Abstract
Objective
To investigate the phenotypic and functional characteristics of peripheral and tissue-infiltrating stem cells, called fibrocytes in patients with idiopathic orbital inflammation (IOI).
Methods
Seven patients with IOI were studied. In the three patients requiring orbital biopsy, fibrocytes were identified in orbital tissue from patients with IOI compared to healthy controls using immunohistochemistry. Fibrocytes from the peripheral blood of all seven patients and controls were quantified and phenotyped by flow cytometry and immunofluorescence for expression of CD34, alpha smooth muscle actin, CD40 and Collagen 1. Quantitation of CD40-mediated IL-6 production was measured using ELISA.
Results
Orbital biopsy specimens from patients with IOI demonstrate tissue infiltration by fibrocytes (n=3). Fibrocytes are present in the peripheral blood of IOI patients (n= 7) but are scarce in healthy donors (n=19). Fibrocytes from IOI patients express substantial levels of CD40 and ligation of CD40 increases IL-6 expression.
Conclusions
Fibrocytes are present in the peripheral blood and orbital tissues of patients with IOI and constitutively express CD40 and express IL-6 in response to ligation. This site-specific predilection of CD34+ fibrocytes to sites of orbital inflammation and fibrosis may suggest a role in IOI. Moreover CD40-mediated activation cytokine production may contribute to the proinflammatory and profibrotic features of IOI and may provide a mechanism for future targeted therapy.
Introduction
Idiopathic orbital inflammation (IOI) is defined as extraocular inflammation without an identifiable local or systemic cause.1–3 Histopathologically, IOI is characterized by inflammatory infiltrates of polyclonal lymphocytes, neutrophils, plasma cells, macrophages, and eosinophils with variable degrees of extracellular matrix deposition and fibrosis.4–8 While systemic corticosteroids are the primary treatment of IOI,3 significant side-effects and a high recurrence rate limit their long-term efficacy.2 Further delineation of the underlying pathophysiologic mechanisms is needed to develop more targeted treatment options.
Fibrocytes are bone-marrow derived stem cells that are recruited from the peripheral blood to sites of injury and inflammation, have features of both immune cells and fibroblasts, and appear to play an important role in several inflammatory and fibrotic disease processes.9, 10 Fibrocytes have been implicated in the normal healing response and in fibrotic processes such as asthma, idiopathic pulmonary fibrosis, chronic renal fibrosis, and hepatic fibrosis.10–21 Fibrocytes typically compose less than 0.5% of peripheral blood mononuclear cells (PBMCs) but are dramatically elevated in some autoimmune disease states.22–25 Fibrocytes express CD34, CD45, and Collagen 1 (Col 1)11, 20 and multiple cytokines and chemoattractants such as CXCR4 and CXCL12 appear to regulate fibrocyte trafficking to sites of injury.26–28 Following their initial recruitment, fibrocytes mediate the site-specific immune response through secretion of proinflammatory cytokines including TNF-α, IL-6, IL-8, and metalloproteinases.29
Previously, the role of fibrocytes in orbital and ophthalmic disease has been limited to thyroid eye disease (TED). Fibrocytes have been identified in large numbers in the orbital and thyroid tissue, are increased in peripheral blood, and produce cytokines IL-6 and TNF-α in response to thyroid stimulating hormone.30 Orbital fibroblasts isolated from TED patients are phenotypically analogous to fibrocytes and express CD40. In vitro CD154 ligation of CD40 leads to increased production of IL-6, IL-8, and macrophage chemoattractant protein – 1 (MCP-1).31
Given these prominent roles of fibrocytes in TED, we sought to determine in IOI patients if fibrocytes could be identified in orbital tissue, if fibrocytes are elevated in the peripheral blood, and whether CD40-mediated IL-6 production was greater than in controls.
Methods
Subjects
Seven patients with idiopathic orbital inflammation as well as healthy controls were recruited from the Oculoplastic and orbital surgery services at the W.K.Kellogg Eye Center at the University of Michigan and Jules Stein Eye Institute at the University of California, Los Angeles. IOI was diagnosed after exclusion of other systemic inflammatory or malignant processes via clinical and laboratory testing. Orbital biopsy was performed for three patients. IOI subjects ranged in age from 34–59 years with a mean of 46 and were 86% female. 86% of patients were Caucasian and 14% Hispanic. All patients were treated with steroid therapy. All patients had normal ANCA, FTA-abs, ACE and TSH results. Healthy controls who presented for routine care without previous history of immunosuppresion, autoimmune disease, asthma, chronic inflammation, HIV, recent trauma or active infection were studied as well.
Informed consent was obtained according to policies of the Institutional Review Board of the University of Michigan Health System and Center for Health Sciences at UCLA. Research methods followed the tenets of the Declaration of Helsinki.
Histopathology
Deparaffinized sections of orbital tissues were examined for expression of CD34, α–SMA, and CD31 using immunoperoxidase staining.32 Briefly, deparaffinized tissue sections were pre-incubated in 50% methanol containing 1.5% H2O2 to block endogenous peroxidase activity. The sections were treated with 10% rabbit serum and incubated sequentially with mouse monoclonal antibodies to CD34 (QBEnd10; Dako Corp, Carpinteria, CA), α–SMA (1A4; Ventana Medical Systems, Tucson, AZ), or CD31 (JC70A; Dako) followed by biotinylated rabbit anti-mouse antibody and streptavidin-biotinylated horseradish peroxidase (Dako). Tissue-bound antibody complexes were visualized after development in a substrate solution containing 3–3' diamino-benzadine (Sigma Chemical Co, St Louis, MO) and 0.01% H2O2 in 0.1 M acetate buffer, pH 5.2, to yield a granular, brown reaction product. Tissue sections were counterstained with hematoxylin and mounted in Gel/Mount (Biomeda Corp, Foster City, CA). Staining of sections with irrelevant monoclonal antibodies of the same isotype were used as controls. All antibody incubations were performed in parallel for 30 minutes at 37°C.
Immunofluorescence
Fibrocytes were isolated from whole blood with Ficoll gradient, plated at 1.2 × 106 cells and cultured for 14 days with 10% FBS +DMEM. The cells were then incubated overnight with primary mouse antihuman monoclonal antibodies to CD34 (BD), monoclonal CD 40 (BD), α–SMA (BD), and polyclonal anti-Col 1 (Millipore) or their isotype control antibody. Secondary antibodies were incubated for 1 hour at room temperature in the dark after washes in PBS containing 0.1% Tween 20. 4',6-diamidino-2-phenylindole (DAPI) was used for nuclear counter staining. They were washed, mounted in Vectashield mounting medium, and examined with a confocal microscope (Leica SP5 Microscope, Leica, Wetzlar, Germany).
Flow cytometry of peripheral fibrocytes
PBMCs were obtained and prepared as described previously with modifications 11. Staining for flow cytometry was performed within 24 h of blood collection. Staining buffer (SB) was prepared in phosphate buffered saline (Invitrogen) containing 2% fetal bovine serum (Invitrogen Life Technologies) with 0.1% sodium azide (Sigma Aldrich). 100 μL whole blood was placed in 12×75 mm polypropylene tubes and 2 ml Pharm Lyse solution 1x (Becton Dickinson Biosciences) was added for 10 min at room temperature. Cells were centrifuged at 500× g for 5 min and resuspended in SB. After two additional washes, cell pellets were resuspended in 100 μL SB and the following anti-human fluorochrome-conjugated MoAbs were added: CD45-PERCP, CD34-PE, and CD40-PE (BD), and isotype control MoAbs. Following 20 min incubation in the dark at 40 C, cells were resuspended and washed three times in SB. They were permeabilized with CytoFix/CytoPerm (BD) for 20 min at 40C, washed twice and resuspended in Perm/Wash buffer (BD). Incubation with biotinylated goat anti-human collagen type I polyclonal Ab (Millipore) and subsequent incubation with Streptavidin conjugated FITC (BD) was performed. Cells were washed twice and fixed with 1% paraformaldehyde. Acquisition and analysis was performed using a FACSCalibur flowcytometer (BD). Percent positive expression was defined as the fraction of cells with increased fluorescent intensity compared with isotype control. Fibrocyte quantification (fibrocyte index, FI) was taken as the percentage of CD14+ monocytes co-expressing CD45, Col-1, and CD34. Expression of surface CD40 molecule on fibrocytes is presented as mean fluorescence intensity (MFI) and was calculated as a ratio of mean fluorescence sample over mean fluorescence isotype control. MFI of one indicates same fluorescent intensity as background or isotype.
CD40-mediated IL-6 production
Fibrocytes were isolated as above by Ficoll gradient. The PBMCs were resuspended at a concentration of 10 × 106 cells per well in 10%FBS + DMEM. After 14 days of incubation, fibrocytes were washed twice with PBS, and then shifted to 1%FBS + DMEM and incubated for 24 hours. Then, cells were stimulated with CD154 (10ng/ml) and incubated for 24 hours. The supernatant was harvested IL-6 production was measured with Luminex assay according to manufacturer specifications (Luminex Corp, Austin, TX).
Statistical analysis
Data values are reported as the mean +/− SD. Statistical analysis was performed using a two- tailed student t- test with a confidence interval greater than 95%.
The data in Figures 3 are not normally distributed (Shapiro–Wilk goodness-of-fit test value: P < 0.0001); therefore, group differences were tested with Kolmogorov–Smirnov test. A t-test on arcsine square root transformed data, used to normalized percentage data, was also performed.33 These tests resulted in statistically significant differences between IOI and healthy groups, with all values of P < 0.001.
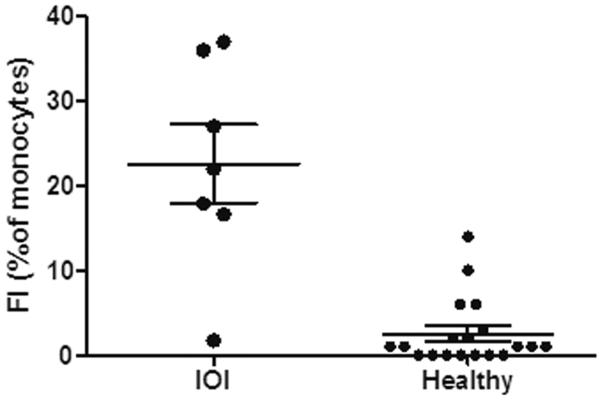
Figure 3.
Increased frequency of CD45+/ Col1 +/ CD34+ fibrocytes in peripheral blood from patients with IOI compared to healthy donors (IOI mean FI= 22.6 +/− 12.2, n= 7 vs Healthy mean FI= 2.5 +/− 3.6, n= 19, p=0.001). Data presented as fraction of monocytes (fibrocyte index, FI) co-expressing CD45, Col1 and CD34 calculated and analyzed by flow cytometry
Results
Fibrocytes infiltrate orbital tissue
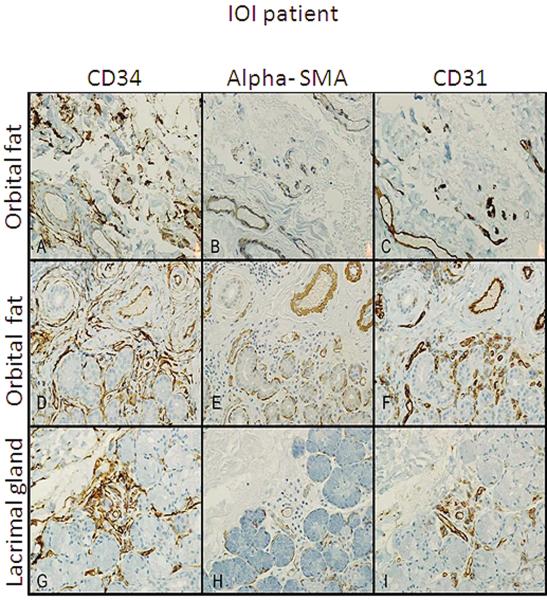
Of the three patients who required orbital biopsy, the presence of fibrocytes in the orbital fat (n=2) and the lacrimal gland (n=1) of patients with IOI was examined. Immunohistochemistry demonstrated CD34+ cells in the fibrotic areas of intraconal fat and lacrimal gland. These cells were spindle or stellate shaped consistent with previously reported fibrocyte morphology and phenotype (Figure 1 A, D, G). CD31 identified vascular endothelial cells and further distinguished fibrocytes (CD31−). α–SMA staining identifies myofibroblasts34 or cells with contractile properties. α–SMA immunoreactivity was sparse with no visible spindle or stellate shaped cells and staining was limited to lacrimal gland acini (1 H) and smooth muscles cells of blood vessels (1 B,E) which was in agreement with CD31 immunoreactivity (1 C, F, I).
Figure 1.
Immunohistochemistry of orbital fat from three IOI patients stained with CD34 alpha SMA and CD31. Spindle-shaped CD34+ cells (A, D,and G), are present in a perivascular distribution with early fibrosis and mild chronic inflammation. α–SMA+ cells (B, E, and H) showed staining of only smooth muscle of blood vessels and myoepithelial cells in sequential tissue sections. CD31+ cell staining was limited to blood vessel endothelial cells(C, F, and I). All 400X of the same region.
Thus CD34+ fibrocytes were present in orbital tissue of IOI patients but these cells were α–SMA− demonstrating lack of myofibroblast differentiation.
Peripheral fibrocyte identification
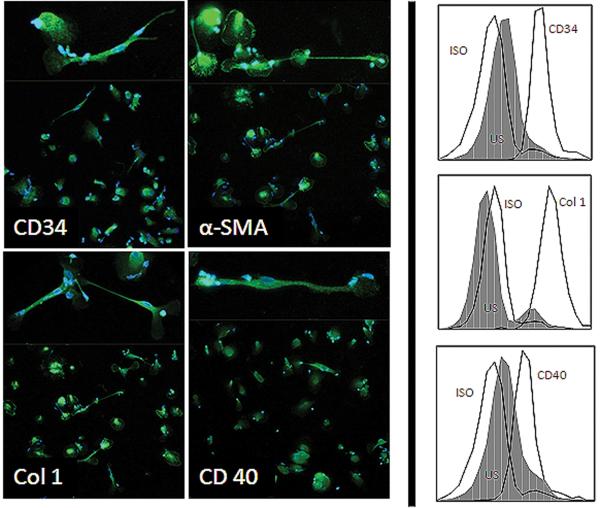
Given the histopathologic evidence for fibrocyte infiltration into orbital tissue of IOI patients, fibrocytes in the peripheral blood were then identified. Fibrocytes were identified expressing high levels of CD34, Col 1, CD 40, and α–SMA as determined by immunofluorescence and flow cytometry (Figure 2). Consistent with previous reports, fibrocytes from these patients were oblong, spindle-shaped cells. Some had arborizing processes and others have nuclear prominence and a round configuration.
Figure 2.
Cultured fibrocytes from an IOI patient express CD34 (A), Col-1 (B), α–SMA (C), and CD40 (D) shown by immunofluorescence staining (left panel) and by flow cytometry (right panel). Spindle or stellate-shaped cellular morphology is consistently seen (insets). ISO (isotope) serves as a positive control.
Peripheral fibrocytes can be elevated in IOI
Peripheral fibrocyte quantification was determined as the percentage of monocytes co-expressing CD45, Col1, and/or CD34 (Fibrocyte Index:FI). Fibrocytes could be identified in the peripheral blood of all seven patients examined at multiple time points during the disease course. The proportion of fibrocytes from IOI patients was increased compared to healthy control donors (Figure 3. IOI mean FI= 22.6 +/− 12.2, n= 7 vs Healthy mean FI= 2.5 +/− 3.6, n= 19, p=0.001).
Fibrocyte response to CD40 ligation
The surface expression of CD40 is increased on orbital fibroblasts derived from TED patients compared to those from healthy donors.31 Given the importance of CD40 expression for immune costimulation and the potential role of infiltrating orbital fibrocytes, we sought to determine if fibrocytes from IOI patients express functional CD40.
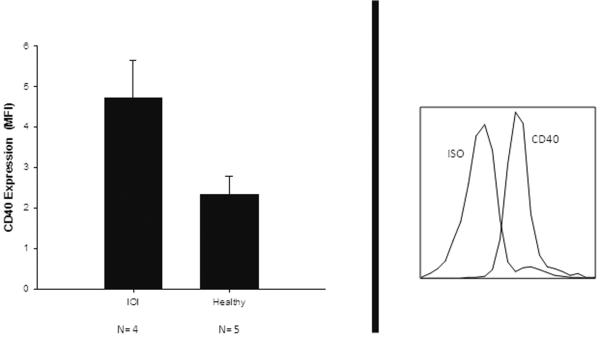
Fibrocytes were isolated from the PBMC of IOI patients and expression of CD40 was assessed by flow cytometry. Surface expression of CD40 on fibrocytes from IOI patients, as measured by MFI, was significantly greater than that seen in healthy controls (Figure 4. IOI MFI= 4.71 +/− 0.94, n=4 vs. healthy MFI=2.33 +/− 0.45, n=5, p= 0.001).
Figure 4.
Increased CD40 surface expression on blood derived fibrocytes from IOI patients compared to healthy controls. Left panel (IOI MFI= 4.71 +/− 0.94, n=4 vs. healthy MFI=2.33 +/− 0.45, n=5, p= 0.001). Mean fluorescence intensity (MFI) analyses and calculated by flow cytometry. Representative flow cytometry histogram from an IOI patient showing CD40 expression, left panel. Isotype antibody control (ISO).
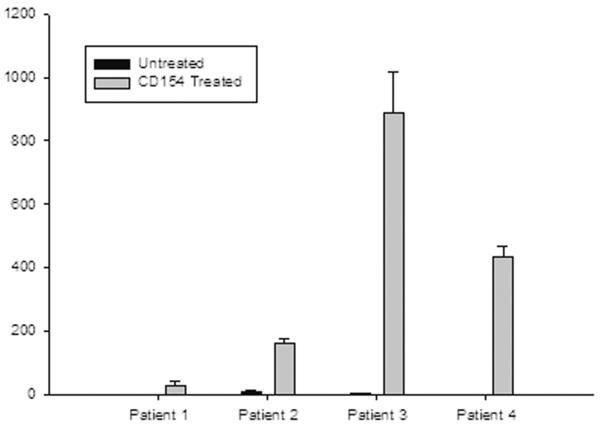
As previously reported, ligation of CD40 with CD154 induces several cytokines depending on the cell type. CD40-mediated IL- 6 production from fibrocytes of IOI patients was measured next. While the magnitude of IL-6 expression in response to CD40 ligation was heterogeneous, fibrocytes from all patients expressed IL-6 upon stimulation (Figure 5. Range 28.86 to 886.7 pg/mL, n= 4, p=0.013).
Figure 5.
CD154 treatment of cultured fibrocytes from IOI patients increases the secretion of IL-6 protein. Concentration of protein (pg/ml) measured in media of cell cultures by Luminex assay.
Discussion
Fibrocyte infiltration has been implicated in diverse diseases associated with tissue destruction and fibrosis. TED patients demonstrate increased levels of both circulating and orbital fibrocytes.30 CD40 is also overexpressed by orbital fibroblasts from TED patients and these fibroblasts exhibit exaggerated cytokine production in response to CD40 ligation.31 This report is the first to demonstrate fibrocytes in the orbits and peripheral blood of IOI patients.
While no evidence of fibrocytes has been previously found in normal orbital tissue,30 fibrocytes in our IOI subjects were localized in the septal and perivascular regions surrounded by an admixture of mild chronic inflammation and early fibrosis (Figure 1). Prior studies have shown spindle-shaped CD34+ fibrocytes migrating to wounds in response to various cytokines and chemoattractants.27, 28 In contrast, areas of advanced scarring in our orbital specimens showed α–SMA positive myofibroblasts but demonstrated minimal CD34 positive fibrocytes. These findings are consistent with previous reports describing decreased CD34 expression in mature wounds.35 Fibrocyte infiltration and cytokine production early in the wound healing process is associated with elevated local levels of IL-1.29 As IL-1 levels decrease during wound remodeling, fibrocytes express α–SMA and produce more collagen.36
Furthermore our studies implicate fibrocytes CD40 expression and activation in this inflammatory process. CD40, initially described as a B cell surface receptor, is costimulatory molecule mediating a variety of proinflammatory signaling cascades when activation occurs with its ligand (CD154). Expression of CD40 has been identified on many immune cells, including platelets, mast cells, and basophils, monocytes and T cells 37, 38 In animal models, blockade of CD40:CD154 signaling cascade inhibits induction of thyroiditis, uveitis, and inflammatory bowel disease.40–43 In addition to blocking induction of autoimmune disease, CD40:CD154 blockade can treat ongoing disease by interrupting macrophage and monocyte interactions with T cells.44 CD40 ligation in the localized site of inflammation reciprocally affects T cell function 45 and has been shown to be present in orbital fibroblasts in TED.31, 46
IL-6 was initially discovered as a B lymphocyte stimulator of immunoglobulin production47 but also plays a crucial role in the pathogenesis of many chronic inflammatory and autoimmune diseases including rheumatoid arthritis, multiple sclerosis, uveitis, asthma and inflammatory bowel disease through its effects on T lymphocytes. IL-6 also stimulates acute phase protein production and promotes a proinflammatory local environment. 48–50 In humans, IL-6 receptor blockade has been shown to suppress inflammation in rheumatoid arthritis, juvenile idiopathic arthritis, and Castleman's disease.51 IL-6 has been shown to be increased in the serum of Graves' patients compared to healthy donors.52–54
Long-term sequelae of refractory IOI can include, pain, diplopia, and loss of vision. Given the poor side-effect profile of corticosteroids currently used as mainstay therapy for IOI, more specific therapies are needed. Indeed, successful treatment with biologic agents targeting B and T cells, as well as monoclonal antibody agents directed against proinflammatory cytokines, have recently been described.56–61 Further characterization of the role of these orbital fibrocytes may provide insight into the pathophysiology of IOI leading to more targeted therapeutic treatments.
Acknowledgements
David M Reed, Ph.D. from the Molecular Biology Vision Core in the Department of Ophthalmology and Visual Sciences provided statistical assistance. This work used the Core Center for Vision Research funded by EY007003 from the National Eye Institute.
Funding: This research was supported by the National Institutes of Health Grants EY008976, EY011708, F026930, and DK063121. Cosmetic Surgery Foundation Grant N012375. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Competing Interests: The authors have declared no competing interests exist.
References
- 1.Gordon LK. Orbital inflammatory disease: a diagnostic and therapeutic challenge. Eye (Lond) 2006;20(10):1196–206. doi: 10.1038/sj.eye.6702383. [DOI] [PubMed] [Google Scholar]
- 2.Yuen SJ, Rubin PA. Idiopathic orbital inflammation: distribution, clinical features, and treatment outcome. Arch Ophthalmol. 2003;121(4):491–9. doi: 10.1001/archopht.121.4.491. [DOI] [PubMed] [Google Scholar]
- 3.Rubin PA, Foster CS. Etiology and management of idiopathic orbital inflammation. Am J Ophthalmol. 2004;138(6):1041–3. doi: 10.1016/j.ajo.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 4.Demirci H, Shields CL, Shields JA, Honavar SG, Mercado GJ, Tovilla JC. Orbital tumors in the older adult population. Ophthalmology. 2002;109(2):243–8. doi: 10.1016/s0161-6420(01)00932-0. [DOI] [PubMed] [Google Scholar]
- 5.Garner A. Pathology of 'pseudotumours' of the orbit: a review. J Clin Pathol. 1973;26(9):639–48. doi: 10.1136/jcp.26.9.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shields JA, Shields CL, Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions: The 2002 Montgomery Lecture, part 1. Ophthalmology. 2004;111(5):997–1008. doi: 10.1016/j.ophtha.2003.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Shinder R, Al-Zubidi N, Esmaeli B. Survey of orbital tumors at a comprehensive cancer center in the United States. Head Neck. 2010 doi: 10.1002/hed.21498. [DOI] [PubMed] [Google Scholar]
- 8.Weber AL, Romo LV, Sabates NR. Pseudotumor of the orbit. Clinical, pathologic, and radiologic evaluation. Radiol Clin North Am. 1999;37(1):151–68. xi. doi: 10.1016/s0033-8389(05)70084-1. [DOI] [PubMed] [Google Scholar]
- 9.Mori L, Bellini A, Stacey MA, Schmidt M, Mattoli S. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp Cell Res. 2005;304(1):81–90. doi: 10.1016/j.yexcr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171(1):380–9. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 11.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1(1):71–81. [PMC free article] [PubMed] [Google Scholar]
- 12.Holland AJ, Tarran SL, Medbury HJ, Guiffre AK. Are fibrocytes present in pediatric burn wounds? J Burn Care Res. 2008;29(4):619–26. doi: 10.1097/BCR.0b013e31817db90a. [DOI] [PubMed] [Google Scholar]
- 13.Nihlberg K, Larsen K, Hultgardh-Nilsson A, Malmstrom A, Bjermer L, Westergren-Thorsson G. Tissue fibrocytes in patients with mild asthma: a possible link to thickness of reticular basement membrane? Respir Res. 2006;7:50. doi: 10.1186/1465-9921-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saunders R, Siddiqui S, Kaur D, et al. Fibrocyte localization to the airway smooth muscle is a feature of asthma. J Allergy Clin Immunol. 2009;123(2):376–84. doi: 10.1016/j.jaci.2008.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang CH, Huang CD, Lin HC, et al. Increased circulating fibrocytes in asthma with chronic airflow obstruction. Am J Respir Crit Care Med. 2008;178(6):583–91. doi: 10.1164/rccm.200710-1557OC. [DOI] [PubMed] [Google Scholar]
- 16.Wang JF, Jiao H, Stewart TL, Shankowsky HA, Scott PG, Tredget EE. Fibrocytes from burn patients regulate the activities of fibroblasts. Wound Repair Regen. 2007;15(1):113–21. doi: 10.1111/j.1524-475X.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 17.Yang L, Scott PG, Giuffre J, Shankowsky HA, Ghahary A, Tredget EE. Peripheral blood fibrocytes from burn patients: identification and quantification of fibrocytes in adherent cells cultured from peripheral blood mononuclear cells. Lab Invest. 2002;82(9):1183–92. doi: 10.1097/01.lab.0000027841.50269.61. [DOI] [PubMed] [Google Scholar]
- 18.Noble PW, Homer RJ. Idiopathic pulmonary fibrosis: new insights into pathogenesis. Clin Chest Med. 2004;25(4):749–58. vii. doi: 10.1016/j.ccm.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Andersson-Sjoland A, de Alba CG, Nihlberg K, et al. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40(10):2129–40. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Moeller A, Gilpin SE, Ask K, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179(7):588–94. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 21.Sakai N, Wada T, Matsushima K, et al. The renin-angiotensin system contributes to renal fibrosis through regulation of fibrocytes. Journal of hypertension. 2008;26(4):780–90. doi: 10.1097/HJH.0b013e3282f3e9e6. [DOI] [PubMed] [Google Scholar]
- 22.Gillespie EF, Papageorgiou KI, Fernando R, et al. Increased expression of TSH receptor by fibrocytes in thyroid-associated ophthalmopathy leads to chemokine production. J Clin Endocrinol Metab. 2012;97(5):E740–6. doi: 10.1210/jc.2011-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(12):6307–12. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galligan CL, Siminovitch KA, Keystone EC, Bykerk V, Perez OD, Fish EN. Fibrocyte activation in rheumatoid arthritis. Rheumatology (Oxford, England) 2010;49(4):640–51. doi: 10.1093/rheumatology/kep265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomer RH. Circulating progenitor cells and scleroderma. Curr Rheumatol Rep. 2008;10(3):183–8. doi: 10.1007/s11926-008-0031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishida Y, Kimura A, Takayasu T, Eisenmenger W, Kondo T. Detection of fibrocytes in human skin wounds and its application for wound age determination. International journal of legal medicine. 2009;123(4):299–304. doi: 10.1007/s00414-009-0320-4. [DOI] [PubMed] [Google Scholar]
- 27.Mehrad B, Burdick MD, Strieter RM. Fibrocyte CXCR4 regulation as a therapeutic target in pulmonary fibrosis. Int J Biochem Cell Biol. 2009;41(8–9):1708–18. doi: 10.1016/j.biocel.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips RJ, Burdick MD, Hong K, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114(3):438–46. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160(1):419–25. [PubMed] [Google Scholar]
- 30.Douglas RS, Afifiyan NF, Hwang CJ, et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2010;95(1):430–8. doi: 10.1210/jc.2009-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang CJ, Afifiyan N, Sand D, et al. Orbital fibroblasts from patients with thyroid-associated ophthalmopathy overexpress CD40: CD154 hyperinduces IL-6, IL-8, and MCP-1. Investigative ophthalmology & visual science. 2009;50(5):2262–8. doi: 10.1167/iovs.08-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elner VM, Dutt S, Pavilack MA, Sugar A, Foster CS, Elner SG. Intercellular adhesion molecule-1 (ICAM-1) and HLA-DR antigens in herpes keratitis. Ophthalmology. 1992;99(9):1400–7. doi: 10.1016/s0161-6420(92)31801-9. [DOI] [PubMed] [Google Scholar]
- 33.Sheskin D. Handbook of Parametric and Nonparametric Statistical Procedures. 3rd ed. Chapman and Hall-CRC; Boca Raton: 2004. [Google Scholar]
- 34.Nagamoto T, Eguchi G, Beebe DC. Alpha-smooth muscle actin expression in cultured lens epithelial cells. Investigative ophthalmology & visual science. 2000;41(5):1122–9. [PubMed] [Google Scholar]
- 35.Aiba S, Tagami H. Inverse correlation between CD34 expression and proline-4-hydroxylase immunoreactivity on spindle cells noted in hypertrophic scars and keloids. J Cutan Pathol. 1997;24(2):65–9. doi: 10.1111/j.1600-0560.1997.tb01098.x. [DOI] [PubMed] [Google Scholar]
- 36.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166(12):7556–62. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 37.Henn V, Slupsky JR, Grafe M, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391(6667):591–4. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 38.Gauchat JF, Henchoz S, Mazzei G, et al. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature. 1993;365(6444):340–3. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]
- 39.Henn V, Steinbach S, Buchner K, Presek P, Kroczek RA. The inflammatory action of CD40 ligand (CD154) expressed on activated human platelets is temporally limited by coexpressed CD40. Blood. 2001;98(4):1047–54. doi: 10.1182/blood.v98.4.1047. [DOI] [PubMed] [Google Scholar]
- 40.Carayanniotis G, Masters SR, Noelle RJ. Suppression of murine thyroiditis via blockade of the CD40-CD40L interaction. Immunology. 1997;90(3):421–6. doi: 10.1111/j.1365-2567.1997.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bagenstose LM, Agarwal RK, Silver PB, et al. Disruption of CD40/CD40-ligand interactions in a retinal autoimmunity model results in protection without tolerance. J Immunol. 2005;175(1):124–30. doi: 10.4049/jimmunol.175.1.124. [DOI] [PubMed] [Google Scholar]
- 42.Namba K, Ogasawara K, Kitaichi N, et al. Amelioration of experimental autoimmune uveoretinitis by pretreatment with a pathogenic peptide in liposome and anti-CD40 ligand monoclonal antibody. J Immunol. 2000;165(6):2962–9. doi: 10.4049/jimmunol.165.6.2962. [DOI] [PubMed] [Google Scholar]
- 43.Danese S, Sans M, Fiocchi C. The CD40/CD40L costimulatory pathway in inflammatory bowel disease. Gut. 2004;53(7):1035–43. doi: 10.1136/gut.2003.026278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howard LM, Miga AJ, Vanderlugt CL, et al. Mechanisms of immunotherapeutic intervention by anti-CD40L (CD154) antibody in an animal model of multiple sclerosis. J Clin Invest. 1999;103(2):281–90. doi: 10.1172/JCI5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Evans HG, Gullick NJ, Kelly S, et al. In vivo activated monocytes from the site of inflammation in humans specifically promote Th17 responses. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(15):6232–7. doi: 10.1073/pnas.0808144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sempowski GD, Rozenblit J, Smith TJ, Phipps RP. Human orbital fibroblasts are activated through CD40 to induce proinflammatory cytokine production. The American journal of physiology. 1998;274(3 Pt 1):C707–14. doi: 10.1152/ajpcell.1998.274.3.C707. [DOI] [PubMed] [Google Scholar]
- 47.Hirano T, Yasukawa K, Harada H, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324(6092):73–6. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- 48.Jones GW, McLoughlin RM, Hammond VJ, et al. Loss of CD4+ T cell IL-6R expression during inflammation underlines a role for IL-6 trans signaling in the local maintenance of Th17 cells. J Immunol. 2010;184(4):2130–9. doi: 10.4049/jimmunol.0901528. [DOI] [PubMed] [Google Scholar]
- 49.Rabe B, Chalaris A, May U, et al. Transgenic blockade of interleukin 6 transsignaling abrogates inflammation. Blood. 2008;111(3):1021–8. doi: 10.1182/blood-2007-07-102137. [DOI] [PubMed] [Google Scholar]
- 50.Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine & growth factor reviews. 2010 doi: 10.1016/j.cytogfr.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 51.Kishimoto T. IL-6: from its discovery to clinical applications. International immunology. 2010;22(5):347–52. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- 52.Salvi M, Girasole G, Pedrazzoni M, et al. Increased serum concentrations of interleukin-6 (IL-6) and soluble IL-6 receptor in patients with Graves' disease. J Clin Endocrinol Metab. 1996;81(8):2976–9. doi: 10.1210/jcem.81.8.8768861. [DOI] [PubMed] [Google Scholar]
- 53.Jyonouchi SC, Valyasevi RW, Harteneck DA, Dutton CM, Bahn RS. Interleukin-6 stimulates thyrotropin receptor expression in human orbital preadipocyte fibroblasts from patients with Graves' ophthalmopathy. Thyroid. 2001;11(10):929–34. doi: 10.1089/105072501753210984. [DOI] [PubMed] [Google Scholar]
- 54.Raychaudhuri N, Douglas RS, Smith TJ. PGE2 induces IL-6 in orbital fibroblasts through EP2 receptors and increased gene promoter activity: implications to thyroid-associated ophthalmopathy. PloS one. 2010;5(12):e15296. doi: 10.1371/journal.pone.0015296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salvi M, Pedrazzoni M, Girasole G, et al. Serum concentrations of proinflammatory cytokines in Graves' disease: effect of treatment, thyroid function, ophthalmopathy and cigarette smoking. Eur J Endocrinol. 2000;143(2):197–202. doi: 10.1530/eje.0.1430197. [DOI] [PubMed] [Google Scholar]
- 56.Garrity JA, Coleman AW, Matteson EL, Eggenberger ER, Waitzman DM. Treatment of recalcitrant idiopathic orbital inflammation (chronic orbital myositis) with infliximab. Am J Ophthalmol. 2004;138(6):925–30. doi: 10.1016/j.ajo.2004.06.077. [DOI] [PubMed] [Google Scholar]
- 57.Lim L, Suhler EB, Smith JR. Biologic therapies for inflammatory eye disease. Clin Experiment Ophthalmol. 2006;34(4):365–74. doi: 10.1111/j.1442-9071.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 58.Miquel T, Abad S, Badelon I, et al. Successful treatment of idiopathic orbital inflammation with infliximab: an alternative to conventional steroid-sparing agents. Ophthal Plast Reconstr Surg. 2008;24(5):415–7. doi: 10.1097/IOP.0b013e318182a522. [DOI] [PubMed] [Google Scholar]
- 59.Osborne SF, Sims JL, Rosser PM. Short-term use of Infliximab in a case of recalcitrant idiopathic orbital inflammatory disease. Clin Experiment Ophthalmol. 2009;37(9):897–900. doi: 10.1111/j.1442-9071.2009.02188.x. [DOI] [PubMed] [Google Scholar]
- 60.Wilson MW, Shergy WJ, Haik BG. Infliximab in the treatment of recalcitrant idiopathic orbital inflammation. Ophthal Plast Reconstr Surg. 2004;20(5):381–3. doi: 10.1097/01.iop.0000139521.38345.ba. [DOI] [PubMed] [Google Scholar]
- 61.Witzig TE, Inwards DJ, Habermann TM, et al. Treatment of benign orbital pseudolymphomas with the monoclonal anti-CD20 antibody rituximab. Mayo Clin Proc. 2007;82(6):692–9. [PubMed] [Google Scholar]