Abstract
Use of personal care products is widespread in the United States but tends to be greater among African Americans than whites. Of special concern is the possible hazard of absorption of chemicals with estrogenic activity (EA) or anti-EA (AEA) in these products. Such exposure may have adverse health effects, especially when it occurs during developmental windows (e.g., prepubertally) when estrogen levels are low. We assessed the ethanol extracts of eight commonly used hair and skin products popular among African Americans for EA and AEA using a cell proliferation assay with the estrogen sensitive MCF-7:WS8 cell line derived from a human breast cancer. Four of the eight personal care products tested (Oil Hair Lotion, Extra-dry Skin Lotion, Intensive Skin Lotion, Petroleum Jelly) demonstrated detectable EA, whereas three (Placenta Hair Conditioner, Tea-Tree Hair Conditioner, Cocoa Butter Skin Cream) exhibited AEA. Our data indicate that hair and skin care products can have EA or AEA, and suggest that laboratory studies are warranted to investigate the in vivo activity of such products under chronic exposure conditions as well as epidemiologic studies to investigate potential adverse health effects that might be associated with use of such products.
Keywords: cosmetics, endocrine disruptors, estrogen receptor binding, estrogenic activity, anti-estrogenic activity, personal care products
INTRODUCTION
Personal care products are a 40 billion dollar industry in the United States, whereas worldwide, the industry is worth more than $200 billion.1 Environmental and human health concerns regarding these products are increasing,2,3 but they do not fall under regulatory scrutiny in the United States unless a health claim is made for a product or damage to the environment warrants investigation.
Personal care products enter the environment in a number of ways including down-the-drain disposal, leaching from landfills, and direct dispersal (e.g., sunscreen wash-off from swimmers). Components of these products are now among the most commonly detected trace chemicals (in the nanogram to microgram per liter range) in surface water4,5 and groundwater.6 As some ingredients of personal care products degrade slowly and some ingredients concentrate in lipids, these substances have the potential to bioaccumulate in the environment, thus indirectly exposing wildlife and humans.4,7 However, substantially higher exposures to humans would be expected from personal use of these products than from environmental exposures, and reports linking direct exposures to adverse health effects have begun to appear. Use of products with estriol concentrations ranging from 9 to 24 mg/g8–13 or containing components with estrogenic or antiandrogenic activity has been associated with premature sexual development and prepubertal gynecomastia in case reports. More extensive observational studies have indicated a relationship between self-reported product use and hormonally mediated outcomes.10,13
In the United States, African Americans report heavier use of many personal care products compared with whites,10,14,15 and may therefore be at higher risk for any possible adverse health effects. National Health and Nutrition Examination Survey (NHANES) data show that African Americans have higher levels of urinary parabens16 and monoethyl phthalate, a metabolite of diethyl phthalate;17 these chemicals are common ingredients in personal care products.18 The ethnic disparity may be especially marked for children, with black children exhibiting far higher levels compared with white children for the two most commonly detected parabens in NHANES.16
Of most concern are the potential endocrine disrupting effects of these products, given that some may contain endogenous estrogens19 or ingredients found to have estrogenic effects.20 However, estrogenic (or anti-estrogenic) effects of the personal care products as commercial mixtures have not been evaluated. In this study, we used an in vitro roboticized version of the E-SCREEN assay that quantifies estrogen receptor (ER)-mediated proliferation of breast cancer MCF-7 cells21 to perform a hazard assessment of ethanol extracts of eight off-the-shelf hair and skin care products that are popular among African American women for estrogenic activity (EA) or anti-EA (AEA).
MATERIALS AND METHODS
Cell lines, Media, and Products
MCF-7:WS8 cells were a gift from Dr. V. Craig Jordan (Northwestern University, Chicago, IL, USA). In this cell line, > 90% of ER is ERα and the remaining < 10% is ERβ. The expression level of GPR30, a membrane bound ER that has been associated with reduction in cell growth, is unknown.22 Chemicals having EA bind to ERs, producing genomic estrogen activation if EA or inhibiting activation if AEA, thereby stimulating MCF-7 cell proliferation or suppressing the ability of E2 to stimulate MCF-7 cell proliferation, respectively.21,23,24 We use the MCF-7 EA assay to evaluate the ability of extracts to stimulate cell proliferation, relative to an E2-positive control. Cell proliferation by a test substance is verified as via ER by the ability of ICI 182,780, a well-known ER antagonist, to interfere with test substance-induced cell proliferation (i.e., to displace EA-containing chemicals from binding to ERs). The AEA MCF-7 assay examines if extracts decrease MCF-7 cell proliferation induced by lower, non-saturating (2.0 × 10−12 M) and higher, saturating (2.0 × 10−9 M) concentrations of E2, relative to the decrease in cell proliferation produced by ICI 182,780, used as the AEA-positive control. As reported by Yang et al.,24 these MCF-7 EA and AEA assays have a 100% concordance with classifications for a set of ICCVAM meta-analyses of test chemicals and are at least as accurate as the validated test methods accepted by the US Environmental Protection Agency and the Organisation for Economic Cooperation and Development. This assay is very sensitive with a half-maximal response (EC50) to E2 of ~1 × 10−12 M E2.24
Media used to initially grow and maintain the cells was RPMI-1640 (Roswell Park Memorial Institute, Buffalo, NY, USA) with non-essential amino acids, 10 µg/ml phenol red, 4 mM l-glutamine, 6 ng/ml insulin, 100 units/ml penicillin, 100 µg/ml streptomycin, and 10% fetal bovine serum (FBS). Media and media supplements were purchased from Invitrogen (Grand Island, NY, USA). 17β-Estradiol (E2) was purchased from Sigma-Aldrich (St Louis, MO, USA) and ICI 182,780 (ICI) was purchased from Tocris Bioscience (Minneapolis, MN, USA). EA and AEA assays were performed in estrogen-free medium (EFM) that is modified from cell maintenance media by replacing 10% FBS with 1% charcoal-stripped FBS and 4% charcoal-stripped calf serum, and substituting phenol red-containing RMPI-1640 with phenol red-free RPMI-1640. The vehicle control (VC) was 1% ethanol (EtOH) in EFM.
As previously described,23,24 every 2–3 months, stocks were regrown from the same MCF-7 primary source, and cells were seeded onto 96-well plates at 5000 cells/well in 200 µl EFM in 96-well plates and incubated for 72 hours. Cells were then treated with E2 or a test extract for 6 days and solutions were changed every other day. Each dilution of E2 or an extract was placed in three wells in different regions of 96-well plates. Data points on graphs are the (normalized) EA ± the standard deviation (SD) for those three wells (see below). The (normalized) VC mean and SD were calculated from all VC wells of a given assay run. Variation of the same dilution of E2 or an extract for wells within a single run was usually so small that the error bars showing the SD were typically hidden by the symbol showing the mean value.
Cell proliferation was assessed by a modified diphenylamine (DPA) reaction25,26 to measure the amount of DNA in each well containing VC, VC + a given concentration of test extract, or VC + a given E2 concentration, using a Bio-Tek PowerWavex 96-well plate reader spectrophotometer, as previously described.23,24 The DNA content in each well was compared with a DNA standard curve using this DPA reaction after incubating the 96-well plates and DNA standard plate 24 h at 37 °C by measuring the absorbance as the difference between OD590 and OD690 with a Bio-Tek ELx 808 plate reader. Each experimental run had a DNA standard curve for calibration. The absorbance was converted to µg DNA/well by a third degree polynomial curve fit of the DNA standard curve.27 The DNA standard curve fit typically had a correlation coefficient >0.99 and accurately estimates the number of MCF-7 cells.23,24
Personal Care Product Protocols
Eight personal care products were identified as popular among young African American women who participated in focus groups in preparation for conducting a study of uterine fibroids (Study of Environment, Lifestyle & Fibroids (www.detroitself.org)). These included four hair care products (HP1: Hair Relaxer, HP2: Placenta Hair Conditioner, HP3: Tea-Tree Hair Conditioner, HP4: Oil Hair Lotion) and four skin care products (SP1: Cocoa Butter Skin Cream, SP2c and SP2y: Extra-dry Skin Lotion, SP3: Intensive Skin Lotion, SP4: Petroleum Jelly); these are general product terms and not the actual product names. Products were purchased from retailers in Raleigh–Durham, NC, USA. Each product was assigned an assay number upon receipt and ingredient information from the label was recorded (Supplementary Table). The products were stored at room temperature as recommended by their manufacturer before testing.
Extraction Procedures
A small aliquot of each product was weighed in a 12 ml glass tube and 100% EtOH was added at a ratio of 1 ml to 1 g product. Each mixture in the glass tube was vortexed, the tubes were placed on a shaker for 2–3 h at room temperature, and then centrifuged at 445 g for 10 min at room temperature, typically producing a pellet of unextracted material and a transparent phase. A transparent phase at the initial g/ml concentration could not be obtained for three of the eight samples (HP2, HP3 (hair conditioners), SP4 (petroleum jelly)). For these three samples, additional EtOH was added so that the final concentration was 0.5 g product/ml and the initial dilution factor was twice that of the other products. One sample (SP2 (Extra-dry Skin Lotion)) separated into a transparent colorless (SP2c) and transparent yellow liquid (SP2y) phase plus a pellet. The liquid phase of each extract was transferred into a 1.5 ml tube, centrifuged at 15,700 g for 15 min at room temperature, and the transparent liquid phases were transferred to a 1.5 ml tube before diluting and testing for EA and AEA.
Protocols for EA and AEA Assays
Protocols for the EA and AEA assays were as previously described23 using a robotic version of the MCF-7 E-SCREEN cell proliferation assay.21 The positive control was an E2 dose–response curve and the extract vehicle was the negative control. An EpMotion 5070 robotic workstation (Eppendorf, Hamburg, Germany) was used to seed cells in 96-well plates, prepare serial dilutions of the test chemicals in tissue-culture medium, change media, and collect samples for the DNA quantification assays. DNA quantification, based on the DPA reaction, was conducted using a Bio-Tek PowerWavex 96-well plate reader spectrophotometer.
For the EA assay, aliquots of extracts were diluted 100× with EFM to obtain the first (highest) test concentration of 0.01 g/ml for all but three of the samples. The HP2, HP3, and SP4 samples, which were further diluted in the extraction phase, started at a test concentration of 0.005 g/ml. The second through sixth test concentrations were obtained by a threefold serial dilution with the VC (e.g., the second testing concentration was obtained by mixing two volumes of VC and one volume of the first testing concentration). For the AEA assay, aliquots of extracts were diluted 100× with 4.0 × 10−12 M E2 (low E2) in EFM to obtain the first test concentration and then serially diluted in Low E2 to produce the second through sixth test concentrations. Each extract concentration was tested in triplicate or quadruplicate on 96-well plates containing MCF-7 cells in EFM. Cells were fed on Day 0 and fresh medium was added with the test extracts every other day up to Day 6. After 6 days of exposure, the amount of DNA/well, an indication of cell proliferation, was assayed using a microplate modification of the Burton DPA assay.25,26
Calculation of EA
The EA of extracts was calculated as the relative maximum %E2 (%RME2, a measure of response amplitude), a percentage of the maximum DNA/well produced by an extract at any dilution relative to the maximum DNA/well produced by E2 (positive control) at any dilution, adjusted by the DNA response to the vehicle (negative) control according to Equation 1:
The EA of a test extract was considered detectable if it produced cell proliferation greater than 15% RME2, a value greater than 3 SDs of the VC.23
Stimulation of MCF-7 proliferation by an extract was confirmed to be an estrogenic effect (versus non-specific effects) by an EA confirmation assay. If the stimulation of MCF-7 proliferation by an extract was suppressed at least 2SD by co-incubation with 1.0 × 10−8 M ICI (a strong anti-estrogen), then the EA of the extract was confirmed. That is, the unsuppressed and ICI-suppressed data points were significantly different at P < 0.05. An extract was classified as not having detectable EA if it did not induce MCF-7 cell proliferation greater than 15% RME2, or if it induced proliferation that could not be inhibited by ICI.
For any EA assay run of an extract to be acceptable, the VC, E2 induction, half-maximum response of E2 (EC50), and E2 R2 had to meet strict acceptance criteria. That is, the mean DNA µg/well for the VC in this experiment was no more than the laboratory historical VC mean + 3SD (0.13 µg/well + 3(0.04 µg/well) = 0.25 µg DNA/well). The maximum proliferation induced by E2 (positive control) was at least 3 times the VC proliferation. The E2 EC50 was no more than the historical E2 EC50 mean + 3SD (3.38 × 10−12M + 3(2.99 × 10−12 M) = 1.2 × 10−11 M). Lastly, the E2 concentration–response curve had a coefficient of determination (E2 R2) ≥0.9 as calculated by Hill kinetics.27
Calculation of AEA
The AEA of extracts was calculated according to Equation 2 as the relative maximum %ICI (% RMICILow E2), a measure of response amplitude. %RMICILow E2 is interpreted as the degree to which the extract reduced cell proliferation relative to the inhibition of cell proliferation produced by ICI (a strong anti-estrogen; positive control).
Low E2 was set at 4.0 × 10−12 M E2, which was historically equal to 60–80% of the maximum E2 response (EC60–EC80) in our MCF-7 assay. The concentration of ICI that produced the maximum reduction of low E2-induced cell proliferation (i.e., the least µg DNA/well) was defined as RMICILow E2. %RMICILow E2 was normalized to 100% for each experimental run. The DNA value for low E2 stimulation (without ICI or test chemical) was normalized to 0%. The AEA of a test extract was considered detectable if it suppressed low E2-stimulated cell proliferation by at least 3SD for at least one dilution of the extract (i.e., %RMICILow E2 for an extract≥ 3SD of low E2 for at least one dilution tested).
Suppression of low E2-stimulated MCF-7 proliferation induced by an extract was confirmed to be anti-estrogenic (rather than non-specific cytotoxicity) in an AEA “confirmation” assay.24 If the suppression of MCF-7 proliferation by an extract (in the presence of low E2) was reversed by at least 2SD after co-incubation with 2.0 × 10−9 M E2 (high E2), the AEA of the extract was confirmed. That is, to confirm AEA, the 2SD error bars of low E2 and high E2 curves for an extract must not overlap. An extract was classified as not having detectable AEA if it did not suppress E2-stimulated MCF-7 cell proliferation, or if it suppressed E2-stimulated cell proliferation that could not be reversed by high E2.
For an AEA assay of an extract to be acceptable, the acceptance criteria had to be met for the VC as described for the EA Assay. In addition, ICI had to reduce the low E2 proliferation by at least 1.5 times, calculated by dividing DNALow E2 by the least value of DNAICI in Low E2. Low E2 (µg DNA/well) induction also had to be within three times the mean + SD of our historical low E2 (0.65 µg/well + 3(0.20 µg/well) = 1.3 µg/well). Finally, the ICI concentration–response curve had to have a negative slope that included at least one data point on its linear portion.
Cytotoxicity Test
After cell treatment with any extract, and before removing cell culture medium in each confirmation assay for EA or AEA described above, cell viability was scored by visual inspection as evaluated and approved by the Interagency Coordinating Committee on the Validation of Alternative Methods as part of the MCF-7 validation process.24 Normal cell morphology was scored as one, low cytotoxicity (<50% of cells with altered morphology) was scored as two, moderate cytotoxicity (50% to <90% of cells with altered morphology) was scored as three, and high cytotoxicity (few or no cells visible) was scored as four. If an extract dilution had a cytotoxicity score of ≥2, that data point was excluded from data analyses (i.e., no cytotoxicity was acceptable). The presence of cytotoxicity in this proliferation-based assay to detect EA can significantly affect the EA–response curve to reduce EA detection and could also result in a false-positive assessment for AEA. However, as described above, we performed AEA confirmation assays of products that reduced E2-induced MCF-7 proliferation to avoid a false-positive AEA conclusion.
RESULTS
EA Assays of Personal Care Products
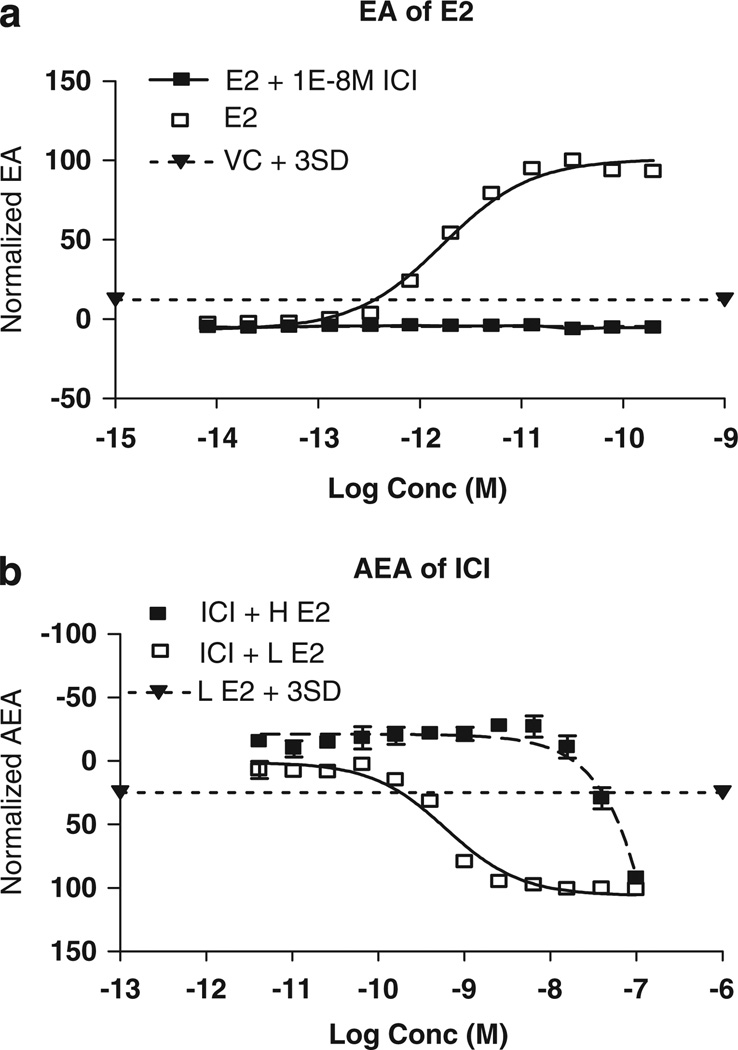
A sample dose–response curve for the positive control (E2) is shown in Figure 1a, illustrating a typical sigmoidal cell proliferation response (y axis) to increasing concentrations of E2 (x axis) that is subsequently suppressed by the anti-estrogen ICI. The VC (1% EtOH in EFM) shows no cell proliferation. A positive and VC were included for each assay run, and the maximum normalized cell proliferation with E2 was set to 100% RME2 and the normalized proliferation with the VC was set to 0% RME2. The response of the test sample was evaluated as a percent of RME2 (plotted as normalized EA).
Figure 1.
(a) Log concentration (moles, abscissa) versus normalized EA (%, ordinate) calculated as given in Equation 1 for E2 as the positive control. Each data point and associated error bars in this and other figures is the normalized mean ± SD of three wells on the same plate. (SD is often so small that error bars are hidden by the symbol plotting the mean.) Unfilled squares and solid line: concentration–response curve for E2. Filled squares and solid line: concentration–response curve for E2 plus 10−8 M ICI to confirm that estrogenic activity is suppressed by an inhibitor of estrogen receptor binding. Filled downward triangle joined by dotted line: VC + 3SD. (b) Log concentration (moles, abscissa) versus normalized AEA (%, ordinate) calculated as given in Equation 2 for ICI as the positive control. Unfilled squares and solid line: concentration–response curve for ICI plus low (4 × 10−12 M) E2. Filled squares and solid line: concentration–response curve for ICI plus high (10−9 M) E2 to confirm that any suppression of estrogenic activity in low E2 is reversed by high E2. Filled downward triangles joined by dotted line: low E2 + 3SD.
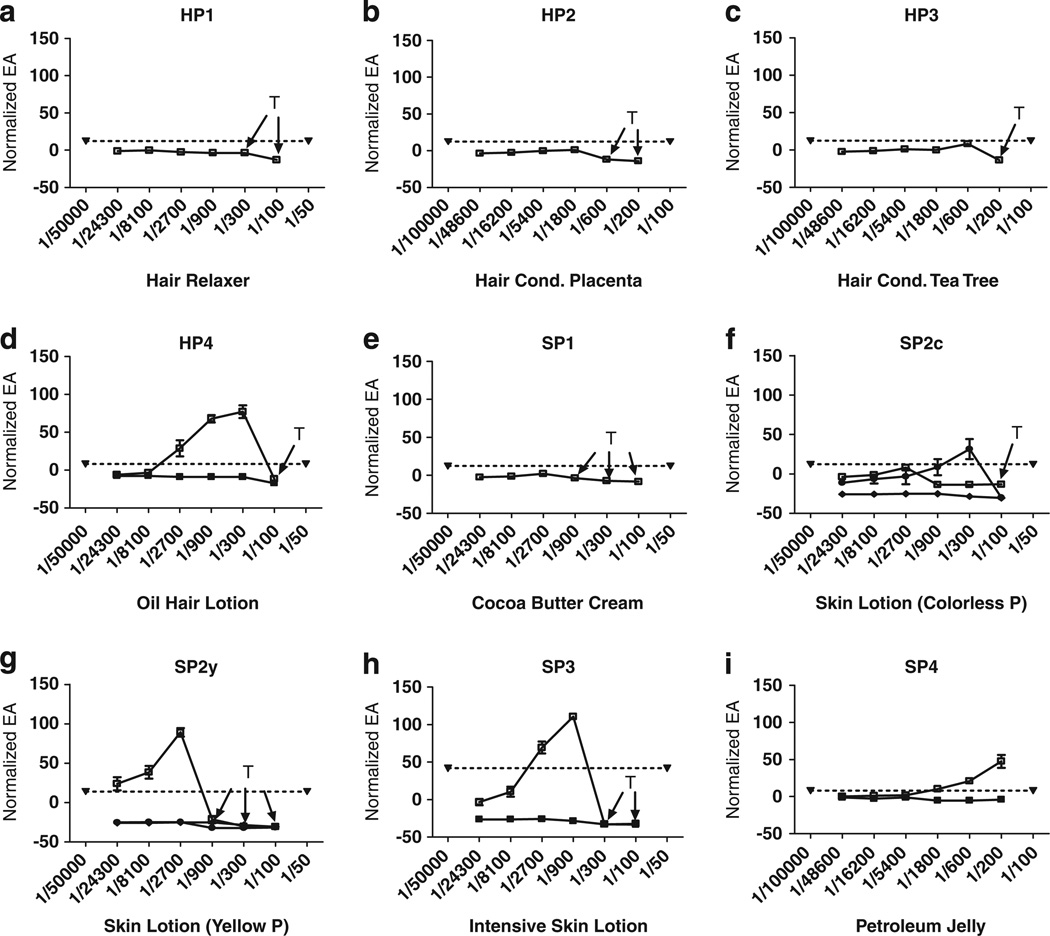
Figure 2 shows sample EA dose–response curves for the nine extracts of eight hair (HP1-4) and skin products (SP1-4; SP2 had a transparent colorless (SP2c) and transparent yellow (SP2y) extract and both were analyzed). Positive EA is seen for HP4 (Figure 2d) and all but one (SP1) of the four skin care products (Figure 2f–i). All extracts were tested at least twice for EA, and the maximum response level (Normalized EA) for each separate run is listed in Table 1. Five of the nine extracts demonstrated EA, and the runs for an individual product showed consistent presence or absence of EA with one exception: SP2c exhibited EA in two trials and no detectable EA in a third trial (a trial showing no activity and one of those showing EA are shown in Figure 2f). The level of Normalized EA was consistently above 40 for HP4, SP2y, and SP4 (Table 1). Although SP3 exhibited some EA, it was below 40 in all but one of the runs. The highest dilution (i.e. lowest concentration) exhibiting EA varied from 1/24,300 (SP2y) to 1/1,800 (SP4).
Figure 2.
(a–i) Dilution–response curves for EA for each of four Hair or four Skin Product extracts as indicated, based on the normalized mean ± SD of three wells on the same plate. (SD is often so small that error bars are hidden by the symbol plotting the mean). Ordinate: normalized EA response (%RME2); Abcissa: extraction dilution. Panels (d, f–i) show confirmation assay for products exhibiting detectable EA. Filled downward triangle joined by dotted line: VC + 3SD. Unfilled square and solid line: concentration–response curve for extracts. Filled squares and solid line: concentration–response curve for the extract plus 10−8 M ICI to confirm that estrogenic activity of the extract is suppressed by an inhibitor of estrogen receptor binding. T: concentrations exhibiting cytotoxicity by visual observations.
Table 1.
Estrogenic activity (EA) and anti-estrogenic activity (AEA) of eight personal care products.
| Product code | Product description | Normalized EA (%RME2) | Normalized AEA (%RMICI) |
|---|---|---|---|
| Hair products (HP) | |||
| HP1 | Hair relaxer | ND, ND, ND | ND |
| HP2 | Hair conditioner with placenta and jojoba oil | ND, ND, ND | 63 |
| HP3 | Hair conditioner with tea-tree oil | ND, ND, ND | 79 |
| HP4 | Oil hair lotion | 50, 77 | ND |
| Skin products (SP) | |||
| SP1 | Cocoa butter cream | ND, ND | 52 |
| SP2c | Extra-dry skin lotion (colorless phase) | ND, 16, 77 | ND |
| SP2y | Extra-dry skin lotion (yellow phase) | 43, 89 | ND |
| SP3 | Intensive skin lotion | 19, 16, 111, 37, 24 | ND |
| SP4 | Petroleum jelly | 64, 48, 77, 54, 43 | ND |
Abbreviation: ND, not detected, refers to individual assay results that did not meet the criteria for EA or AEA.
The extra-dry skin lotion sample produced two extracts differing by color.
ER-mediated EA is confirmed by ICI suppression of MCF-7 cell proliferation. Products having detectable EA were subjected to this confirmation assay, and suppression was demonstrated in all cases of EA (see sample curves showing inhibition by ICI in Figure 2d, f–i). That is, cell proliferation induced by an extract of any product was confirmed to be due to ER binding rather than to any non-specific mechanism.
AEA Assays of Personal Care Products
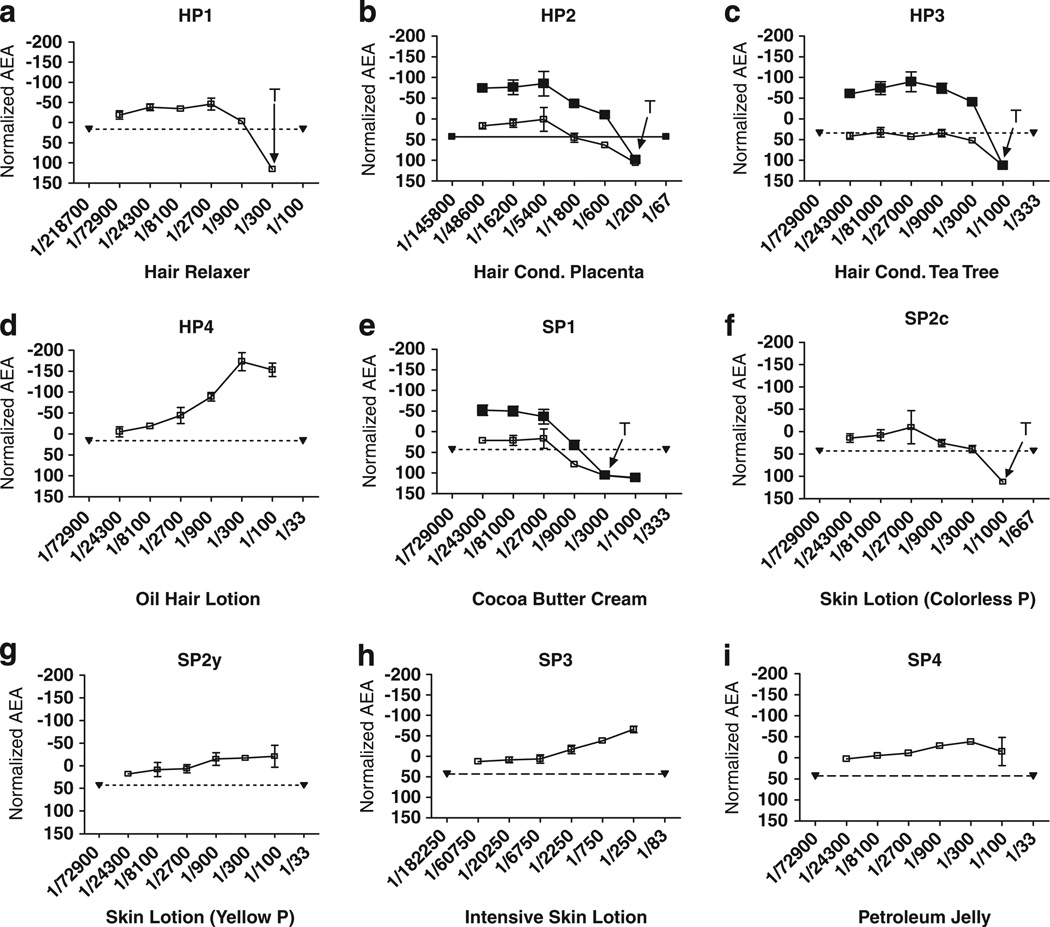
The response pattern of AEA for the positive control, ICI, is shown in Figure 1b. The concentration-dependent response for ICI (in low E2) shows a descending curve, illustrating the inhibition of E2-induced cell proliferation as ICI concentration increases. This suppression of cell proliferation illustrates the dose-dependent increases in AEA, plotted from 0–100 as normalized AEA (%RMICI). High concentration E2 (ICI + high E2) reverses this inhibition.
The hair and skin product extracts were similarly examined for each extract’s ability to suppress the proliferation of MCF-7 cells produced by low E2. Figure 3 shows dilution–response curves for all extracts, plotted as normalized AEA. Both hair conditioners (HP2 & HP3) as well as the Cocoa Butter Skin Cream (SP1) exhibited AEA (Figure 3b, c, and e). The three products having detectable AEA were confirmed because high E2 produced a recovery of cell proliferation of at least 2SD. That is, suppression of cell proliferation induced by an extract was indeed due to binding of E2 to ERs, rather than non-specific suppression due to cytotoxic or other effects. The level of normalized AEA for each of the three products was from 52% to 79% (Table 1).
Figure 3.
(a–i) Dilution–response curves for AEA for each of four Hair or four Skin Product extracts, based on the normalized mean ± SD of three wells on the same plate. (SD is often so small that error bars are hidden by the symbol plotting the mean). Ordinate: normalized AEA response (%RMICI); Abcissa: extraction dilution. Panels b, c & e show AEA Confirmation Assays for these products having detectable AEA. Filled downward triangles joined by dotted line: low E2 + 3SD. Unfilled squares and solid line: AEA concentration–response curve for extracts plus low E2. Filled squares and solid line: concentration–response curve for extract plus high E2 to confirm that anti-estrogenic activity of the extract is reversed by adding a higher concentration of E2. T: concentrations exhibiting cytotoxicity by visual observations.
The normalized AEA curves for the hair and skin products that had exhibited EA are also noteworthy. For these products, the cell proliferation tended to increase with increasing concentrations (lower dilutions) (Figure 3d, f–i). This indicates that the proliferative effects of the low E2 and the proliferative effects of the personal care product tended to be additive.
DISCUSSION
To our knowledge, this is the first report of tests for EA and AEA of off-the-shelf hair and skin care products, rather than selected single ingredients of such products. Seven of eight tested products exhibited EA or AEA. No product exhibited both EA and AEA.
Except for SP4 (Petroleum Jelly), all products had lengthy ingredient lists, with 74 total listed ingredients for the eight products (Supplementary Table). To our knowledge, most of the listed ingredients have not been tested for EA, much less for AEA.28 Placenta and tea-tree oil are thought to have EA,9,15 but the two hair conditioners with these ingredients demonstrated AEA instead. Six of the eight products contained parabens (Supplementary Table), preservatives that have demonstrated varying degrees of EA depending on the type (e.g. ethyl, methyl, propyl).20,29,30 In our studies, three of these paraben-containing products showed EA and the other three showed AEA. Petroleum Jelly (SP4) exhibited moderate EA in our tests and it was among the top-five ingredients in four other products (HP1, HP4, SP1 and SP3). However, EA was observed only in two of the products, HP4 and SP3, whereas HP1 showed no activity and SP1 showed AEA. Thus, the EA or AEA of these products was not predictable by examining their listed ingredients.
Perhaps the most surprising finding about any one product was the EA of SP4 (Petroleum Jelly). This product is often used alone but is also commonly used in other skin care products31 and as a vehicle for dermal drug-delivery preparations.32 Petroleum jelly products are also often used on infants as low-cost therapies for common problems such as diaper rash33 and recurring nosebleeds.34 Petroleum jelly, also called petrolatum, was first produced and patented in the 1870s from a waste product of crude oil made up of a complex mixture of straight chain, branched chain, and cyclic hydrocarbons.35 It has never been synthetically reproduced but continues to be manufactured by refinement of the crude petroleum product.36 Thus, commercial petrolatum may differ among production batches and manufacturing sites. For example, when petrolatum is used as a vehicle for drug delivery, drug release can vary by manufacturer.37 Petroleum jelly has been considered to have low skin penetrance,38 but it includes cyclic hydrocarbons, and a ring structure is considered essential for EA or AEA.39 The question of petrolatum penetrance needs further study in order to evaluate the risk of endocrine disruption due to its topical application.
Given the limited number of products tested and the variety of ingredients in different personal care products, our data do not allow for generalizations about product type. In other words, the presence or absence of EA or AEA in the specific products we tested is not an indicator of EA or AEA in similar (non-tested) product types. We used ethanol as our extraction solvent and some chemicals having EA might not be soluble in ethanol. Therefore, using a single solvent as an extract might have led to false negatives, as was seen for extracts of plastic products.23 Finally, all products tested were sold with plastic packaging. Estrogen-active compounds (e.g., BPA) often leach from plastics23 and could contribute to EA detected in a personal care product. It would be of interest in a follow-up study to identify, through chemical analyses and testing of the identified peaks for EA and AEA, the active ingredient(s) in each product exhibiting significant activity. However, such an evaluation is beyond the scope of the current project and the results would not alter the significance of the finding that the extracts of several of the products examined here exhibit such activity in vitro.
One limitation of in vitro testing systems is the difficulty in extrapolating to in vivo effects in humans. The MCF-7 assay takes into account effects on cellular metabolism such as cytochrome P450 enzyme metabolism that is functional in this breast cancer cell line. However, although ERα was implicated in our observations, any potential effects due to the membrane bound estrogen receptor, GPR30, were not investigated. Nor have we evaluated possible effects of these products directly on downstream factors such as aromatase. Thus, further study with other in vitro systems could increase our understanding of the modes of action and the effects of these products. In addition, important questions about whole-body and organ-specific responses remain unanswered.
A major factor for hair and skin product effects is the extent of dermal absorption. Dermal absorption has been well studied and documented for medications that can be delivered via creams (e.g., testosterone and progesterone) or skin patches (e.g., synthetic estrogen and progestin for contraception). Absorption of specific personal care product ingredients has also been implicated. Examples include parabens, especially the short chain methyl, ethyl, and propyl parabens,16,40,41 and short chain phthalates.42 However, skin absorption of most ingredients has not been tested, and the effects of product mixtures are mostly unknown.
Few studies have examined health effects resulting from the use of personal care products. Possible increase in breast cancer risk is a concern,43–46 especially after a report that parabens were found to be present in tumor tissue at levels similar to those found to induce uterine growth in a rodent model.47 Furthermore, a recent study indicated that high urinary monoethyl phthalate levels may be related to increased breast cancer risk and suggested that it may be related to personal care product use.11 However, no study has examined breast cancer risk in relation to personal care product use. Case reports have linked exposure to personal care products and premature female sexual development,12,48 as well as gynecomastia in boys8,9,49 and men.50 But one study which measured urinary excretion of parabens in a small group of pregnant women found no significant association with anogenital distance in their male offspring.51
We are aware of only three epidemiologic studies examining personal care product use and adverse health outcomes. The first is a pilot study examining the association between hair product use in childhood and early age at menarche. Women in New York city were interviewed about their hair product use as children, using pictures of possible products to increase accuracy of self-report.10 Results showed that hair oil use as a child was most prevalent among African American women and was significantly associated with earlier menarche after adjustment for race/ethnicity and year of birth. The second study found an association between hair relaxer use and uterine fibroids among participants in the Black Women’s Health Study.13 The third study investigated the incidence of endometriosis and use of personal care products containing benzophenone-type UV filters in a population of women undergoing laparoscopy/laparotomy or pelvic imaging; women with elevated urinary levels of an estrogenic metabolite of the UV filters were more likely to be diagnosed with endometriosis.52
If the use of certain personal care products does have significant risks for EA or AEA effects, health outcomes of interest would not only include age at menarche and benign gynecologic conditions, but also include menstrual cycle patterns, fertility, and hormonally mediated cancers. Effects in males are also plausible. Perhaps the most vulnerable exposure periods would be during infancy and childhood when natural hormone levels are normally very low. The estrogenic effects of parabens alone are estimated to exceed normal endogenous EA in prepubertal girls.53 Thus, national data showing that black children have five times the urinary paraben levels seen in white children is of special concern.16
We wish to emphasize that this paper describes the use of a sensitive and accurate in vitro assay to perform a hazard analysis for chemicals having EA or AEA in eight personal care products, rather than a risk analysis. Given the many variables briefly discussed above, such as multiple potential sources to absorb or ingest substances with EA, multiple routes of excretion or metabolism, unknown synergistic or antagonistic interactions, short- versus longterm exposures to various levels of substances with EA, varying sensitivity to EA or AEA of different ethnic groups or developmental stages, etc., it is premature to conduct a risk analysis based on these results alone. However, we believe that studies like ours and recent efforts to develop methods to assess product use54,55 and evaluate biomarker associations with product use56,57 will facilitate the much needed research into effects on health outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the Divisions of Intramural Research and the National Toxicology Program of the National Institute of Environmental Health Sciences at the National Institutes of Health (NIH), and by NIH grant 1R43/44 ES014806, 01-03 to C.Z.Y. This article may be the work product of an employee or group of employees of the NIH; however, the statements, opinions or conclusions contained therein do not necessarily represent the statements, opinions or conclusions of NIH or the United States government. We thank Aimee D’Aloisio, Elizabeth Maull, and Kyla Taylor who critically reviewed an earlier draft of the manuscript.
Footnotes
CONFLICT OF INTEREST
C.Z.Y. is employed by and owns stock in CertiChem (CCi). GDB owns stock in and is a consultant CEO of CCi. All authors had freedom to design, conduct, interpret, and publish research uncompromised by any controlling sponsor All other authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Journal of Exposure Science and Environmental Epidemiology website (http://www.nature.com/jes)
REFERENCES
- 1. Business.com Inc. [cited 4 September 2012];Beauty and personal care products industry overview. 2011 Available at http://www.business.com/guides/beauty-and-personal-care-products-industry-overview-21128/ [Google Scholar]
- 2.Boxall AB, Rudd MA, Brooks BW, Caldwell DJ, Choi K, Hickmann S, et al. Pharmaceuticals and personal care products in the environment: what are the big questions? Environ Health Perspect. 2012;120:1221–1229. doi: 10.1289/ehp.1104477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daughton CG, Ternes TA. Pharmaceuticals and personal care products in the environment: agents of subtle change. Environ Health Perspect. 1999;107(Suppl 6):907–938. doi: 10.1289/ehp.99107s6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brausch JM, Rand GM. A review of personal care products in the aquatic environment: environmental concentrations and toxicity. Chemosphere. 2011;82:1518–1532. doi: 10.1016/j.chemosphere.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Murray KE, Thomas SM, Bodour AA. Prioritizing research for trace pollutants and emerging contaminants in the freshwater environment. Environ Pollut. 2010;158:3462–3471. doi: 10.1016/j.envpol.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Lapworth DJ, Baran N, Stuart ME, Ward RS. Emerging organic contaminants in groundwater: a review of sources, fate and occurrence. Environ Pollut. 2012;163:287–303. doi: 10.1016/j.envpol.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 7.Coogan MA, Edziyie RE, La Point TW, Venables BJ. Algal bioaccumulation of triclocarban, triclosan, and methyl-triclosan in a North Texas wastewater treatment plant receiving stream. Chemosphere. 2007;67:1911–1918. doi: 10.1016/j.chemosphere.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Felner EI, White PC. Prepubertal gynecomastia: indirect exposure to estrogen cream. Pediatrics. 2000;105:E55. doi: 10.1542/peds.105.4.e55. [DOI] [PubMed] [Google Scholar]
- 9.Henley DV, Lipson N, Korach KS, Bloch CA. Prepubertal gynecomastia linked to lavender and tea tree oils. New Engl J Med. 2007;356:479–485. doi: 10.1056/NEJMoa064725. [DOI] [PubMed] [Google Scholar]
- 10.James-Todd T, Terry MB, Rich-Edwards J, Deierlein A, Senie R. Childhood hair product use and earlier age at menarche in a racially diverse study population: a pilot study. Ann Epidemiol. 2011;21:461–465. doi: 10.1016/j.annepidem.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.López-Carrillo L, Hernández-Ramírez RU, Calafat AM, Torres-Sánchez L, Galván-Portillo M, Needham LL, et al. Exposure to phthalates and breast cancer risk in northern Mexico. Environ Health Perspect. 2010;118:539–544. doi: 10.1289/ehp.0901091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tiwary CM. Premature sexual development in children following the use of estrogen- or placenta-containing hair products. Clin Pediatr (Phila) 1998;37:733–739. doi: 10.1177/000992289803701204. [DOI] [PubMed] [Google Scholar]
- 13.Wise LA, Palmer JR, Reich D, Cozier YC, Rosenberg L. Hair relaxer use and risk of uterine leiomyomata in African-American women. Am J Epidemiol. 2012;175:432–440. doi: 10.1093/aje/kwr351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiwary CM, Ward JA. Use of hair products containing hormone or placenta by US military personnel. J Pediatr Endocrinol Metab. 2003;16:1025–1032. doi: 10.1515/jpem.2003.16.7.1025. [DOI] [PubMed] [Google Scholar]
- 15.Tiwary CM. A survey of use of hormone/placenta-containing hair preparations by parents and/or children attending pediatric clinics. Mil Med. 1997;162:252–256. [PubMed] [Google Scholar]
- 16.Calafat AM, Ye X, Wong LY, Bishop AM, Needham LL. Urinary concentrations of four parabens in the U.S. population: NHANES 2005–2006. Environ Health Perspect. 2010;118:679–685. doi: 10.1289/ehp.0901560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect. 2004;112:331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dodson RE, Nishioka M, Standley LJ, Perovich LJ, Brody JG, Rudel RA. Endocrine disruptors and asthma-associated chemicals in consumer products. Environ Health Perspect. 2012;120:935–943. doi: 10.1289/ehp.1104052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olson AC, Link JS, Waisman JR, Kupiec TC. Breast cancer patients unknowingly dosing themselves with estrogen by using topical moisturizers. J Clin Oncol. 2009;27:e103–e104. doi: 10.1200/JCO.2009.23.1225. [DOI] [PubMed] [Google Scholar]
- 20.Boberg J, Taxvig C, Christiansen S, Hass U. Possible endocrine disrupting effects of parabens and their metabolites. Reprod Toxicol. 2010;30:301–312. doi: 10.1016/j.reprotox.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Soto AM, Sonnenschein C, Chung KL, Fernandez MF, Olea N, Serrano FO. The E-SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environ Health Perspect. 1995;103(Suppl 7):113–122. doi: 10.1289/ehp.95103s7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ariazi EA, Brailoiu E, Yerrum S, Shupp HA, Slifker MJ, Cunliffe HE, et al. The G protein-coupled receptor GPR30 inhibits proliferation of estrogen receptor-positive breast cancer cells. Cancer Res. 2010;70:1184–1194. doi: 10.1158/0008-5472.CAN-09-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang CZ, Yaniger SI, Jordan VC, Klein DJ, Bittner GD. Most plastic products release estrogenic chemicals: a potential health problem that can be solved. Environ Health Perspect. 2011;119:989–996. doi: 10.1289/ehp.1003220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang CZ, Casey W, Stoner MA, Kollessery GJ, Wong AW, Bittner GD. A robotic MCF-7:WS8 cell proliferation assay to detect agonist and antagonist estrogenic activity. Toxicol Sci. 2014;137:335–349. doi: 10.1093/toxsci/kft250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burton K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956;62:315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Natarajan N, Shambaugh Gr, Elseth K, Haines G, Radosevich J. Adaptation of the diphenylamine (DPA) assay to a 96-well plate tissue culture format and comparison with the MTT assay. BioTechniques. 1994;17:166–171. [PubMed] [Google Scholar]
- 27.Hill AV. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J Physiol (Lond) 1910;40:iv–vii. [Google Scholar]
- 28.EPA. ACToR Database. [cited 29 March 2012];2012 Available at http://actor.epa.gov/actor/faces/ACToRHome.jsp;jsessionid=50659B59A0DD4B4E4AE067E41401B3BC
- 29.Gomez E, Pillon A, Fenet H, Rosain D, Duchesne MJ, Nicolas JC, et al. Estrogenic activity of cosmetic components in reporter cell lines: Parabens, UV screens, and musks. J Toxicol Environ Health A. 2005;68:239–251. doi: 10.1080/15287390590895054. [DOI] [PubMed] [Google Scholar]
- 30.Lemini C, Jaimez R, Avila ME, Franco Y, Larrea F, Lemus AE. In vivo and in vitro estrogen bioactivities of alkyl parabens. Toxicol Ind Health. 2003;19:69–79. doi: 10.1191/0748233703th177oa. [DOI] [PubMed] [Google Scholar]
- 31.Draelos ZD. Active agents in common skin care products. Plast Reconstr Surg. 2010;125:719–724. doi: 10.1097/PRS.0b013e3181c83192. [DOI] [PubMed] [Google Scholar]
- 32.Ogita Y, Takahashi Y, Iwata M, Sasatsu M, Onishi H, Hashimoto S, et al. Comparison of physical properties and drug-releasing characteristics of white petrolatums. Pharmazie. 2010;65:801–804. [PubMed] [Google Scholar]
- 33.Borkowski S. Diaper rash care and management. Pediatr Nurs. 2004;30:467–470. [PubMed] [Google Scholar]
- 34.Loughran S, Spinou E, Clement WA, Cathcart R, Kubba H, Geddes NK. A prospective, single-blind, randomized controlled trial of petroleum jelly/Vaseline for recurrent paediatric epistaxis. Clin Otolaryngol Allied Sci. 2004;29:266–269. doi: 10.1111/j.1365-2273.2004.00813.x. [DOI] [PubMed] [Google Scholar]
- 35.Al Aboud KM, Khachemoune A. Vaseline: a historical perspective. Dermatol Nurs. 2009;21:143–144. [PubMed] [Google Scholar]
- 36.Meyer E. White Mineral Oil and Petroleum and their Related Products. New York: Chemical Publishing Company; 1968. [Google Scholar]
- 37.Konur Hekimoĝlu S, Kislal Loĝlu S, Hmcal A. In vitro release properties of caffeine II. Effect of type of petrolatum and sodium benzoate. Drug Dev Ind Pharm. 1987;13:1093–1105. [Google Scholar]
- 38.Kornreich C, Zheng ZS, Xue GZ, Prystowsky JH. A simple method to predict whether topical agents will interfere with phototherapy. Cutis. 1996;57:113–118. [PubMed] [Google Scholar]
- 39.Blair RM, Fang H, Branham WS, Hass BS, Dial SL, Moland CL, et al. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands. Toxicol Sci. 2000;54:138–153. doi: 10.1093/toxsci/54.1.138. [DOI] [PubMed] [Google Scholar]
- 40.Buist HE, van Burgsteden JA, Freidig AP, Maas WJ, van de Sandt JJ. New in vitro dermal absorption database and the prediction of dermal absorption under finite conditions for risk assessment purposes. Regul Toxicol Pharmacol. 2010;57:200–209. doi: 10.1016/j.yrtph.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 41.CIR. Cosmetic Ingedient Review Expert Panel (CIR) Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27(Suppl 4):1–82. doi: 10.1080/10915810802548359. [DOI] [PubMed] [Google Scholar]
- 42.Blount BC, Silva MJ, Caudill SP, Needham LL, Pirkle JL, Sampson EJ, et al. Levels of seven urinary phthalate metabolites in a human reference population. Environ Health Perspect. 2000;108:979–982. doi: 10.1289/ehp.00108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darbre PD. Environmental oestrogens, cosmetics and breast cancer. Best Pract Res Clin Endocrinol Metab. 2006;20:121–143. doi: 10.1016/j.beem.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Darbre PD, Charles AK. Environmental oestrogens and breast cancer: evidence for combined involvement of dietary, household and cosmetic xenoestrogens. Anticancer Res. 2010;30:815–827. [PubMed] [Google Scholar]
- 45.Donovan M, Tiwary CM, Axelrod D, Sasco AJ, Jones L, Hajek R, et al. Personal care products that contain estrogens or xenoestrogens may increase breast cancer risk. Med Hypotheses. 2007;68:756–766. doi: 10.1016/j.mehy.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 46.Harvey PW, Darbre P. Endocrine disrupters and human health: could oestrogenic chemicals in body care cosmetics adversely affect breast cancer incidence in women. J Appl Toxicol. 2004;24:167–176. doi: 10.1002/jat.978. [DOI] [PubMed] [Google Scholar]
- 47.Darbre PD, Aljarrah A, Miller WR, Coldham NG, Sauer MJ, Pope GS. Concentrations of parabens in human breast tumours. J Appl Toxicol. 2004;24:5–13. doi: 10.1002/jat.958. [DOI] [PubMed] [Google Scholar]
- 48.Edidin DV, Levitsky LL. Prepubertal gynecomastia associated with estrogen-containing hair cream. Am J Dis Child. 1982;136:587–588. doi: 10.1001/archpedi.1982.03970430019005. [DOI] [PubMed] [Google Scholar]
- 49.DiRaimondo CV, Roach AC, Meador CK. Gynecomastia from exposure to vaginal estrogen cream. New Engl J Med. 1980;302:1089–1090. [PubMed] [Google Scholar]
- 50.Gottswinter JM, Korth-Schütz S, Ziegler R. Gynecomastia caused by estrogen containing hair lotion. J Endocrinol Invest. 1984;7:383–386. doi: 10.1007/BF03351021. [DOI] [PubMed] [Google Scholar]
- 51.Shirai S, Suzuki Y, Yoshinaga J, Shiraishi H, Mizumoto Y. Urinary excretion of parabens in pregnant Japanese women. Reprod Toxicol. 2013;35:96–101. doi: 10.1016/j.reprotox.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 52.Kunisue T, Chen Z, Buck Louis GM, Sundaram R, Hediger ML, Sun L, et al. Urinary concentrations of benzophenone-type UV filters in U.S. women and their association with endometriosis. Environ Sci Technol. 2012;46:4624–4632. doi: 10.1021/es204415a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boberg J, Taxvig C, Christiansen S, Hass U. Update on uptake, distribution, metabolism and excretion (ADME) and endocrine disrupting activity of parabens. [cited 29 March 2012];Report for Danish Environmental Protection Agency. 2009 Available at http://www.mst.dk/NR/rdonlyres/1D32086E-65A4-4B2C-B74F-6A6C6753DBB7/0/Updateonuptakedistributionmetabolismandexcretion_ADME_pdf. [Google Scholar]
- 54.Bennett DH, Wu XM, Teague CH, Lee K, Cassady DL, Ritz B, et al. Passive sampling methods to determine household and personal care product use. J Expo Sci Environ Epidemiol. 2012;22:148–160. doi: 10.1038/jes.2011.40. [DOI] [PubMed] [Google Scholar]
- 55.Wu KM, Dou J, Ghantous H, Chen S, Bigger A, Birnkrant D. Current regulatory perspectives on genotoxicity testing for botanical drug product development in the U.S.A. Regul Toxicol Pharmacol. 2010;56:1–3. doi: 10.1016/j.yrtph.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 56.Buckley JP, Palmieri RT, Matuszewski JM, Herring AH, Baird DD, Hartmann KE, et al. Consumer product exposures associated with urinary phthalate levels in pregnant women. J Expo Sci Environ Epidemiol. 2012;22:468–475. doi: 10.1038/jes.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Janjua NR, Frederiksen H, Skakkebaek NE, Wulf HC, Andersson AM. Urinary excretion of phthalates and paraben after repeated whole-body topical application in humans. Int J Androl. 2008;31:118–130. doi: 10.1111/j.1365-2605.2007.00841.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.