Abstract
Melatonin has anticarcinogenic properties in experimental models. We undertook a case–cohort study of 928 Icelandic men without prostate cancer (PCa) nested within the Age, Gene/Environment Susceptibility (AGES)-Reykjavik cohort to investigate the prospective association between first morning-void urinary 6-sulfatoxymelatonin (6-STM) levels and the subsequent risk for PCa, under the hypothesis that men with lower 6-STM levels have an increased risk for advanced PCa. We used weighted Cox proportional hazards models to assess the association between first morning-void 6-STM level and PCa risk, adjusting for potential confounders. A total of 111 men were diagnosed with incident PCa, including 24 with advanced disease. Men who reported sleep problems at baseline had lower morning 6-STM levels compared with those who reported no sleep problems. Men with morning 6-STM levels below the median had a fourfold statistically significant increased risk for advanced disease compared with men with levels above the median (hazard ratio: 4.04; 95% confidence interval, 1.26–12.98). These results require replication in larger prospective studies with longer follow-up.
Keywords: Circadian rhythm, Melatonin levels, Prostate cancer
The circadian rhythm regulates diverse physiologic and metabolic activities [1]. Melatonin is a hormone secreted by the pineal gland in a 24-h circadian rhythm; under normal conditions, production peaks at night. Melatonin secretion can be inhibited by many factors, including light exposure at night. Most epidemiologic studies support a positive association between measures of circadian disruption or sleep loss and prostate cancer (PCa) risk [2]. In experimental studies, melatonin exhibits chemopreventive properties [3]. Cross-sectionally, men with PCa had lower melatonin levels compared with men with benign prostatic hyperplasia [4]. No prior study has evaluated the prospective association between prediagnostic 6-sulfatoxymelatonin (6-STM) levels, the primary melatonin metabolite, and PCa [5].
We undertook a case–cohort study (Supplemental Fig. 1) within the Age, Gene/Environment Susceptibililty-Reykjavik (AGES-Reykjavik) study to investigate the association between morning urinary 6-STM level and PCa risk. We leveraged questionnaire data on sleep disruption, previously linked with PCa risk [6], to investigate cross-sectional associations with 6-STM level. Full study methods are provided in the online Supplement. Briefly, AGES-Reykjavik collected information via physical examination, questionnaire, and biologic specimens during a 2-day assessment between 2002 and 2006. Subjects collected a first morning-void urine sample. Urine samples were assayed for 6-STM using the melatonin-sulfate enzyme-linked immunosorbent assay (IBL International, Toronto, ON, Canada).
PCa diagnosis and cause of death were identified by linkage with the nationwide Icelandic Cancer Registry and Statistics Iceland using unique identification numbers. We studied risk for total PCa, advanced cancer (extraprostatic stage T3a or higher, N1/M1, cancer death), and lethal cancer (N1/M1 or cancer death).
Participant characteristics were summarized by 6-STM levels dichotomized at the subcohort median. We used Cox proportional hazards regression, modified for the case–cohort design using the Prentice method to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between 6-STM level and incident PCa. Models were adjusted for age and creatinine level, and additionally for family history of PCa, beta-blocker use, depression, sleep problems, and diabetes. Results were similar when additionally adjusted for cortisol.
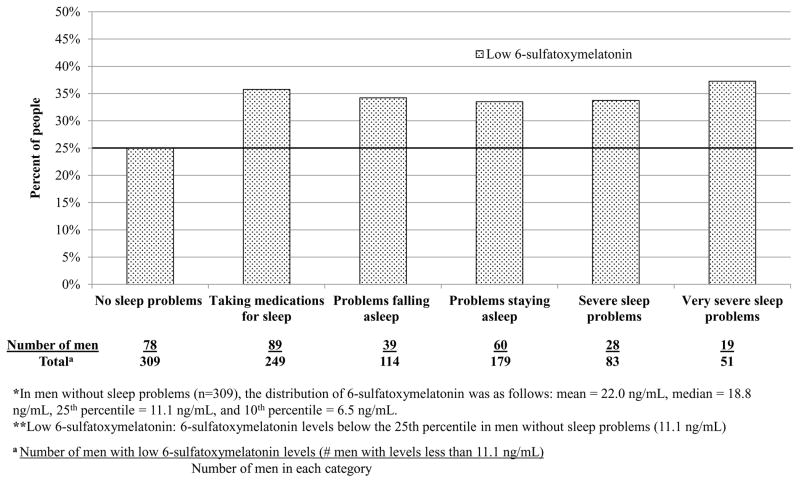
Men with lower 6-STM levels tended to drink less alcohol and had slightly lower creatinine levels, but were more likely to have diabetes and to be taking beta-blockers or psychotropic drugs (Supplemental Table 1). There were no material differences in season of urine collection. 6-STM levels were lower among men who reported sleep problems compared to men without problems (Supplemental Table 1). We determined the 25th percentile of 6-STM in men without sleep problems and defined values less than the 25th percentile as low. Twenty-five percent of men without sleep problems had low 6-STM levels (Fig. 1). In contrast, 35% of men taking medications for sleep and 34% of men with severe sleep problems had low 6-STM levels. Men with 6-STM levels below the median had a 47% higher, although not statistically significant, PCa risk overall compared to men with higher levels (Table 1). Men with lower 6-STM levels had a fourfold increased risk for advanced disease (HR: 4.04; 95% CI, 1.26–12.98); results were similar for lethal PCa (Table 1). In subanalyses to consider potential biases (Supplemental Table 3), including limiting analyses to men who returned their urine samples in the morning or to men reporting no sleep problems, or starting follow-up 2 yr after urine collection, point estimates remained similar but were not statistically significant. In this prospective study of older men, we found an inverse association between urinary 6-STM level and advanced or lethal PCa. We are unaware of any prior prospective evaluation of melatonin, as measured by its urinary metabolite, and PCa, although the findings are broadly in line with prior epidemiologic and experimental studies. A study showed serum melatonin levels measured over 24 h were lower in PCa patients than in controls [4]. Experimental studies suggest that melatonin has anticarcinogenic properties, as melatonin inhibits prostate tumor growth in vitro and in vivo [3,7].
Fig. 1.
Percent of people in each sleep disruption category with low 6-sulfatoxymelatonin (6-STM) levels in the Age, Gene/Environment Susceptibility (AGES)-Reykjavik case–cohort study.
*In men without sleep problems (n = 309), the distribution of 6-STM was as follows: mean = 22.0 ng/ml, median = 18.8 ng/ml, 25th percentile = 11.1 ng/ml, and 10th percentile = 6.5 ng/ml.
**Low 6-STM: 6-STM levels below the 25th percentile in men without sleep problems (11.1 ng/ml).
† Number of men with low 6-STM levels (ie, <11.1 ng/ml).
Table 1.
Association between 6-sulfatoxymelatoninlevels and risk of prostate cancer : Age, Gene/Environment Susceptibility (AGES)-Reykjavik case–cohort study 2002–2009
| High | Low | p value | |
|---|---|---|---|
| Overall PCa, no. | |||
| PCa cases/subcohort control participants | 52/432 | 59/432 | – |
|
| |||
| HR (95% CI) | |||
| Age and creatinine level adjusted | Ref. | 1.31 (0.85–2.04) | 0.22 |
| Fully adjusted** | Ref. | 1.47 (0.94–2.30) | 0.09 |
|
| |||
| Nonadvanced PCa†, no. | |||
| PCa cases/subcohort control participants | 45/432 | 42/432 | – |
|
| |||
| HR (95% CI) | |||
| Age and creatinine level adjusted | Ref. | 1.07 (0.66–1.72) | 0.79 |
| Fully adjusted ** | Ref. | 1.11 (0.67–1.82) | 0.69 |
|
| |||
| Advanced PCa§, no. | |||
| PCa cases/subcohort control participants | 7/432 | 17/432 | – |
|
| |||
| HR (95% CI) | |||
| Age and creatinine level adjusted | Ref. | 2.92 (1.00–8.56) | 0.05 |
| Fully adjusted** | Ref. | 4.04 (1.26–12.99) | 0.02 |
|
| |||
| Lethal PCa^, no. | |||
| PCa cases/subcohort control participants | 4/432 | 14/432 | – |
|
| |||
| HR (95% CI) | |||
| Age and creatinine level adjusted | Ref. | 4.39 (1.18–16.31) | 0.03 |
| Fully adjusted** | Ref. | 4.83 (1.26–18.45) | 0.02 |
PCa = prostate cancer; HR = hazard ratio; CI = confidence interval.
6-sulfatoxymelatonin (6-STM) levels were dichotomized at the median in subcohort (17.14 ng/ml); HR compares low 6- STM levels (below the median) to high levels (above the median).
Fully adjusted model adjusted for age (as time scale), creatinine level, family history of PCa, history of depression, history of diabetes, severe sleep problems, and current beta-blocker use.
Nonadvanced PCa defined as less than stage T3a at diagnosis without distant metastases or death due to PCa.
Advanced PCa defined as stage T3a or T3b at diagnosis, distant metastases at diagnosis or death from PCa over follow-up.
Lethal PCa defined as distant metastases at diagnosis or death from PCa over follow-up.
This study adds to accumulating epidemiologic data investigating associations between circadian disruption or sleep loss and PCa [2], and provides a potential mechanism and framework for understanding prior results. Night-shift work, which disrupts circadian rhythms and suppresses melatonin secretion through nocturnal exposure to artificial light, was categorized as a probable human carcinogen by the International Agency for Research on Cancer, based primarily on data in breast cancer. Night-shift work has been associated with increased risk of PCa [8] as well as elevated prostate-specific antigen (PSA) levels among men without PCa [9]. Moreover, blind men, some of whom may have uninhibited melatonin secretion, have lower PCa incidence compared to the general population [10].
Our results rest on a single morning urinary 6-STM measurement, which may not represent long-term levels. We were also limited by a small number of events. Follow-up is short and men may have had underlying disease at the time of exposure assessment. We attempted to address the possibility of reverse association, although power was limited given the median follow-up time from urine collection to diagnosis of 2.3 yr. Although we had broad information on various covariates and were able to control for possible confounders, we lacked information on factors such as vitamin D levels. We also lacked information on Gleason grade, screening history, or PSA levels, and stage information was missing for 35% of cases. Finally, this study was restricted to elderly men in Iceland; levels of melatonin in this population may vary from those of other populations, such as those with less extreme variation in daylight during the year or younger men, although it is noteworthy that levels did not vary by season of collection. While this limits generalizability of our findings, it is unlikely that underlying biologic impact on PCa pathogenesis would differ.
In summary, men with lower morning 6-STM levels were at increased risk for advanced or lethal PCa. These results require replication in larger prospective cohort studies with longer follow-up and more detailed clinical information. Given that disruption of melatonin levels is potentially avoidable, further studies of melatonin and PCa risk should be a priority.
Supplementary Material
Take-home message.
In this report, we evaluated the prospective association between urinary 6-sulfatoxymelatonin levels and risk of prostate cancer in an Icelandic population. We found that lower levels of 6-sulfatoxymelatonin were associated with an increased risk of advanced prostate cancer.
Patient summary.
In this report, we evaluated the prospective association between urinary 6-STM levels and risk of PCa in an Icelandic population. We found that lower levels of 6-STM were associated with an increased risk for advanced PCa.
Acknowledgments
Funding/Support and role of the sponsor: This study was supported in part by RANNIS (the Icelandic Research Fund); the Harvard Catalyst Award (awards UL1-RR-025758 and KL2-RR-025757) from the National Institutes of Health (NIH) National Center for Research Resources; the Icelandic Cancer Society; NIH National Cancer Institute training grants (grants R25-CA-098566 and T32-CA-09001-35 to SCM); and the Prostate Cancer Foundation (to LAM). The AGES-Reykjavik is supported by Contract N01-AG-12100 from the NIH National Institutes on Aging (NIA) Intramural Research Program, the Icelandic Heart Association, and the Icelandic Parliament. CAC was supported in part by NIH NIA grant P01-AG-009975. The funding agencies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
The researchers are indebted to the participants for their willingness to participate in the study and we thank the Icelandic Heart Association clinic staff for their invaluable contribution.
Footnotes
Author contributions: Sarah C. Markt had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sigurdardottir, Markt, Rider, Fall, Batista, Launer, Harris, Aspelund, Stampfer, Gudnason, Czeisler, Lockley, Valdimarsdóttir, Mucci.
Acquisition of data: Sigurdardottir, Markt, Valdimarsdóttir.
Analysis and interpretation of data: Sigurdardottir, Markt, Rider, Haneuse, Fall, Schernhammer, Tamimi, Flynn-Evans, Batista, Launer, Harris, Aspelund, Stampfer, Gudnason, Czeisler, Lockley, Valdimarsdóttir, Mucci.
Drafting of the manuscript: Sigurdardottir, Markt.
Critical revision of the manuscript for important intellectual content: Sigurdardottir, Markt, Rider, Haneuse, Fall, Schernhammer, Tamimi, Flynn-Evans, Batista, Launer, Harris, Aspelund, Stampfer, Gudnason, Czeisler, Lockley, Valdimarsdóttir, Mucci.
Statistical analysis: Sigurdardottir, Markt.
Obtaining funding: Valdimarsdóttir, Mucci.
Administrative, technical, or material support: None.
Supervision: Valdimarsdóttir, Mucci.
Other (specify): None.
Financial disclosures: Sarah C. Markt certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/ affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Charles Czeisler has received consulting fees from or served as a paid member of scientific advisory boards for the following: Bombardier Inc, Boston Red Sox, Boston Celtics, Cephalon Inc (acquired by Teva Pharmaceutical Industries Ltd. October 2011), the Jackson family, Koninklijke Philips Electronics NV, Novartis, United Parcel Service, Vanda Pharmaceuticals Inc, and Zeo Inc; owns an equity interest in Lifetrac Inc, Somnus Therapeutics Inc, and Vanda Pharmaceuticals Inc; received royalties from McGraw Hill, Penguin Press/Houghton Mifflin Harcourt, and Philips Respironics Inc.; and has received research support from Cephalon, National Football League Charities, ResMed, and Philips Respironics. Dr. Czeisler is the incumbent of an endowed professorship provided to Harvard University by Cephalon Inc, holds a number of process patents in the field of sleep/circadian rhythms, and since 1985 has also served as an expert witness on various legal matters related to sleep and/or circadian rhythms.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blask DE, Dauchy RT, Sauer LA. Putting cancer to sleep at night: the neuroendocrine/circadian melatonin signal. Endocrine. 2005;27:179–88. doi: 10.1385/ENDO:27:2:179. [DOI] [PubMed] [Google Scholar]
- 2.Sigurdardottir LG, Valdimarsdóttir UA, Fall K, et al. Circadian disruption, sleep loss, and prostate cancer risk: a systematic review of epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2012;21:1002–11. doi: 10.1158/1055-9965.EPI-12-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moretti RM, Marelli MM, Maggi R, Dondi D, Motta M, Limonta P. Antiproliferative action of melatonin on human prostate cancer LNCaP cells. Oncol Rep. 2000;7:347–51. [PubMed] [Google Scholar]
- 4.Bartsch C, Bartsch H, Schmidt A, Ilg S, Bichler KH, Flüchter SH. Melatonin and 6-sulfatoxymelatonin circadian rhythms in serum and urine of primary prostate cancer patients: evidence for reduced pineal activity and relevance of urinary determinations. Clin Chim Acta. 1992;209:153–67. doi: 10.1016/0009-8981(92)90164-l. [DOI] [PubMed] [Google Scholar]
- 5.Bojkowski CJ, Aldhous ME, English J, et al. Suppression of nocturnal plasma melatonin and 6-sulphatoxymelatonin by bright and dim light in man. Horm Metab Res. 1987;19:437–40. doi: 10.1055/s-2007-1011846. [DOI] [PubMed] [Google Scholar]
- 6.Sigurdardottir LG, Valdimarsdóttir UA, Mucci LA, et al. Sleep disruption among older men and risk of prostate cancer. Cancer Epidemiol Biomark Prev. 2013;22:872–9. doi: 10.1158/1055-9965.EPI-12-1227-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiu SYW, Law IC, Lau KW, Tam PC, Yip AWC, Ng WT. Melatonin slowed the early biochemical progression of hormone-refractory prostate cancer in a patient whose prostate tumor tissue expressed MT1 receptor subtype. J Pineal Res. 2003;35:177–82. doi: 10.1034/j.1600-079x.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 8.Straif K, Baan R, Grosse Y, et al. Carcinogenicity of shift-work, painting, and firefighting. Lancet Oncol. 2007;8:1065–6. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- 9.Flynn-Evans EE, Mucci L, Stevens RG, Lockley SW. Shiftwork and prostate-specific antigen in the National Health and Nutrition Examination Survey. J Natl Cancer Inst. 2013;105:1292–7. doi: 10.1093/jnci/djt169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feychting M, Osterlund B, Ahlbom A. Reduced cancer incidence among the blind. Epidemiol Camb Mass. 1998;9:490–4. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.