Abstract
Positive allosteric modulators of ionotropic glutamate receptors have emerged as a target for treating cognitive impairment and neurodegeneration, but also mental illnesses such as major depressive disorder. The possibility of creating a new class of pharmaceutical agent to treat refractive mental health issues has compelled researchers to redouble their efforts to develop a safe, effective treatment for memory and cognition impairments. Coupled with the more robust research methodologies that have emerged, including more sophisticated high-throughput-screens, higher resolution structural biology techniques, and more focused assessment on pharmacokinetics, the development of positive modulators of AMPA receptors holds great promise. We describe recent approaches that improve our understanding of the basic physiology underlying memory and cognition, and their application towards promoting human health.
INTRODUCTION
Ion channels are transmembrane proteins that facilitate communication between cells to allow the intake of information from the environment and output an appropriate response to change. The glutamate receptor family of ion channels binds to synaptically-released glutamate, and in turn, initiates either a change in second messenger signaling (via the metabotropic glutamate receptors) or permit ions (sodium, potassium, chloride, calcium) to flow between the intracellular and extracellular compartments, via electrochemical signaling [1]. The family of ionotropic glutamate receptors (iGluRs) includes the N-methyl-D-aspartate receptors (NMDARs, GluN1-3), the alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPARs, GluA1-4), the kainate receptors (GluK1-5) and the “orphan” receptors (GluD1-2) [2]. Here, we focus on recent developments in the positive allosteric modulation of the AMPAR family of ionotropic glutamate receptors.
AMPARs are considered an appropriate target for drug discovery and development efforts because they play a critical role in synaptic plasticity, the cellular mechanism that is thought to underlie learning and memory, including long term potentiation (LTP) and long term depression (LTD) (reviewed in [3]). Perhaps the single most important demonstration of the importance of AMPAR in drug development was the finding in 2008 that AMPAR were implicated in the cellular mechanisms underlying the observed antidepressive effects of subanesthetic doses of ketamine [4]. In the present review we consider the research findings over the past 2–3 years regarding synaptic physiology, AMPAR structure and function, drug discovery and development, and neuropharmacology. The results from basic and clinical research, as well as the progress from PHARMA, offer a hopeful perspective on the future of this class of compound.
The Role of AMPAR in Synaptic Plasticity
Cellular and network plasticity require activation of the NMDAR and subsequent elevation of intracellular calcium, followed by upregulation of AMPAR activity and subsequent induction of long term potentiation (LTP) [3]. LTP has several components, including local CaMKII activation leading to triggering of signaling cascades and the subsequent activity-dependent phosphorylation of AMPAR, which regulates AMPAR channel conductance. Perhaps the most intriguing new information about AMPAR’s role in synaptic plasticity relates to the dynamic regulation of its trafficking inside the neuron and to the cell surface. The concept of “silent” synapses being mobilized is a critical feature in thinking of how these receptors can be upregulated to maintain LTP [5]. Auxiliary proteins (transmembrane AMPAR regulatory proteins, TARPs), such as stargazin and scaffolding proteins, appear to be dynamically regulated, and it remains unclear how mobilization of silent synapses is impacted by positive allosteric modulators, although preliminary experiments seemed to suggest an important impact of TARP binding on the pharmacology of AMPAR positive allosteric modulation [6]. Recent experiments that map the sites of protein-protein interaction between AMPAR and TARPs suggest that at least one site of interaction overlaps a positive allosteric modulatory binding site on AMPAR [7].
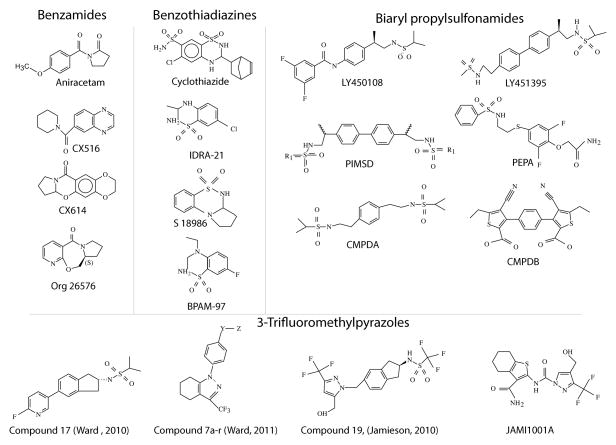
Chemotypes of Positive Allosteric Modulators
Most AMPAR positive allosteric modulators to fall into four major classifications: the benzamides (including aniracetam [8] and its derivatives, the CX ampakines such as CX516 [9], CX614 [10], CX717 [11], CX929 [12] and Org 26576 [13]); the benzothiadiazines (including cyclothiazide [14], IDRA-21 [15], S 18986 [16], and BPAM-97 [17,18]); the biaryl propylsulfonamides (including LY404187 [19], LY451395 [20], (R,R)-2a, and -2b, or PIMSD [21], PEPA [22], and CMPDA and CMPDB [23]), which recapitulate structural features of the first two classes; and the 3-trifluoromethylpyrazoles (including a series of compounds developed by Ward et al. [24,25] and Jamieson et al. [26–28] (Figure 1). Pirotte et al. [29] document, from their review of patents filed between 2008–2012, that two smaller classes, including pyrrole/thiophenecarboxylic acids described by Lilly and phenyliminothiazoles described by GlaxoSmithKline may be further developed but currently do not seem to represent a major discovery target. The limited number of chemotypes is perceived to be an impediment to getting compounds to the clinical trials pipeline [30].
Fig. 1. Chemotypes of AMPAR positive allosteric modulators.
The structures shown provide a representative snapshot of past and present AMPAR positive modulators that have been studied (originating references are provided in the text).
Mechanisms of Action
AMPAR are ~1000 amino-acid, transmembrane proteins, with an extracellular glutamate binding site, which assemble as either homo- or hetero-tetrameric receptor complexes [31]. A significant advance that stimulated the development of new positive allosteric modulators was the crystallization of the isolated ligand-binding domain (LBD) to which both agonist (glutamate) [32] and positive allosteric modulators bind. The secondary structures within a polypeptide chain form a dimer of dimers with the neighboring polypeptide chain, through protein-protein interactions between subunits; however, the ATDs interact with one subunit whereas the LBD interacts with another subunit, likely as a mechanism to enhance allosteric action.
The LBD is shaped as an open “clamshell”, with an upper and lower lid, joined by “hinges”. Activation of an AMPAR occurs when glutamate enters the clamshell and forms interactions with the upper lobe of the clamshell, which drives the movement of the lower shell away from the lipid bilayer and towards the upper shell. This clamshell closure drives subsequent rearrangements at the transmembrane pore to permit ion flow [33]. Agonist can dissociate, allowing the receptor complex to deactivate and return to rest. Like many ligand-binding receptors, prolonged exposure to agonist can also lead to a refractory state, desensitization, in which agonist presumably remains locked within the closed clamshell, but the pore returns to the closed state. Receptor desensitization occurs through a major rearrangement and subsequent disruption of the dimer interface, allowing uncoupling of the LBD and the ion-conducting pore [34,35]. Recent work suggests that receptor desensitization causes global rearrangement of the AMPAR quaternary structure, such that the ATD is pulled down towards the LBD, compressing the extracellular structure, and that LBD itself becomes flattened, inducing a mechanical pulling force on the transmembrane domains [34]. Interestingly, there is accumulating evidence that the desensitized state is characterized by substantial conformational heterogeneity [36]. The allosteric modulators discussed here all bind at the interface between two LBDs, in a solvent-accessible pocket formed by residues from each of the two dimers.
AMPAR positive allosteric modulators exert their effect by slowing the conformational transitions that permit rearrangement of the dimer interface, thereby slowing the macroscopic rate of receptor desensitization, and in some cases, receptor deactivation. RNA splicing results in heterogeneity within this pocket (at the Ser/Asn site; [37]), such that positive allosteric modulators can be splice isoform selective (for either the flip or flop isoform) or non-selective [38]. Recent work [39] delineates five subdomains of the modulator binding pocket, and provides careful structure-function analysis with derivatized benzothiadiazine modulators to understand potency and efficacy [18,40]. Modulators have been shown to bind in three “modes” related to occupancy of this pocket [35]: 1) two molecules of modulator binding perpendicular to the 2-fold axis of symmetry of the LBD dimer, such as cyclothiazide; 2) two overlapping conformations of modulator within the central site (subsite A), such as CX614; and 3) chemically symmetrical compounds that bind to subsite A with a single conformation, such as CMPDA. Structure-function work on positive allosteric modulators that interact with this site consistently reveal that modifications of either moieties on the compound or the amino acid sidechains on the receptor, which alter the number of contacts or the strength of the interactions between the compounds, water molecules and protein sidechains within the five subsites, determine both efficacy and affinity [10,41]. However, our understanding of the interactions had not seemed robust enough to allow completely accurate predictive modeling of the activity of newly synthesized compounds. Recently, (R, R)-2b, was used in a groundbreaking series of mechanistic studies demonstrating that positive allosteric modulators that are structural “hybrids” of CTZ and aniracetam permit both the stabilization of the dimer interface, preventing receptor desensitization, and interact with the “hinges” between the two clamshells, stabilizing the LBD in an agonist-bound conformation [34].
An interesting development in the area of protein-protein interactions at the dimer interface relates further to modulator stoichiometry. Increasing the number and strength of molecular interactions between amino acids of adjacent subunits through mutations of specific amino acids of glutamate receptors slows the kinetics of deactivation and/or desensitization [42–44], similar to allosteric modulators. Norholm et al. (2013) [45] have synthesized a new benzothiadiazine compound, PIMSD, by optimizing its structure into a forced dimer, and then facilitating its testing by using the L483Y AMPAR mutation to promote a stable dimer of LBDs and therefore optimize the modulator binding pocket. This compound showed submicromolar binding affinity, as assessed by the increasingly popular method of measuring the thermodynamics of modulator-receptor binding using isothermal titration calorimetry (ITC). Testing compounds with forced LBD dimers may enhance high through-put screening of modulators. In a complementary study, Ptak et al. (2013) [46] used small-angle X-ray scattering (SAXS) to detect subtle differences in protein structure in the presence of cyclothiazide and other benzothiadiazines, and this study revealed that manipulating the stoichiometry of the modulator permitted enhanced dimerization of the protein. They suggest a novel use for AMPAR positive modulators in driving glutamate receptor dimerization, essentially allowing for the ready isomerization of the protein to an activated state. They propose that this strategy could have broad implications in medicinal chemistry.
A recent patent review on AMPAR modulators showed that this receptor is quite actively being pursued as a drug target [29]. The authors’ interpretation of patented compounds and their activities was that rational drug design to optimize potency and efficacy in vitro led to larger and more complicated molecules, for which further development was constrained by poorer performance on pharmacokinetic profiles and in vivo efficacy. These authors predicted a return to the development of lower molecular weight molecules with perhaps lower in vivo efficacy, but for which pharmacokinetics were more favorable. This may be consistent with an emerging trend to design small molecules that can modulate multiple receptor types within a behavioral circuit. Strategies to control protein dimerization, the use of robust FLIPR technology to facilitate rapid screening of activity [47], structure based drug design [28], and approaches to co-test efficacy with bioavailability and pharmacokinetics [25] are technological advances that should increase the discovery of new lead compounds.
Modulation of Activity – Modulation of Behavior
The consequence of binding a positive allosteric modulator by an AMPAR is, by definition, an enhanced net charge transfer. Either by slowing receptor deactivation, desensitization, or both, the net influx of positive ions into the post-synaptic neuron increases due to the modulator. What is the downstream consequence of enhanced current flow into the postsynaptic cell, and how does that change translate to a change in behavior? There is not a good consensus in the field regarding this important point. In order to change behavior, the AMPAR modulator must have an impact on the circuits that regulate that behavior. This concept was recently modeled by Bouteiller at al. [48], who demonstrate how a change in receptor kinetics changes the firing pattern of a single neuron, and ultimately, the activity of a network of neurons, using multiscale modeling. Starting with a 16-state kinetic model from the Howe group [49], simulating the actions of a positive allosteric modulator (CX614) on single cells, to a network of hippocampal neurons, their model predicts that CX614 would have complicated effects on neuronal excitability, with both a shifting of neuronal spike timing and an increase in the number of evoked spikes. At higher concentrations, continuous spiking was seen, suggesting the possibility of the formation of epileptiform activity.
There is considerable interest in understanding the relationship between the induction of LTP, the maintenance of LTP, and morphological changes in synaptic connections within a specific circuit. It seems clear that a critical consequence of the increased electrical activity caused by modulators is a subsequent involvement in neurotrophin-mediated, especially BDNF-mediated, sculpting of postsynaptic spines [50]. In fact, BDNF itself has emerged as target for development in the treatment of cognitive deficits. There are a number of studies demonstrating a positive effect of AMPAR positive modulators on behavior in animal models, including a fairly wide sampling of different AMPAR positive modulator chemotypes. Kramar et al [51] demonstrated an important proof of concept in a model of middle aged, ovariectomized rats. Actin polymerization in spines, reduced in ovariecomized rats, was restored by infusion of BDNF or by daily injections of the positive modulator, CX929 [12]. The most parsimonious explanation for these results was that the ampakine treatment recapitulated infusion by BDNF because it directly upregulated BDNF or a factor very similar to BDNF.
S 18986, a Servier benzothiadiazine, enhanced AMPAR currents (EC50 =130 micromolar), increased induction and maintenance of LTP, as well as expression of BDNF, and improved cognition in procedural, spatial, episodic, working and declarative memory in young and middle aged rats [16]. Rats that received a single dose of S 18986 infused into the prelimbic cortex enhanced acquisition and retention of an odor-reward association [52]. The dimeric modulator, PIMSD, which was shown to have promising in vitro attributes at an EC50 = 0.73 micromolar, was used in a rat scopolamine-induced impairment model [53]. Place learning using a water maze was tested 25 minutes after subcutaneous injection, and PIMSD was found to partially alleviate the symptoms of the scopolamine-induced impairment. Org 26576 was injected intraperitoneally in a rat 6-hydroxydopamine lesion-induced hyperactivity model (an animal model of attention-deficit hyperactivity disorder) [54]. In the rat model, a dose-dependent inhibition of locomotor hyperactivity was found. Another important proof-of-concept animal model study, using intraperitoneal injections of CX929 (5 mg/kg), was performed in a mouse model of the human neurodevelopment disorder, Angelman Syndrome (AS) [55]. Mice with abnormal expression of an E3 ligase and a transcriptional co-factor, genes deleted in human AS, exhibited abnormal dendritic spine morphology and impairment of LTP. When treated with the ampakine CX929 (twice a day for five days) the mutant mice demonstrated WT-like LTP, theta-burst stimulation-induced actin polymerization within dendritic spines, and long-term memory scores in fear conditioning. Another ampakine, CX1739, was tested in a mouse model of autism, and was found to reduce the sociability deficit in these animals [56]. A third ampakine, BCP-1, was tested in mice using daily intraperitoneal injections for two weeks in a model of exercise-induced fatigue, and found to increase swim times to exhaustion, thereby extending the endurance capacity and facilitating recovery from fatigue [57]. Thus, recent progress in animal models of modulating different types of animal behavior, using different chemotypes of AMPAR positive modulators, continues to show promise. And yet, there are differing views on whether positive results in animal models will be predictive of positive results in human clinical trials [30,58–60].
Human Clinical Trials
A broad approach for therapeutic impact has been taken against the glutamatergic network as a whole, including agents for major depressive disorder, bipolar disorder [61] and schizophrenia [62]. In addition, there are a number of clinical trials reported in both clinicaltrials.gov and the International Clinical Trials Registry Platform for compounds with a direct action on AMPAR. Most of the trials are closed, but emerging publications report positive, if not robust, results. For example, Phase 1 trials of the safety, tolerability and pharmacokinetic data on healthy and depressed humans were useful, particularly in supporting that Org 26576 was well-tolerated at higher dose in depressed patients than in healthy volunteers [63]. The in vivo assessment of Org 26576 led to the Phase 1b trial on depressive symptomology, cognition, BDNF and endocrine responses, as well as to the assessment of relevant biomarkers [64]. All treatment groups (including placebo control) showed a mean improvement in depressive symptomology and key biomarkers, and changes from baseline tended to be higher in the treatment group, but these findings were not conclusive. This may be a reflection of the work that needs to be done by the field in determining what should be measured if one wishes to demonstrate enhanced cognition (see below).
Another clinical use for AMPA modulators relates to attention, alertness and sleep. Org 26576 was tested in human ADHD adults, but again, the suggestion of therapeutic benefit was not confirmed by a clear dose-response. High doses of CX717 (whose structure has not been published, apparently due to DARPA restrictions) were found to counteract the effects of sleep deprivation on attention-based tasks in healthy human volunteers [65]. Again, although the findings were not robust, they suggest an improved performance on some attention-based tasks. CX717 is not currently listed on the Cortex Pharmaceuticals Product Pipeline website (http://www.cortexpharm.com/product/index.html), although CX1739 appears to be advancing for multiple studies.
The Future
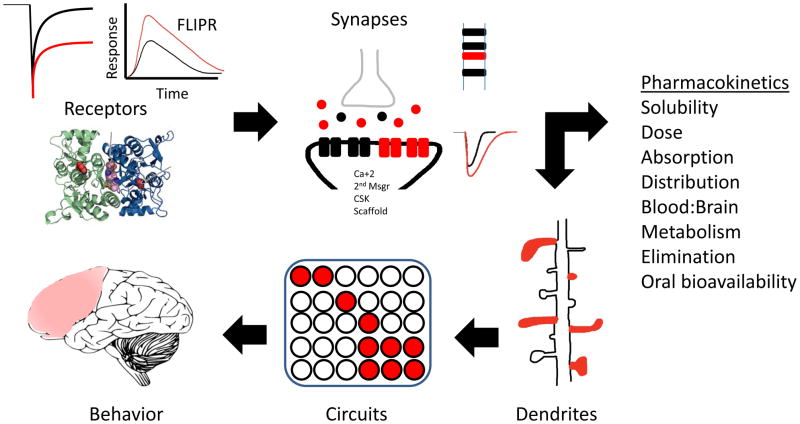
Given that there are advances in technology that may hasten the discovery of new lead compounds, and promising preclinical and clinical results on a few compounds, it is clear that the assessment of efficacy of a potential new ampakine needs to be done at the level of the receptor, the synapse, the dendrite, the circuit, and the behavior (Figure 2). More importantly, perhaps, is appropriate consideration of the questions posed by Lynch et al. (2014) in a recent review on pharmacological enhancement of memory and cognition in normal human volunteers [66]. What is the relationship between memory and cognition in normal subjects, and in what types of behavior (circuits)? Without a clear definition of terms, appreciation of circuitry underlying different behaviors, and rigor of using appropriate animals to measure appropriate behaviors, it is not clear that new lead compounds will become new treatments. There seems to be much wisdom in the immediate goal of combining network mapping with localization of specific synaptic changes in order to understand the effects of a compound on network substrates of specific cognitive operations.
Fig. 2. Screening of new AMPAR positive allosteric modulators.
Recent improvements in how new lead compounds are tested suggest that initial in vitro tests may include electrophysiology, calcium imaging and structure-based design. The modulator may then be tested in cells or slices, using electrophysiology, immunocytochemistry and biomarker biochemistry. It is now more common to see the in vitro work accompanied by a battery of pharmacokinetics, as well as analysis of morphology, circuitry and behavior in vivo. Thus, even initial reports of new lead compounds have an impressive array of methodologies supporting the further development of that compound. In this cartoon, the red images represent the expected experimental results in the presence of a putative new AMPA positive allosteric modulator.
Highlights.
Better AMPAR positive allosteric modulators are being developed
New methodologies and approaches enhance the analysis of their activity in vitro
AMPA positive modulators show promise in healthy volunteers
Mental illnesses such as major depressive disorder and schizophrenia are targets
Acknowledgments
The author thanks the graduate students, undergraduate students, postdoctoral fellows, and research associates in her lab who have contributed to the work cited within this manuscript. We gratefully acknowledge support of the National Institutes of Health, National Institute for Mental Health, award #R01MH064700.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol Rev. 2010;62(3):405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. 2009;56(1):2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3*.Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: The last 25 years. Neuron. 2013;80(3):704–717. doi: 10.1016/j.neuron.2013.10.025. Interesting historical perspective on our understanding of synaptic plasticity; every trainee under the age of 50 should be required to read this. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: Role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biological Psychiatry. 2008;63(4):349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 5*.Haering SC, Tapken D, Pahl S, Hollmann M. Auxiliary subunits: Shepherding ampa receptors to the plasma membrane. Membranes. 2014;4(3):469–490. doi: 10.3390/membranes4030469. Comprehensive discussion of familes and functions of auxilliary proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomita S, Sekiguchi M, Wada K, Nicoll RA, Bredt DS. Stargazin controls the pharmacology of ampa receptor potentiators. Proc Natl Acad Sci U S A. 2006;103(26):10064–10067. doi: 10.1073/pnas.0603128103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cais O, Herguedas B, Krol K, Cull-Candy SG, Farrant M, Greger IH. Mapping the interaction sites between ampa receptors and tarps reveals a role for the receptor n-terminal domain in channel gating. Cell Reports. 2014;9(2):728–740. doi: 10.1016/j.celrep.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isaacson JS, Nicoll RA. Aniracetam reduces glutamate receptor desensitization and slows the decay of fast excitatory synaptic currents in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:10936–10940. doi: 10.1073/pnas.88.23.10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krintel C, Harpsoe K, Zachariassen LG, Peters D, Frydenvang K, Pickering DS, Gajhede M, Kastrup JS. Structural analysis of the positive AMPA receptor modulators CX516 and me-CX516 in complex with the glua2 ligand-binding domain. Acta Crystallogr D Biol Crystallogr. 2013;69(Pt 9):1645–1652. doi: 10.1107/S0907444913011839. [DOI] [PubMed] [Google Scholar]
- 10.Jin R, Clark S, Weeks AM, Judman JT, Gouaux E, Partin KM. Mechanism of positive allosteric modulators acting on AMPA receptors. J Neurosci. 2005;25(39):9027–9036. doi: 10.1523/JNEUROSCI.2567-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porrino LJ, Daunais JB, Rogers GA, Hampson RE, Deadwyler SA. Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates. PLoS biology. 2005;3(9):e299. doi: 10.1371/journal.pbio.0030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmons DA, Mehta RA, Lauterborn JC, Gall CM, Lynch G. Brief ampakine treatments slow the progression of Huntington’s Disease phenotypes in r6/2 mice. Neurobiology of Disease. 2011;41(2):436–444. doi: 10.1016/j.nbd.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordan GR, McCulloch J, Shahid M, Hill DR, Henry B, Horsburgh K. Regionally selective and dose-dependent effects of the ampakines ORG 26576 and ORG 24448 on local cerebral glucose utilisation in the mouse as assessed by 14c-2-deoxyglucose autoradiography. Neuropharmacology. 2005;49(2):254–264. doi: 10.1016/j.neuropharm.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Yamada K, Tang CM. Benzothiadiazines inhibit rapid glutamate receptor desensitization and enhance glutamatergic synaptic currents. Journal of Neuroscience. 1993;13:3904–3915. doi: 10.1523/JNEUROSCI.13-09-03904.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zivkovic D, Thompson DM, Bertolino M, DiBella M, Costa E, Guidotti A. 7-chloro-3-methyl-3-4-dihydro-2h-1,2,4 benzothiadiazine s,s-dioxide (idra21): A benzothiadiazine derivative that enhances cognition by attenuating dl-amino-2,3-dihydro-5-methyl-3-oxo-4-isoxazolepropanoic acid (ampa) receptor desensitization. The Journal of Pharmacology and Experimental Therapeutics. 1995;272:300–309. [PubMed] [Google Scholar]
- 16.Bernard K, Danober L, Thomas JY, Lebrun C, Munoz C, Cordi A, Desos P, Lestage P, Morain P. Drug focus: S 18986: A positive allosteric modulator of ampa-type glutamate receptors pharmacological profile of a novel cognitive enhancer. CNS Neuroscience & Therapeutics. 2010;16(5):e193–212. doi: 10.1111/j.1755-5949.2009.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francotte P, Tullio P, Goffin E, Dintilhac G, Graindorge E, Fraikin P, Lestage P, Danober L, Thomas JY, Caignard DH, Pirotte B. Design, synthesis, and pharmacology of novel 7-substituted 3,4-dihydro-2h-1,2,4-benzothiadiazine 1,1-dioxides as positive allosteric modulators of AMPA receptors. J Med Chem. 2007;50(13):3153–3157. doi: 10.1021/jm070120i. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed AH, Ptak CP, Oswald RE. Molecular mechanism of flop selectivity and subsite recognition for an ampa receptor allosteric modulator: Structures of GluA2 and GluA3 in complexes with PEPA. Biochemistry. 2010;49(13):2843–2850. doi: 10.1021/bi1000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quirk JC, Nisenbaum ES. Ly404187: A novel positive allosteric modulator of AMPA receptors. CNS Drug Reviews. 2002;8(3):255–282. doi: 10.1111/j.1527-3458.2002.tb00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zmijewski M, Gillespie TA, Jackson DA, Schmidt DF, Yi P, Kulanthaivel P. Application of biocatalysis to drug metabolism: Preparation of mammalian metabolites of a biaryl-bis-sulfonamide ampa (alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid) receptor potentiator using actinoplanes missouriensis. Drug Metabolism and Disposition: the Biological Fate of Chemicals. 2006;34(6):925–931. doi: 10.1124/dmd.106.009522. [DOI] [PubMed] [Google Scholar]
- 21.Kaae BH, Harpsoe K, Kastrup JS, Sanz AC, Pickering DS, Metzler B, Clausen RP, Gajhede M, Sauerberg P, Liljefors T, Madsen U. Structural proof of a dimeric positive modulator bridging two identical AMPA receptor-binding sites. Chemistry & Biology. 2007;14(11):1294–1303. doi: 10.1016/j.chembiol.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Sekiguchi M, Nishikawa K, Aoki S, Wada K. A desensitization-selective potentiator of AMPA-type glutamate receptors. Br J Pharmacol. 2002;136(7):1033–1041. doi: 10.1038/sj.bjp.0704804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timm DE, Benveniste M, Weeks AM, Nisenbaum ES, Partin KM. Structural and functional analysis of two new positive allosteric modulators of GluA2 desensitization and deactivation. Mol Pharmacol. 2011;80(2):267–280. doi: 10.1124/mol.110.070243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward SE, Harries M, Aldegheri L, Andreotti D, Ballantine S, Bax BD, Harris AJ, Harker AJ, Lund J, Melarange R, Mingardi A, et al. Discovery of n-[(2s)-5-(6-fluoro-3-pyridinyl)-2,3-dihydro-1h-inden-2-yl]-2-propanesulfo namide, a novel clinical ampa receptor positive modulator. J Med Chem. 2010;53(15):5801–5812. doi: 10.1021/jm1005429. [DOI] [PubMed] [Google Scholar]
- 25.Ward SE, Harries M, Aldegheri L, Austin NE, Ballantine S, Ballini E, Bradley DM, Bax BD, Clarke BP, Harris AJ, Harrison SA, et al. Integration of lead optimization with crystallography for a membrane-bound ion channel target: Discovery of a new class of AMPA receptor positive allosteric modulators. J Med Chem. 2010 doi: 10.1021/jm100679e. [DOI] [PubMed] [Google Scholar]
- 26.Jamieson C, Basten S, Campbell RA, Cumming IA, Gillen KJ, Gillespie J, Kazemier B, Kiczun M, Lamont Y, Lyons AJ, Maclean JK, et al. A novel series of positive modulators of the AMPA receptor: Discovery and structure based hit-to-lead studies. Bioorg Med Chem Lett. 2010;20(19):5753–5756. doi: 10.1016/j.bmcl.2010.07.138. [DOI] [PubMed] [Google Scholar]
- 27.Jamieson C, Campbell RA, Cumming IA, Gillen KJ, Gillespie J, Kazemier B, Kiczun M, Lamont Y, Lyons AJ, Maclean JK, Martin F, et al. A novel series of positive modulators of the AMPA receptor: Structure-based lead optimization. Bioorg Med Chem Lett. 2010;20(20):6072–6075. doi: 10.1016/j.bmcl.2010.08.063. [DOI] [PubMed] [Google Scholar]
- 28.Jamieson C, Maclean JK, Brown CI, Campbell RA, Gillen KJ, Gillespie J, Kazemier B, Kiczun M, Lamont Y, Lyons AJ, Moir EM, et al. Structure based evolution of a novel series of positive modulators of the AMPA receptor. Bioorg Med Chem Lett. 2011 doi: 10.1016/j.bmcl.2010.11.098. [DOI] [PubMed] [Google Scholar]
- 29**.Pirotte B, Francotte P, Goffin E, de Tullio P. AMPA receptor positive allosteric modulators: A patent review. Expert Opinion on Therapeutic Patents. 2013;23(5):615–628. doi: 10.1517/13543776.2013.770840. Thorough review with information on patents for positive modulators that is not available through reading published papers; nice insight into the big picture of AMPAR drug discovery. [DOI] [PubMed] [Google Scholar]
- 30.Ward SE, Bax BD, Harries M. Challenges for and current status of research into positive modulators of AMPA receptors. Br J Pharmacol. 2010;160(2):181–190. doi: 10.1111/j.1476-5381.2010.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar J, Mayer ML. Functional insights from glutamate receptor ion channel structures. Annual Review of Rhysiology. 2013;75:313–337. doi: 10.1146/annurev-physiol-030212-183711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armstrong N, Sun Y, Chen GQ, Gouaux E. Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature. 1998;395:913–917. doi: 10.1038/27692. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, Olson R, Horning M, Armstrong N, Mayer ML, Gouaux E. Mechanism of glutamate receptor desensitization. Nature. 2002;417:245–253. doi: 10.1038/417245a. [DOI] [PubMed] [Google Scholar]
- 34**.Durr KL, Chen L, Stein RA, De Zorzi R, Folea IM, Walz T, McHaourab HS, Gouaux E. Structure and dynamics of AMPA receptor GluA2 in resting, pre-open, and desensitized states. Cell. 2014;158(4):778–792. doi: 10.1016/j.cell.2014.07.023. The most important current article on AMPAR strcuture, function and modulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35**.Green T, Nayeem N. The multifaceted subunit interfaces of ionotropic glutamate receptors. The Journal of Physiology. 2014 doi: 10.1113/jphysiol.2014.273409. Outstanding and accessible review of the different ways in which subunit interfaces regulate glutamate receptor activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyerson JR, Kumar J, Chittori S, Rao P, Pierson J, Bartesaghi A, Mayer ML, Subramaniam S. Structural mechanism of glutamate receptor activation and desensitization. Nature. 2014;514(7522):328–334. doi: 10.1038/nature13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sommer B, Keinänen K, Verdoorn TA, Wisden W, Burnashev N, Herb A, Köhler M, Takagi T, Sakmann B, Seeburg PH. Flip and flop: A cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990;249:1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- 38.Partin KM, Fleck MF, Mayer ML. AMPA receptor flip/flop mutants affecting deactivation, desensitization and modulation by cyclothiazide, aniracetam and thiocyanate. Journal of Neuroscience. 1996;16:6634–6647. doi: 10.1523/JNEUROSCI.16-21-06634.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ptak CP, Ahmed AH, Oswald RE. Probing the allosteric modulator binding site of GluR2 with thiazide derivatives. Biochemistry. 2009;48(36):8594–8602. doi: 10.1021/bi901127s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed AH, Oswald RE. Piracetam defines a new binding site for allosteric modulators of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors. J Med Chem. 2010;53(5):2197–2203. doi: 10.1021/jm901905j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weeks AM, Harms JE, Partin KM, Benveniste M. Functional insight into development of positive allosteric modulators of AMPA receptors. Neuropharmacology. 2014;85:57–66. doi: 10.1016/j.neuropharm.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stern-Bach Y, Russo S, Neuman M, Rosenmund C. A point mutation in the glutamate binding site blocks desensitization of AMPA receptors. Neuron. 1998;21:907–918. doi: 10.1016/s0896-6273(00)80605-4. [DOI] [PubMed] [Google Scholar]
- 43.Horning MS, Mayer ML. Regulation of AMPA receptor gating by ligand binding core dimers. Neuron. 2004;41:379–388. doi: 10.1016/s0896-6273(04)00018-2. [DOI] [PubMed] [Google Scholar]
- 44.Furukawa H, Singh SK, Mancusso R, Gouaux E. Subunit arrangement and function in NMDA receptors. Nature. 2005;438(7065):185–192. doi: 10.1038/nature04089. [DOI] [PubMed] [Google Scholar]
- 45.Norholm AB, Francotte P, Olsen L, Krintel C, Frydenvang K, Goffin E, Challal S, Danober L, Botez-Pop I, Lestage P, Pirotte B, et al. Synthesis, pharmacological and structural characterization, and thermodynamic aspects of GluA2-positive allosteric modulators with a 3,4-dihydro-2h-1,2,4-benzothiadiazine 1,1-dioxide scaffold. J Med Chem. 2013;56(21):8736–8745. doi: 10.1021/jm4012092. [DOI] [PubMed] [Google Scholar]
- 46.Ptak CP, Hsieh CL, Weiland GA, Oswald RE. Role of stoichiometry in the dimer-stabilizing effect of AMPA receptor allosteric modulators. ACS Chemical Biology. 2014;9(1):128–133. doi: 10.1021/cb4007166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whiteaker KL, Sullivan JP, Gopalakrishnan M. Cell-based assays using the fluorometric imaging plate reader (FLIPR) In: Enna SJ, editor. Current Protocols in Pharmacology. Unit 9. Chapter 9. 2001. p. 2. [DOI] [PubMed] [Google Scholar]
- 48.Bouteiller JM, Allam SL, Hu EY, Greget R, Ambert N, Keller AF, Bischoff S, Baudry M, Berger TW. Integrated multiscale modeling of the nervous system: Predicting changes in hippocampal network activity by a positive AMPA receptor modulator. IEEE Transactions on Bio-Medical Engineering. 2011;58(10):3008–3011. doi: 10.1109/TBME.2011.2158605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robert A, Armstrong N, Gouaux JE, Howe JR. AMPA receptor binding cleft mutations that alter affinity, efficacy, and recovery from desensitization. J Neurosci. 2005;25(15):3752–3762. doi: 10.1523/JNEUROSCI.0188-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.Zagrebelsky M, Korte M. Form follows function: BDNF and its involvement in sculpting the function and structure of synapses. Neuropharmacology. 2014;76(Pt C):628–638. doi: 10.1016/j.neuropharm.2013.05.029. Very good review of the most current data supporting a critical function for BDNF in synaptic plasticity; accessible for the non-specialist. [DOI] [PubMed] [Google Scholar]
- 51.Kramar EA, Chen LY, Lauterborn JC, Simmons DA, Gall CM, Lynch G. BDNF upregulation rescues synaptic plasticity in middle-aged ovariectomized rats. Neurobiology of Aging. 2012;33(4):708–719. doi: 10.1016/j.neurobiolaging.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yefimenko N, Portero-Tresserra M, Marti-Nicolovius M, Guillazo-Blanch G, Vale-Martinez A. The AMPA receptor modulator S18986 in the prelimbic cortex enhances acquisition and retention of an odor-reward association. Neurosci Lett. 2013;548:105–109. doi: 10.1016/j.neulet.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 53.Mala H, Chen Y, Worm VH, Kure J, Kaae BH, Madsen U, Badolo L, Pickering DS, Mogensen J. Cognitive enhancing effects of an AMPA receptor positive modulator on place learning in mice. Behavioural Brain Research. 2012;226(1):18–25. doi: 10.1016/j.bbr.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 54.Adler LA, Kroon RA, Stein M, Shahid M, Tarazi FI, Szegedi A, Schipper J, Cazorla P. A translational approach to evaluate the efficacy and safety of the novel AMPA receptor positive allosteric modulator org 26576 in adult attention-deficit/hyperactivity disorder. Biological Psychiatry. 2012;72(11):971–977. doi: 10.1016/j.biopsych.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 55.Baudry M, Kramar E, Xu X, Zadran H, Moreno S, Lynch G, Gall C, Bi X. Ampakines promote spine actin polymerization, long-term potentiation, and learning in a mouse model of Angelman syndrome. Neurobiology of Disease. 2012;47(2):210–215. doi: 10.1016/j.nbd.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Silverman JL, Oliver CF, Karras MN, Gastrell PT, Crawley JN. Ampakine enhancement of social interaction in the btbr mouse model of autism. Neuropharmacology. 2013;64:268–282. doi: 10.1016/j.neuropharm.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fan W, Wu X, Pan Y, Li C, Niu Y, Zhai Y, Mei Q. 1-(1,3-benzodioxol-5-yl-carbo-nyl) piperidine, a modulator of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor, ameliorates exercise-induced fatigue in mice. Biological & Pharmaceutical Bulletin. 2014;37(1):13–17. doi: 10.1248/bpb.b13-00399. [DOI] [PubMed] [Google Scholar]
- 58.Black MD. Therapeutic potential of positive AMPA modulators and their relationship to AMPA receptor subunits. A review of preclinical data. Psychopharmacology (Berl) 2005;179(1):154–163. doi: 10.1007/s00213-004-2065-6. [DOI] [PubMed] [Google Scholar]
- 59.Bowie D. Ionotropic glutamate receptors & cns disorders. CNS & neurological disorders drug targets. 2008;7(2):129–143. doi: 10.2174/187152708784083821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lynch G, Palmer LC, Gall CM. The likelihood of cognitive enhancement. Pharmacol Biochem Behav. 2011;99(2):116–129. doi: 10.1016/j.pbb.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Machado-Vieira R, Ibrahim L, Henter ID, Zarate CA., Jr Novel glutamatergic agents for major depressive disorder and bipolar disorder. Pharmacol Biochem Behav. 2012;100(4):678–687. doi: 10.1016/j.pbb.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Menniti FS, Lindsley CW, Conn PJ, Pandit J, Zagouras P, Volkmann RA. Allosteric modulators for the treatment of schizophrenia: Targeting glutamatergic networks. Current Topics in Medicinal Chemistry. 2013;13(1):26–54. doi: 10.2174/1568026611313010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nations KR, Bursi R, Dogterom P, Ereshefsky L, Gertsik L, Mant T, Schipper J. Maximum tolerated dose evaluation of the AMPA modulator ORG 26576 in healthy volunteers and depressed patients: A summary and method analysis of bridging research in support of phase ii dose selection. Drugs in R&D. 2012;12(3):127–139. doi: 10.2165/11634360-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nations KR, Dogterom P, Bursi R, Schipper J, Greenwald S, Zraket D, Gertsik L, Johnstone J, Lee A, Pande Y, Ruigt G, et al. Examination of org 26576, an AMPA receptor positive allosteric modulator, in patients diagnosed with major depressive disorder: An exploratory, randomized, double-blind, placebo-controlled trial. Journal of Psychopharmacology. 2012;26(12):1525–1539. doi: 10.1177/0269881112458728. [DOI] [PubMed] [Google Scholar]
- 65.Boyle J, Stanley N, James LM, Wright N, Johnsen S, Arbon EL, Dijk DJ. Acute sleep deprivation: The effects of the ampakine compound cx717 on human cognitive performance, alertness and recovery sleep. Journal of Psychopharmacology. 2012;26(8):1047–1057. doi: 10.1177/0269881111405353. [DOI] [PubMed] [Google Scholar]
- 66**.Lynch G, Cox CD, Gall CM. Pharmacological enhancement of memory or cognition in normal subjects. Frontiers in Systems Neuroscience. 2014;8(90) doi: 10.3389/fnsys.2014.00090. Thoughtful, forward-looking view into how the field needs to provide some discipline in its thinking of meaningful tests of potential modulators on human behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]