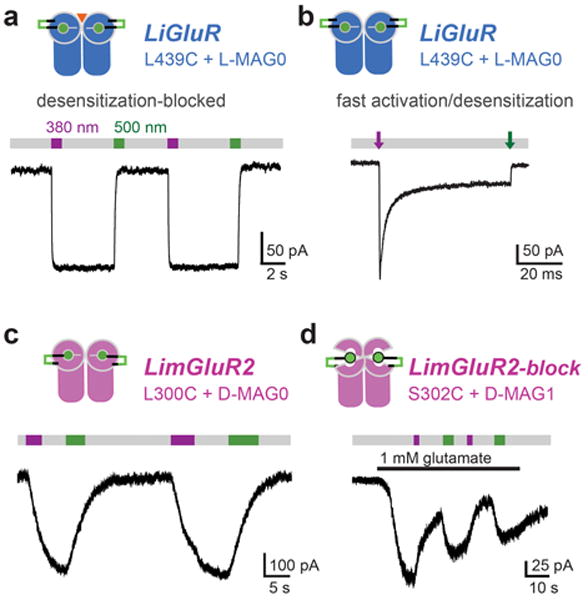
(a,b) LiGluR photoswitching after labeling of GluK2(L439C) with L-MAG0. Illumination with ∼380 nm light (violet bar) isomerizes MAG to the cis configuration, which results in receptor activation as demonstrated in HEK cell voltage-clamp recordings. Illumination with ∼500 nm light (green bar) reverses MAG to its trans configuration and causes receptor deactivation. (a) Reversible LiGluR photoswitching recorded in the presence of concanavalin A to suppresses desensitization. Regular MAG is ‘bistable’ since it stays in the cis state for minutes after the light is turned off (grey bar). (b) Fast photoswitching (100 μs light pulse with high intensity) leads to receptor activation in less than a millisecond and concomitant desensitization (τdesen = 4 ms), which resembles receptor gating induced by brief pulses of glutamate (from Reiner et al. [32]). (c) LimGluR2 photo-agonism obtained with D-MAG0 labeling at L300C. Gβγ-mediated activation of coexpressed GIRK1 channels was used as a read-out of mGluR2 activation (for details see Levitz et al. [29]). (d) LimGluR2 photo-antagonism obtained with D-MAG0 labeling at S302C. At this position cis-MAG suppresses glutamate-induced activation of mGluR2 (from [29]).
PTL-based approaches provide a high degree of modularity, since receptor and ligand can be changed in a rational and largely independent way. The cysteine subsitution for photoswitch attachment allows targeting of specific receptor subtypes (e.g. mGluR2 vs mGluR3). Additionally, the mutation for installment of the photoswitch can be combined with other mutations, e.g. to change the affinity of the light-gated receptor to endogenous glutamate or the ion selectivity. An extreme example is the construction of HyLighter, a chimeric iGluR that incorporates a K+-selective pore domain to permit light-induced hyperpolarization [33].
Modifications can also be made to the MAG photoswitch. The first generation regular MAG is isomerized to cis with ∼380 nm and back to trans with ∼500 nm light (Figure 3a). Recently, we described MAG460 with a push-pull azobenzene core, which shifts the activation spectrum by ∼100 nm and allows for efficient cis-activation with 460 nm blue light (Figure 2d) [34]. In contrast to regular MAG, which is bistable due to its slow cis-to-trans relaxation over tens of minutes, MAG460 relaxes within a second, which means it can be operated as single wavelength photoswitch. An important consequence is that MAG460 can be used to detect different light intensities, as well as dynamic changes in light intensity. Other MAG versions, namely MAG2P and MAGA2P, were specifically developed for 2-photon photoswitching [35]. Recently, we described toCl-MAG, a tetra-ortho-chloro-substituted azobenzene that can be used for receptor activation with 630 nm light [36]. A significant advantage of the PTL approach is that it relies on a genetically modified receptor, which provides highly specific control over individual receptor subunits, as well as genetic targeting to specific cells.
Optical control of glutamate receptors with temporal resolution and genetic specificity: from molecules to systems
Optical techniques afford a high temporal precision. PTLs allow particularly precise control, since the photoisomerization of azobenzene is a picosecond process and binding is not limited by diffusion. For GluK2, in combination with a MAG0 photoswitch, we recently achieved maximal activation with light pulses as short as 10 μs, which resulted in channel opening in less than millisecond (Figure 3b) [32]. This photoactivation of GluK2 was followed by fast desensitization, similar to gating elicited by brief pulses of glutamate. Furthermore, by utilizing the fact that ligand binding is strictly limited to subunits carrying the photoswitch, we were able to show that the amount of desensitization depends on the subunit occupancy of the receptors and that both GluK2 and GluK5 subunits have to be occupied to cause receptor desensitization. Beyond biophysical applications, fast photoswitching makes it possible to precisely mimic synaptic activation profiles.
In recent years, optical manipulation has also been used to probe the role of GluRs at the level of the synapse (Figure 4a). One major advantage here is that the modified receptor is genetically-encoded, which allows for targeting of individual cells in order to distinguish pre- and postsynaptic effects. For instance, LimGluR2 has been used to presynaptically control release probability and short-term plasticity in hippocampal neurons (Figure 4b) [29]. Another example is the exclusive activation of postsynaptic receptors, which allowed Kauwe et al. to discover a novel plasticity mechanism that is induced post-synaptically and expressed pre-synaptically via a retrograde signal at the fly neuromuscular junction (Figure 4d) [37]. LiGluR has also been used to control Ca2+-influx into astrocytes to trigger gliotransmission (Figure 4c) [38], or to trigger exocytosis from neuroendocrine cells [39]. In addition, spatially-targeted 2-photon uncaging of glutamate has been applied at synapses in vivo in mice demonstrating the applicability of chemical based approaches in the mammalian brain (Figure 4e) [7]. Combining photoswitchable tethered ligands with genetic tools should allow for the utilization of the full potential of optical approaches for advanced experimentation at synapses in vivo.
Figure 4. Optical control of GluRs for studying synapses and circuits.
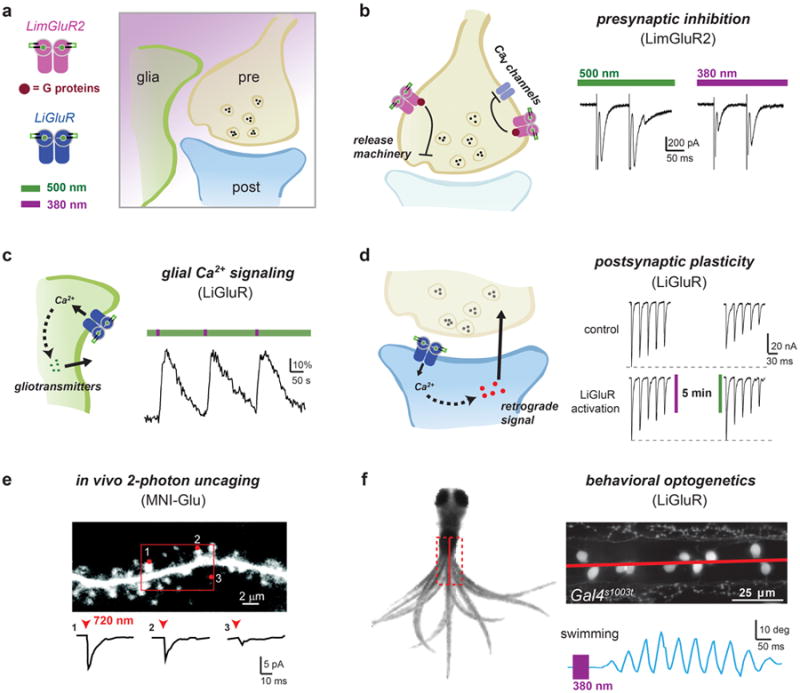
(a) The expression of photoswitchable glutamate receptors can be targeted to the presynaptic, postsynaptic, or glial compartments for studying their role in synaptic signaling. (b) Expression and MAG-labeling of LimGluR2 in autaptic hippocampal neurons allows for light-induced presynaptic inhibition and manipulation of short-term plasticity. LimGluR2 activation (380 nm, violet bar) increases the paired pulse ratio (pulse 2/pulse 1) of evoked EPSCs confirming the presynaptic origin of the effect (from Levitz et al. [29]). (c) Repeated photoactivation of LiGluR in cultured cortical astrocytes produces large, reliable Ca2+–transients that can initiate gliotransmitter release (from Li et al. [38]). (d) Postsynaptic activation of LiGluR expressed in the muscle of the drosophila neuromuscular junction showed that postsynaptic iGluRs can enhance recovery from synaptic depression pointing to a retrograde signaling mechanism (from Kauwe et al. [37]). (e) In vivo 2-photon uncaging of MNI-glutamate at single dendritic spines in mouse cortex demonstrates the spatial and temporal precision afforded by optical activation of glutamate receptors (from Noguchi et al. [7]). (f) Targeted expression of LiGluR (UAS:LiGluR) to the Gal4s1003t in larval zebrafish allowed for the optical induction of a swim response and subsequent identification of key neurons involved in the swim circuit (from Wyart et al. [42]).
Apart from detailed studies in the synaptic context, optical manipulation of GluRs may be used for the general control of neuronal activity. For example, LiGluR can be used to depolarize cells to robustly control firing on both short [40] and long timescales [41]. An optogenetic approach, combining photocontrol with genetic control, offers many experimental possibilities to study signaling on a systems-wide level and in behavioral contexts. Light-gated GluRs can serve as optogenetic tools to identify neuronal subpopulations and to dissect their contributions to circuit function. For instance, targeted expression of LiGluR in transgenic Gal4 driver-lines helped to identify specific neurons in the spinal cord that control swimming of zebrafish larvae (Figure 4f) [42]. Another example is the viral expression of LiGluR in retinal ganglion cells followed by MAG treatment, which restores basic light sensitivity to mice that have lost their vision due to mutations causing photoreceptor cell degeneration in mouse and human [43].
While suitable for general control of neuronal activity or G protein signaling, the key difference of the PTL approach to opsin-based techniques is the ability to probe specific receptors to gain a molecular foothold on systems or behavioral processes. Ultimately, by relying on knock-in animals or targeted genome-editing to introduce photoswitch attachment sites, it will be possible to target specific GluRs, while maintaining their native expression profiles. Alternatively, this can be achieved by expression of subunits that require coassembly with a second, endogenous subunit, as was recently demonstrated for the K2P-channel TREK1 [44]. In addition, antagonistic photoswitches can provide an easily identifiable signature for specific receptors or serve as an alternative to receptor knock-outs.
Conclusions
We compared four approaches that are currently pursued to utilize the unique advantages of light for controlling GluRs in different experimental schemes. Next to caged compounds, tethered photoswitches are particularly promising tools, since they may provide control of unprecedented precision and allow for combination with genetic targeting. Consequently, photoswitchable tethered ligands and modulators have also been developed for other membrane protein classes including, most notably, potassium channels [45,46], nicotinic acetylcholine receptors [19,47], GABAA receptors [48], as well as P2X receptors [49,50].
Both, uncaging and photoswitch applications can be further refined by utilizing the available pharmacological repertoires and by tuning the photophysical properties of photo-photoswitches. In addition, alternative bioconjugation techniques for tethering of photoswitchable ligands might be explored to yield higher specificity or to allow for orthogonal labeling of different receptor subtypes within the same preparation. Also naturally occurring photoreceptor domains, such as LOV and BLUF-domains might provide means to engineer light-sensitive receptors. As these domains rely on endogenously available light-sensitive cofactors (e.g. flavin), they could allow to optically control certain receptor functions in a fully genetically encoded manner. These powerful and emerging optically approaches will continue to enrich our pharmacological toolsets, which in combination with rapidly developing genetic techniques, will enable new, sophisticated applications aiming to gain a complete picture of specific GluR functions at the molecular, synaptic, and systems levels. Eventually this work could help guide therapeutic intervention and define pharmacological targets more precisely.
Highlights.
tools for manipulating GluRs are needed to decipher signaling at excitatory synapses
optical approaches are non-invasive and provide high spatio-temporal resolution
photo-pharmacology can be used to address native GluRs
genetic-encoding allows for targeting of synaptic compartments and cell types
photoswitchable tethered ligands offer ultimate precision and specificity
Acknowledgments
We thank all members of the Isacoff lab and our collaboration partners who contributed to the development and refinement of soluble and tethered photoswitches. Work in our lab was supported by the National Institutes of Health Nanomedicine Development Center for the Optical Control of Biological Function (2PN2EY018241).
Footnotes
Conflict of Interest: The authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: Structure, regulation, and function. Pharm Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niswender CM, Conn PJ. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Ann Rev Pharm Tox. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callaway EM, Katz LC. Photostimulation using caged glutamate reveals functional circuitry in living brain slices. Proc Natl Acad Sci U S A. 1993;90:7661–7665. doi: 10.1073/pnas.90.16.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callaway EM, Yuste R. Stimulating neurons with light. Curr Opi Neurobiol. 2002;12:587–592. doi: 10.1016/s0959-4388(02)00364-1. [DOI] [PubMed] [Google Scholar]
- 5.Ellis-Davies GC. Caged compounds: Photorelease technology for control of cellular chemistry and physiology. Nat Meth. 2007;4:619–628. doi: 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *6.Nikolenko V, Poskanzer KE, Yuste R. Two-photon photostimulation and imaging of neural circuits. Nat Meth. 2007;4:943–950. doi: 10.1038/nmeth1105. Important examples of co-uncaging of glutamate and GABA with different wavelengths. [DOI] [PubMed] [Google Scholar]
- *7.Noguchi J, Nagaoka A, Watanabe S, Ellis-Davies GC, Kitamura K, Kano M, Matsuzaki M, Kasai H. In vivo two-photon uncaging of glutamate revealing the structure-function relationships of dendritic spines in the neocortex of adult mice. J Physiol. 2011;589:2447–2457. doi: 10.1113/jphysiol.2011.207100. In vivo photo-uncaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *8.Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron. 2011;69:1188–1203. doi: 10.1016/j.neuron.2011.02.025. The development of RuBi-glutamate and 2-photon uncaging in brain slices enabled the authors to map how dense inhibitory interneurons connect to pyramidal cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fino E, Araya R, Peterka DS, Salierno M, Etchenique R, Yuste R. RuBi-Glutamate: Two-photon and visible-light photoactivation of neurons and dendritic spines. Front Neur Circuits. 2009;3:2. doi: 10.3389/neuro.04.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *10.Olson JP, Kwon HB, Takasaki KT, Chiu CQ, Higley MJ, Sabatini BL, Ellis-Davies GC. Optically selective two-photon uncaging of glutamate at 900 nm. J Am Chem Soc. 2013;135:5954–5957. doi: 10.1021/ja4019379. This paper describes DEAC450, a diethylaminocoumarin derivative that allows for highly efficient glutamate uncaging with 450 nm (1-p) and 900 nm (2-p) light, and may enable orthogonal 2-photon manipulations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodríguez-Moreno A, Kohl MM, Reeve JE, Eaton TR, Collins HA, Anderson HL, Paulsen O. Presynaptic induction and expression of timing-dependent long-term depression demonstrated by compartment-specific photorelease of a use-dependent NMDA receptor antagonist. J Neurosci. 2011;31:8564–8569. doi: 10.1523/JNEUROSCI.0274-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *12.Palma-Cerda F, Auger C, Crawford DJ, Hodgson AC, Reynolds SJ, Cowell JK, Swift KA, Cais O, Vyklicky L, Corrie JE, Ogden D. New caged neurotransmitter analogs selective for glutamate receptor sub-types based on methoxynitroindoline and nitrophenylethoxycarbonyl caging groups. Neuropharm. 2012;63:624–634. doi: 10.1016/j.neuropharm.2012.05.010. This study describes several new caged iGluR agonists, as well as NPEC-ACPD, a non-selective mGluR agonist, and NPEC-DHPG, a group I selective mGluR agonist. [DOI] [PubMed] [Google Scholar]
- 13.Adesnik H, Nicoll RA, England PM. Photoinactivation of native AMPA receptors reveals their real-time trafficking. Neuron. 2005;48:977–985. doi: 10.1016/j.neuron.2005.11.030. [DOI] [PubMed] [Google Scholar]
- **14.Klippenstein V, Ghisi V, Wietstruk M, Plested AJ. Photoinactivation of glutamate receptors by genetically encoded unnatural amino acids. J Neurosci. 2014;34:980–991. doi: 10.1523/JNEUROSCI.3725-13.2014. This work describes incorporation of Bzp into different ligand-binding domain positions of GluA2, which resulted in UV-induced, state-dependent photo-inactivation of GluA2 homomers and GluA1/GluA2 heteromers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye S, Riou M, Carvalho S, Paoletti P. Expanding the genetic code in Xenopus laevis oocytes. Chembiochem. 2013;14:230–235. doi: 10.1002/cbic.201200515. [DOI] [PubMed] [Google Scholar]
- **16.Zhu S, Riou M, Yao CA, Carvalho S, Rodriguez PC, Bensaude O, Paoletti P, Ye S. Genetically encoding a light switch in an ionotropic glutamate receptor reveals subunit-specific interfaces. Proc Natl Acad Sci U S A. 2014;111:6081–6086. doi: 10.1073/pnas.1318808111. This paper describes the incorporation of Azp into the N-terminal domain of GluN1. This resulted in partial UV-induced inactivation of GluN1/GluN2B, but not GluN1/GluN2A heteromers, highlighting the functional importance of the GluN1/GluN2 NTD interface. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daggett KA, Sakmar TP. Site-specific in vitro and in vivo incorporation of molecular probes to study G-protein-coupled receptors. Curr Opi Chem Biol. 2011;15:392–398. doi: 10.1016/j.cbpa.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Kang JY, Kawaguchi D, Coin I, Xiang Z, O'Leary DD, Slesinger PA, Wang L. In vivo expression of a light-activatable potassium channel using unnatural amino acids. Neuron. 2013;80:358–370. doi: 10.1016/j.neuron.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lester HA, Krouse ME, Nass MM, Wassermann NH, Erlanger BF. A covalently bound photoisomerizable agonist: comparison with reversibly bound agonists at Electrophorus electroplaques. J Gen Physiol. 1980;75:207–232. doi: 10.1085/jgp.75.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beharry AA, Woolley GA. Azobenzene photoswitches for biomolecules. Chem Soc Rev. 2011;40:4422–4437. doi: 10.1039/c1cs15023e. [DOI] [PubMed] [Google Scholar]
- 21.Volgraf M, Gorostiza P, Szobota S, Helix MR, Isacoff EY, Trauner D. Reversibly caged glutamate: a photochromic agonist of ionotropic glutamate receptors. J Am Chem Soc. 2007;129:260–261. doi: 10.1021/ja067269o. [DOI] [PubMed] [Google Scholar]
- 22.Reiter A, Skerra A, Trauner D, Schiefner A. A photoswitchable neurotransmitter analogue bound to its receptor. Biochemistry. 2013;52:8972–8974. doi: 10.1021/bi4014402. [DOI] [PubMed] [Google Scholar]
- 23.Stawski P, Sumser M, Trauner D. A photochromic agonist of AMPA receptors. Angew Chem. 2012;51:5748–5751. doi: 10.1002/anie.201109265. [DOI] [PubMed] [Google Scholar]
- *24.Pittolo S, Gómez-Santacana X, Eckelt K, Rovira X, Dalton J, Goudet C, Pin JP, Llobet A, Giraldo J, Llebaria A, Gorostiza P. An allosteric modulator to control endogenous G protein-coupled receptors with light. Nat Chem Biol. 2014;10:813–815. doi: 10.1038/nchembio.1612. This paper describes Alloswitch-1, a soluble photoswitchable negative allosteric modulator (NAM) with specificity for mGluR5. [DOI] [PubMed] [Google Scholar]
- 25.Fehrentz T, Schönberger M, Trauner D. Optochemical genetics. Angew Chem. 2011;50:12156–12182. doi: 10.1002/anie.201103236. [DOI] [PubMed] [Google Scholar]
- 26.Kramer RH, Mourot A, Adesnik H. Optogenetic pharmacology for control of native neuronal signaling proteins. Nat Neurosci. 2013;16:816–823. doi: 10.1038/nn.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volgraf M, Gorostiza P, Numano R, Kramer RH, Isacoff EY, Trauner D. Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat Chem Biol. 2006;2:47–52. doi: 10.1038/nchembio756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *28.Gorostiza P, Volgraf M, Numano R, Szobota S, Trauner D, Isacoff EY. Mechanisms of photoswitch conjugation and light activation of an ionotropic glutamate receptor. Proc Natl Acad Sci U S A. 2007;104:10865–10870. doi: 10.1073/pnas.0701274104. Photophysics and mechanism attachment and function of the regular MAG photoswitch on LiGluR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **29.Levitz J, Pantoja C, Gaub B, Janovjak H, Reiner A, Hoagland A, Schoppik D, Kane B, Stawski P, Schier AF, Trauner D, et al. Optical control of metabotropic glutamate receptors. Nat Neurosci. 2013;16:507–516. doi: 10.1038/nn.3346. This work describes the development of light-gated mGluRs, including photo-agonistic version for mGluR2 and 3 and photo-antagonistic versions for mGluR2 and 6. LimGluR2 was used to reversibly inhibit synaptic transmitter release in hippocampal neurons and modulate the acoustic startle response in live zebrafish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Numano R, Szobota S, Lau AY, Gorostiza P, Volgraf M, Roux B, Trauner D, Isacoff EY. Nanosculpting reversed wavelength sensitivity into a photoswitchable iGluR. Proc Natl Acad Sci U S A. 2009;106:6814–6819. doi: 10.1073/pnas.0811899106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiner A, Isacoff EY. Photoswitching of cell surface receptors using tethered ligands. Methods Mol Biol. 2014;1148:45–68. doi: 10.1007/978-1-4939-0470-9_4. [DOI] [PubMed] [Google Scholar]
- **32.Reiner A, Isacoff EY. Tethered ligands reveal glutamate receptor desensitization depends on subunit occupancy. Nat Chem Biol. 2014;10:273–280. doi: 10.1038/nchembio.1458. This study describes sub-millisecond photoswitching of LiGluR, which allowed for the detailed analysis of the effect of subunit occupancy on desensitization of GluK2 homotetramers and GluK2/GluK5 heterotetramers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janovjak H, Szobota S, Wyart C, Trauner D, Isacoff EY. A light-gated, potassium-selective glutamate receptor for the optical inhibition of neuronal firing. Nat Neurosci. 2010;13:1027–1032. doi: 10.1038/nn.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *34.Kienzler MA, Reiner A, Trautman E, Yoo S, Trauner D, Isacoff EY. A red-shifted, fast-relaxing azobenzene photoswitch for visible light control of an ionotropic glutamate receptor. J Am Chem Soc. 2013;135:17683–17686. doi: 10.1021/ja408104w. This paper describes MAG460, a PTL that can be isomerized into the active cis state with visible blue light and spontaneously relaxes in the dark. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Izquierdo-Serra M, Gascón-Moya M, Hirtz JJ, Pittolo S, Poskanzer KE, Ferrer E, Alibés R, Busque F, Yuste R, Hernando J, Gorostiza P. Two-photon neuronal and astrocytic stimulation with azobenzene-based photoswitches. J Am Chem Soc. 2014;136:8693–8701. doi: 10.1021/ja5026326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rullo A, Reiner A, Reiter A, Trauner D, Isacoff E, Woolley A. Long wavelength optical control of glutamate receptor ion channels using a tetra-ortho-substituted azobenzene derivative. Chem Comm. 2014;50:14613–14615. doi: 10.1039/c4cc06612j. [DOI] [PubMed] [Google Scholar]
- *37.Kauwe G, Isacoff EY. Rapid feedback regulation of synaptic efficacy during high-frequency activity at the Drosophila larval neuromuscular junction. Proc Natl Acad Sci U S A. 2013;110:9142–9147. doi: 10.1073/pnas.1221314110. In this paper LiGluR was specifically expressed in muscle at the larval Drosophila neuromuscular junction. Photoactivation of postsynaptic receptors altered presynaptic depression during high frequency bursts, apparently due to a retrograde signal that enhances presynaptic cAMP-dependent synaptic vesicle recruitment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li D, Hérault K, Isacoff EY, Oheim M, Ropert N. Optogenetic activation of LiGluR-expressing astrocytes evokes anion channel-mediated glutamate release. J Physiol. 2012;590:855–873. doi: 10.1113/jphysiol.2011.219345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Izquierdo-Serra M, Trauner D, Llobet A, Gorostiza P. Optical control of calcium-regulated exocytosis. Biochim Biophys Acta. 2013;1830:2853–2860. doi: 10.1016/j.bbagen.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Szobota S, Gorostiza P, Del Bene F, Wyart C, Fortin DL, Kolstad KD, Tulyathan O, Volgraf M, Numano R, Aaron HL, Scott EK, et al. Remote control of neuronal activity with a light-gated glutamate receptor. Neuron. 2007;54:535–545. doi: 10.1016/j.neuron.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Hou Q, Gilbert J, Man HY. Homeostatic regulation of AMPA receptor trafficking and degradation by light-controlled single-synaptic activation. Neuron. 2011;72:806–818. doi: 10.1016/j.neuron.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wyart C, Del Bene F, Warp E, Scott EK, Trauner D, Baier H, Isacoff EY. Optogenetic dissection of a behavioural module in the vertebrate spinal cord. Nature. 2009;461:407–410. doi: 10.1038/nature08323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caporale N, Kolstad KD, Lee T, Tochitsky I, Dalkara D, Trauner D, Kramer R, Dan Y, Isacoff EY, Flannery JG. LiGluR restores visual responses in rodent models of inherited blindness. Mol Therapy. 2011;19:1212–1219. doi: 10.1038/mt.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandoz G, Levitz J, Kramer RH, Isacoff EY. Optical control of endogenous proteins with a photoswitchable conditional subunit reveals a role for TREK1 in GABA(B) signaling. Neuron. 2012;74:1005–1014. doi: 10.1016/j.neuron.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH. Light-activated ion channels for remote control of neuronal firing. Nat Neurosci. 2004;7:1381–1386. doi: 10.1038/nn1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandoz G, Levitz J. Optogenetic techniques for the study of native potassium channels. Front Mol Neurosci. 2013;6:6. doi: 10.3389/fnmol.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tochitsky I, Banghart MR, Mourot A, Yao JZ, Gaub B, Kramer RH, Trauner D. Optochemical control of genetically engineered neuronal nicotinic acetylcholine receptors. Nat Chem. 2012;4:105–111. doi: 10.1038/nchem.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin WC, Davenport CM, Mourot A, Vytla D, Smith CM, Medeiros KA, Chambers JJ, Kramer RH. Engineering a light-regulated GABAA receptor for optical control of neural inhibition. ACS Chem Biol. 2014;9:1414–1419. doi: 10.1021/cb500167u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lemoine D, Habermacher C, Martz A, Mery PF, Bouquier N, Diverchy F, Taly A, Rassendren F, Specht A, Grutter T. Optical control of an ion channel gate. Proc Natl Acad Sci U S A. 2013;110:20813–20818. doi: 10.1073/pnas.1318715110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Browne LE, Nunes JP, Sim JA, Chudasama V, Bragg L, Caddick S, North RA. Optical control of trimeric P2X receptors and acid-sensing ion channels. Proc Natl Acad Sci U S A. 2014;111:521–526. doi: 10.1073/pnas.1318582111. [DOI] [PMC free article] [PubMed] [Google Scholar]


