Abstract
Background
Cholinergic projection systems degeneration is associated with dopamine non-responsive features of Parkinson's disease (PD). Cholinergic deficits are variable in non-demented PD. Identification of cholinergic deficits in PD may help with selection of suitable patients for targeted cholinergic drug treatment in PD. The objective of this retrospective multivariate predictor analysis study was to identify clinical markers indicative of cholinergic deficits in PD patients, as assessed by acetylcholinesterase ([11C]PMP) positron emission tomography.
Methods
One hundred thirty-seven PD patients (34 female) participated; median modified Hoehn and Yahr score was 2.5 (range 1–4), average age of 65.6 ± 7.4 years, and average duration of motor disease symptoms of 6.0 ± 4.2 years. Subjects were dichotomized as “normocholinergic” or “hypocholinergic” based on a 5th percentile cutoff from normal for the basal forebrain-cortical and pedunculopontine nucleus-thalamic cholinergic projection systems. Previously identified clinical indices of cholinergic denervation were used for statistical prediction of cholinergic deficits. Logistic regression determined which risk factors predicted cholinergic deficits. Sensitivity, specificity, and accuracy were determined for the (combinations of) significant predictor variables.
Results
There were 49 (35.8%) hypocholinergic PD subjects. The combination of RBD symptoms and fall history showed highest diagnostic accuracy (81.1%) for predicting combined thalamic and cortical cholinergic deficits. A combined assessment of 8.5 meter walk time and lower score on the Montreal cognitive assessment scale provided diagnostic accuracy of 80.7 % for predicting isolated cortical cholinergic denervation.
Conclusion
Assessment of clinical indices of cholinergic denervation may be useful for identifying suitable subjects for trials of targeted cholinergic drug treatments in PD.
Keywords: Parkinson's disease, acetylcholine, acetylcholinesterase, PET, biomarkers
INTRODUCTION
Parkinson's disease (PD) is a multisystem neurodegenerative syndrome with significant heterogeneity of motor and non-motor features.1 Cholinergic projection systems degeneration is associated with specific motor and non-motor features of PD, independent of nigrostriatal dopaminergic denervation; it is associated with impaired cognition,2–7 falling,8–10 slower gait speed,11 REM sleep behavior disorder (RBD),12 and impaired olfaction.6, 13 In addition, older age, longer motor disease symptom duration, and male gender associate with cholinergic system degeneration in PD.7, 14
Cholinergic system degeneration is heterogeneous in PD, and may affect basal forebrain-neocortical or pedunculopontine nucleus-thalamic projections differentially.7 Heterogeneity of cholinergic system degeneration may explain clinical variation in PD. Optimal evaluation of cholinergic replacement therapy requires accurate identification of the subset of PD patients with cholinergic deficits. Clinical trials of cholinergic agents in PD would be facilitated greatly by inclusion of convenient clinical measures as markers of differential cholinergic system loss.
The purpose of this study was to identify convenient, cost-effective, and non-invasive clinical markers of cholinergic deficits in PD subjects. Our previous studies on markers of cholinergic denervation were mainly based on univariate analysis of a single clinical predictor variable. The objective of this retrospective study was to perform a multivariate predictor analysis to identify which combination(s) of clinical markers of cholinergic deficits best predict cholinergic denervation as assessed by acetylcholinesterase (AChE) positron emission tomography (PET).6, 7, 10–14
METHODS
Subjects
This retrospective cross-sectional multivariate predictor study included 137 PD patients (34 female) who are part of an ongoing cohort study (ClinicalTrials.gov Identifier NCT01565473). The median modified Hoehn and Yahr score was 2.5 (range 1–4),15, 16 average age of 65.6 ± 7.4 years, and average duration of motor disease symptoms of 6.0 ± 4.2 years. Patients met the UK Parkinson's Disease Society Brain Bank clinical diagnostic criteria.17 The diagnosis of PD was confirmed by the presence of a typical pattern of nigrostriatal dopaminergic denervation with vesicular monoaminergic transporter-type 2 PET.18 Most subjects were on dopaminergic replacement therapy. None of the subjects were on anti-cholinergic or cholinesterase inhibitor drugs.
Written informed consent was obtained from all subjects prior to research procedures. The University of Michigan Medical School Institutional Review Board for human studies approved the study.
Clinical risk factors for cholinergic deficits
We dichotomized each cholinergic deficit associated clinical feature into a “hypocholinergic risk factor” (risk is present=1, risk is absent=0). Cutoffs for continuous variables were based on 85% specificity cutoff as determined by receiver-operating characteristic (ROC) analysis across all PD subjects. This approach yielded the following clinical indices for cholinergic deficits: age of 71 years or older, duration of motor disease symptoms of 9 years or longer, University of Pennsylvania Smell Identification Test (UPSIT)19, 20 score of 9 or lower, 8.5 meter walk time of 9.6 seconds or longer at self-selected gait speed, Montreal cognitive assessment (MoCA) test21 score of 24 or lower, history of 1 or more falls in the previous year, presence of RBD as assessed by using the informant-based response to question #1 on the Mayo Sleep Questionnaire,22 and male gender. Walk time was assessed in the morning, after overnight withdrawal of dopaminergic drugs (dopaminergic “off” state). All subjects walked 8.5 meters in a hallway and were timed using a stopwatch.11 MoCA and UPSIT assessments were performed in the dopaminergic “on” state. Table 1 provides an overview of average values and percentages for each of the clinical features for the different groups.
Table 1.
Average values and percentages for each of the clinical features associated with cholinergic deficits for the different groups. UPSIT=University of Pennsylvania smell identification test; MoCA=Montreal cognitive assessment; RBD=REM sleep behavior disorder.
| Normocholinergic (N=88) | Cortex and thalamus combined cholinergic deficits (N=23) |
Cortex only cholinergic deficits (N=26) |
|
|---|---|---|---|
| Age (years) | 63.9 ± 7.1 | 69.0 ± 7.4 | 68.4 ± 6.8 |
| % Gender (female / male) | 31.8 / 68.2 | 13.0 / 87.0 | 11.5 / 88.5 |
| Motor disease symptom duration (years) | 5.3 ± 3.8 | 8.2 ± 4.5 | 6.6 ± 5.0 |
| UPSIT | 17.4 ± 8.1 | 14.6 ± 8.0 | 13.8 ± 8.1 |
| 8.5 meter walk time (seconds) | 8.0 ± 1.8 | 10.0 ± 5.9 | 9.1 ± 2.8 |
| MoCA | 26.3 ± 2.4 | 25.1 ± 3.1 | 25.2 ± 2.9 |
| % Falls (no fall Hx / fall Hx) | 78.4 / 21.6 | 47.8 / 52.2 | 73.1 / 26.9 |
| % RBD (no RBD / RBD) | 53.4 / 46.6 | 21.7 / 78.3 | 53.8 / 46.2 |
Brain imaging procedures
All brain imaging procedures have been described in detail previously.23 In short, all subjects underwent brain magnetic resonance imaging (MRI) for anatomic co-registration with [11C]PMP AChE PET24 to enable an MRI-based volume of interest analysis. Thalamic and neocortical [11C]PMP AChE hydrolysis rates per minute (k3), a measure of cholinergic terminal integrity, were estimated using reference tissue-based linear least squares analysis25 with the striatum as the reference tissue.
Cholinergic deficits
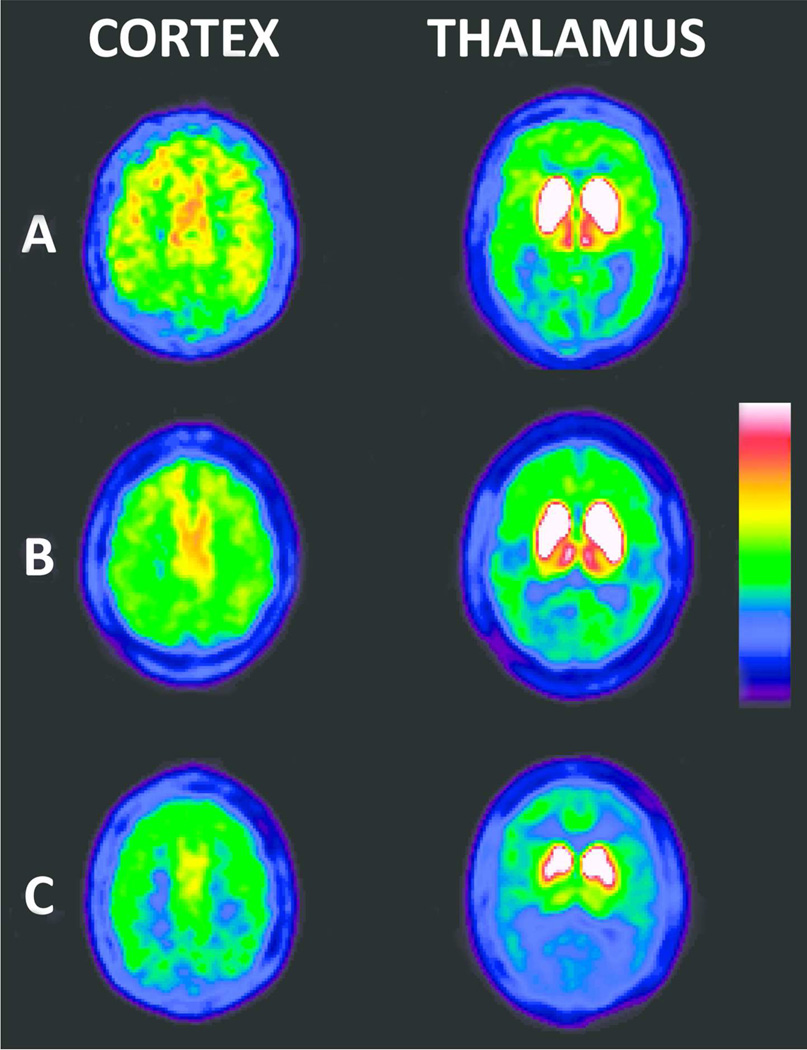
Subjects were characterized as either “normocholinergic” or “hypocholinergic” based on the 5th percentile cut-off from normal range neocortical or thalamic cholinergic innervation. There were 49 (35.8%) hypocholinergic PD subjects; 26 with cortical-only cholinergic denervation, 18 PD subjects with a combination of both cortical and thalamic cholinergic denervation, and 5 with thalamic-only cholinergic denervation. We grouped hypocholinergic subjects either as cortical-only hypocholinergic or as combined cortical and thalamic hypocholinergic (Figure 1).
Figure 1.
Averaged radioactivity from 40 to 70 minutes frames of dynamic [11C]PMP PET for a subject with normal cortical and thalamic cholinergic innervation (row A), a subject with cortical-only cholinergic deficits (row B), and a subject with combined cortical and thalamic cholinergic deficits (row C).
Statistical analysis
Statistical procedures were performed using SPSS Statistics 20 (IBM, Chicago, Ill). Separate analyses were performed for the cortical-only hypocholinergic and the combined cortical and thalamic hypocholinergic groups. Stepwise (likelihood ratio) logistic regression was performed to determine which risk factors best predicted cholinergic group status (normocholinergic or hypocholinergic). For each of the significant predictors and for combinations of significant predictors (i.e. all risk factors are present) the sensitivity (% true positive for risk factor across all hypocholinergic subjects), specificity (% true negative for risk factor across all normocholinergic subjects), and accuracy (proportion of true positives and true negatives across all subjects) were calculated. Area under the curve of ROC analysis was calculated for the combination of significant predictors with highest diagnostic accuracy. The proportion of negative and positive cases for each cholinergic deficits risk factor was compared between the normocholinergic vs. the hypocholinergic group using the χ2 test for contingency tables.
RESULTS
For the combined cortical and thalamic cholinergic denervation group, results of stepwise logistic regression showed significant prediction of cholinergic deficits (2, χ2=18.5, p<0.001) by presence of RBD (Wald=8.7, p=0.003) and fall history (Wald=10.0, p=0.002). The highest diagnostic test accuracy (81.1%; specificity 93.2%) was obtained with the combination of RBD presence and fall history (Table 2). Area under the curve of ROC analysis was 0.64.
Table 2.
χ2 test results for the cholinergic deficits risk factor proportions of combined cortical and thalamic hypocholinergic PD subjects vs. normocholinergic PD subjects, and cortical-only PD subjects vs. normocholinergic PD subjects. Sensitivity, specificity, and accuracy for the different (combinations of) cholinergic deficits risk factors is included. Significant contingency table results are in italics. Table is sorted by increasing accuracy within each of the group comparisons. Hx=history; RBD=REM sleep behavior disorder.
| χ2(p-value) | Sensitivity (%) | Specificity (%) | Accuracy (%) | ||
|---|---|---|---|---|---|
| Combined cortical-thalamic | RBD | 7.3 (0.007) | 78.3 | 53.4 | 58.6 |
| Fall Hx | 8.5 (0.004) | 52.2 | 78.4 | 73.0 | |
| RBD & fall Hx | 13.0 (<0.001) | 34.8 | 93.2 | 81.1 | |
| Cortical-only | Gender | 4.2 (0.041) | 88.5 | 31.8 | 44.7 |
| Walking speed | 4.4 (0.037) | 34.6 | 84.1 | 72.8 | |
| MoCA | 7.3 (0.007) | 42.3 | 83.0 | 73.7 | |
| Walking speed & gender | 3.8 (0.050) | 26.9 | 88.6 | 74.6 | |
| MoCA & gender | 5.9 (0.015) | 34.6 | 86.4 | 74.6 | |
| Age | 8.2 (0.004) | 42.3 | 84.1 | 74.6 | |
| Age & gender | 5.7 (0.017) | 30.8 | 88.6 | 75.4 | |
| MoCA & age & gender | 0.9 (0.349) | 7.7 | 96.6 | 76.3 | |
| MoCA & age | 3.6 (0.057) | 15.4 | 95.5 | 77.2 | |
| Walking speed & age & gender | 3.4 (0.067) | 7.7 | 98.9 | 78.1 | |
| Walking speed & MoCA & age & gender | 3.4 (0.065) | 3.8 | 100 | 78.1 | |
| Walking speed & age | 6.9 (0.009) | 15.4 | 97.7 | 78.9 | |
| Walking speed & MoCA & age | 6.9 (0.009) | 7.7 | 100 | 78.9 | |
| Walking speed & MoCA | 13.2 (<0.001) | 19.2 | 98.9 | 80.7 |
For the cortical-only cholinergic denervation group, results of stepwise logistic regression showed significant prediction of cholinergic deficits (4, χ2=21.8, p<0.001) by a longer walk time (Wald=4.1, p=0.042), lower MoCA score (Wald=5.0, p=0.026), higher age (Wald=7.3, p=0.007), and male gender (Wald=4.2, p=0.040). The highest diagnostic test accuracy (80.7%; specificity 98.9%) was obtained with the combination of walk time (9.6 seconds or longer) and lower MoCA score (24 or lower; Table 2). Area under the curve of ROC analysis was 0.59.
DISCUSSION
The goal of this study was to identify combination(s) of convenient clinical markers that predict cholinergic deficits in PD patients. A history of falling, especially combined with RBD presence, may be indicative of combined cortical and thalamic cholinergic deficits. Longer 8.5 meter walk time in combination with a MoCA score of 24 or lower may be indicative of cortical-only cholinergic deficits.
Ideally, studies should be conducted to identify markers that are specific to cholinergic system degeneration exclusively. However, this may be a challenging goal to achieve given the multisystem neurodegenerative nature of PD26 and the effect of co-morbidities on PD features.27–30 Selection of effective cut-off criteria of the clinical features was based on high specificity but at the expense of lower sensitivity. The reason for this selection approach relates to the multisystem neurodegeneration process of PD where clinical manifestations like cognitive impairment or gait speed can have different contributing pathogenetic factors that are not limited to cholinergic denervation alone, such as noradrenergic denervation or amyloidopathy.31, 32 A more sensitive selection process would consequently include a greater number of PD subjects without cholinergic denervation. Therefore, we believe that in the presence of multisystem neurodegeneration recruitment for cholinergic augmentation therapy should prioritize specificity above sensitivity to maximize clinical response and minimize side-effects.
A prerequisite of clinical trials of novel cholinergic augmentation therapies is the proper identification of the subset of PD subjects with cholinergic deficits. Cholinergic system PET imaging is a highly effective method for identifying cholinergic deficits but with restricted applicability due to relatively high expense and limited availability. We identified fall history in combination with presence of RBD as best predictors of combined cortical and thalamic cholinergic deficits; however, it should be noted that a history of RBD was based on clinical symptoms endorsement of dream enactment behavior and not assessed by polysomnography.12 Time to walk 8.5 meter (9.6 seconds or longer) in combination with a MoCA score of 24 or lower were best predictors of isolated cortical cholinergic deficits. The development of patient stratification tools was recently recommended as the highest priority translational research recommendation in the final report of the “Parkinson's Disease 2014: Advancing Research, Saving Lives” meeting organized by the United States National Institute of Neurological Disorders and Stroke (http://www.ninds.nih.gov/research/parkinsonsweb/PD2014). Although our findings will require validation in an independent cohort, the proposed method of patient selection based on clinical predictor variables may present a cost-effective and efficient method to enrich study populations for trials of cholinergic agents in PD.
ACKNOWLEDGEMENTS
The authors thank Christine Minderovic, Cyrus Sarosh, the PET technologists, cyclotron operators, and chemists for their assistance.
FINANCIAL DISCLOSURES
Müller: Research support from the National Institutes of Health (NIH), Michael J Fox Foundation (MJFF) and the Department of Veteran Affairs (VA).
Bohnen: Research support from the NIH, MJFF and the VA.
Kotagal: Research support from the American Academy of Neurology Clinical Research Training Fellowship.
Scott: Receives Editorial Royalties from Wiley, speaker honoraria from Zevacor Molecular, is an owner of SynFast Consulting, LLC, and has received research funding from the University of Michigan, the Alzheimer's Association, GE Healthcare, Bristol-Myers Squibb, Avid Radiopharmaceuticals / Eli Lilly, Astellas Pharma Inc., and Molecular Imaging Research.
Koeppe: Research support from the NIH.
Frey: Has received research support from the NIH, GE Healthcare, and AVID Radiopharmaceuticals. Consultant for AVID Radiopharmaceuticals, MIMVista, Bayer-Schering, Siemens and GE healthcare. Holds equity (common stock) in GE, Bristol-Myers, Merck, Medtronic, Johnson & Johnson and Novo-Nordisk.
Albin: Research support from the NIH, MJFF, and the VA. Serves on the editorial boards of Neurology, Experimental Neurology, and Neurobiology of Disease. Served on the Data Safety and Monitoring Boards for the QE3 and HORIZON trials. Chair of the Data Safety and Monitoring Board for PRIDE-HD trial.
FUNDING AGENCIES
This work was supported by National Institutes of Health grants P01 NS015655 & R01 NS07085, the Michael J. Fox Foundation, and the Department of Veterans Affairs. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
ABBREVIATIONS
- AChE
acetylcholinesterase
- MoCA
Montreal cognitive assessment
- MRI
magnetic resonance imaging
- PD
Parkinson's disease
- PET
positron emission tomography
- [11C]PMP
[11C]methylpiperidin-4-yl propionate
- RBD
rapid eye movement (REM) sleep behavior disorder
- ROC
receiver-operating characteristic
- UPSIT
University of Pennsylvania smell identification test
Footnotes
RELEVANT CONFLICTS OF INTEREST
The authors have no relevant financial or conflict of interest to disclose.
AUTHOR CONTRIBUTIONS
- Research Project: A. Conception, B. Organization, C. Execution
- Statistical Analysis: A. Design, B. Execution, C. Review and Critique
- Manuscript: A. Writing of the First Draft, B. Review and Critique
Müller: 1A, 1B, 1C, 2A, 2B, 3A
Bohnen: 1A, 1B, 1C, 2A, 2C, 3B
Kotagal: 2C, 3B
Scott: 1B, 1C, 2C, 3B
Koeppe: 1A, 1B, 1C, 2C, 3B
Frey: 1A, 1B, 1C, 2C, 3B
Albin: 1A, 1B, 1C, 2C, 3B
REFERENCES
- 1.Langston JW. The Parkinson's complex: Parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59:591–596. doi: 10.1002/ana.20834. [DOI] [PubMed] [Google Scholar]
- 2.Muller ML, Bohnen NI. Cholinergic dysfunction in Parkinson's disease. Curr Neurol Neurosci Rep. 2013;13:377. doi: 10.1007/s11910-013-0377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Svenningsson P, Westman E, Ballard C, Aarsland D. Cognitive impairment in patients with Parkinson's disease: diagnosis, biomarkers, and treatment. Lancet Neurol. 2012;11:697–707. doi: 10.1016/S1474-4422(12)70152-7. [DOI] [PubMed] [Google Scholar]
- 4.Dubois B, Danze F, Pillon B, Cusimano G, Lhermitte F, Agid Y. Cholinergic-dependent cognitive deficits in Parkinson's disease. Ann Neurol. 1987;22:26–30. doi: 10.1002/ana.410220108. [DOI] [PubMed] [Google Scholar]
- 5.Bohnen NI, Kaufer DI, Hendrickson R, et al. Cognitive correlates of cortical cholinergic denervation in Parkinson's disease and parkinsonian dementia. J Neurol. 2006;253:242–247. doi: 10.1007/s00415-005-0971-0. [DOI] [PubMed] [Google Scholar]
- 6.Bohnen NI, Muller ML, Kotagal V, et al. Olfactory dysfunction, central cholinergic integrity and cognitive impairment in Parkinson's disease. Brain. 2010;133:1747–1754. doi: 10.1093/brain/awq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohnen NI, Muller ML, Kotagal V, et al. Heterogeneity of cholinergic denervation in Parkinson's disease without dementia. J Cereb Blood Flow Metab. 2012;32:1609–1617. doi: 10.1038/jcbfm.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yarnall A, Rochester L, Burn DJ. The interplay of cholinergic function, attention, and falls in Parkinson's disease. Mov Disord. 2011;26:2496–2503. doi: 10.1002/mds.23932. [DOI] [PubMed] [Google Scholar]
- 9.Chung KA, Lobb BM, Nutt JG, Horak FB. Effects of a central cholinesterase inhibitor on reducing falls in Parkinson disease. Neurology. 2010;75:1263–1269. doi: 10.1212/WNL.0b013e3181f6128c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohnen NI, Muller ML, Koeppe RA, et al. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 2009;73:1670–1676. doi: 10.1212/WNL.0b013e3181c1ded6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bohnen NI, Frey KA, Studenski S, et al. Gait speed in Parkinson disease correlates with cholinergic degeneration. Neurology. 2013;81:1611–1616. doi: 10.1212/WNL.0b013e3182a9f558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotagal V, Albin RL, Muller ML, et al. Symptoms of rapid eye movement sleep behavior disorder are associated with cholinergic denervation in Parkinson disease. Ann Neurol. 2012;71:560–568. doi: 10.1002/ana.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bohnen NI, Muller ML. In vivo neurochemical imaging of olfactory dysfunction in Parkinson's disease. J Neural Transm. 2013;120:571–576. doi: 10.1007/s00702-012-0956-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotagal V, Albin RL, Muller ML, Koeppe RA, Frey KA, Bohnen NI. Gender differences in cholinergic and dopaminergic deficits in Parkinson disease. J Neural Transm. 2013;120:1421–1424. doi: 10.1007/s00702-013-1013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. 2004;19:1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 16.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 17.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bohnen NI, Albin RL, Koeppe RA, et al. Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. J Cereb Blood Flow Metab. 2006;26:1198–1212. doi: 10.1038/sj.jcbfm.9600276. [DOI] [PubMed] [Google Scholar]
- 19.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- 20.Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94:176–178. doi: 10.1288/00005537-198402000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 22.Boeve BF, Molano JR, Ferman TJ, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in an aging and dementia cohort. Sleep Med. 2011;12:445–453. doi: 10.1016/j.sleep.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller ML, Albin RL, Kotagal V, et al. Thalamic cholinergic innervation and postural sensory integration function in Parkinson's disease. Brain. 2013;136:3282–3289. doi: 10.1093/brain/awt247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shao X, Hoareau R, Runkle AC, et al. Highlighting the versatility of the Tracerlab synthesis modules. Part 2: fully automated production of [11C]-labeled radiopharmaceuticals using a Tracerlab FXC-Pro. J Labelled Compd Rad. 2011;54:819–838. doi: 10.1002/jlcr.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagatsuka Si S, Fukushi K, Shinotoh H, et al. Kinetic analysis of [11C]MP4A using a high-radioactivity brain region that represents an integrated input function for measurement of cerebral acetylcholinesterase activity without arterial blood sampling. J Cereb Blood Flow Metab. 2001;21:1354–1366. doi: 10.1097/00004647-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 27.Sohn YH, Kim JS. The influence of white matter hyperintensities on the clinical features of Parkinson's disease. Yonsei Med J. 1998;39:50–55. doi: 10.3349/ymj.1998.39.1.50. [DOI] [PubMed] [Google Scholar]
- 28.Kotagal V, Albin RL, Muller ML, Koeppe RA, Frey KA, Bohnen NI. Diabetes is associated with postural instability and gait difficulty in Parkinson disease. Parkinsonism Relat Disord. 2013;19:522–526. doi: 10.1016/j.parkreldis.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotagal V, Albin RL, Muller ML, Koeppe RA, Frey KA, Bohnen NI. Modifiable cardiovascular risk factors and axial motor impairments in Parkinson disease. Neurology. 2014;82:1514–1520. doi: 10.1212/WNL.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee SJ, Kim JS, Yoo JY, et al. Influence of white matter hyperintensities on the cognition of patients with Parkinson disease. Alzheimer Dis Assoc Disord. 2010;24:227–233. doi: 10.1097/WAD.0b013e3181d71a13. [DOI] [PubMed] [Google Scholar]
- 31.Muller ML, Frey KA, Petrou M, et al. β-amyloid and postural instability and gait difficulty in Parkinson's disease at risk for dementia. Mov Disord. 2013;28:296–301. doi: 10.1002/mds.25213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grimbergen YA, Langston JW, Roos RA, Bloem BR. Postural instability in Parkinson's disease: the adrenergic hypothesis and the locus coeruleus. Expert Rev Neurother. 2009;9:279–290. doi: 10.1586/14737175.9.2.279. [DOI] [PubMed] [Google Scholar]