Abstract
While there are now fewer allogeneic stem cell transplants (allo-SCT) performed for chronic myeloid leukemia (CML), leukemic relapse post-transplant remains a persistent problem. To better define clinical and biological parameters determining post-relapse outcome, we studied 59 patients with CML relapsing after HLA-identical sibling allo-SCT between 1993 and 2008. Eighteen (30.5%) were transplanted in advanced phase and 41(69.5%) in chronic phase. With a median follow up from relapse of 7.9 years, 5-year post-relapse survival (PRS) was 62%. Multivariate analysis found disease status at transplant, time to diagnosis of relapse from transplant, and pre- transplant TKI use as significant factors associated with PRS. Analysis of BCR-ABL transcript expression in the hematopoietic progenitor compartment was performed in 36 patients (22 relapsed, 8 non-relapsed, and 6 TKI alone controls). Patients with BCR-ABL expression in their early hematopoietic stem cell compartment (HSC: Lineage−CD34+CD38-CD90+) had worse survival irrespective of the disease status. We conclude disease status remains the strongest clinical prognostic factor for PRS in CML following allo-SCT. The persistence of BCR-ABL expression in the progenitor cell compartment in some patients after SCT emphasizes the need to target CML-leukemia stem cells.
Keywords: HSCT, relapse, CML, leukemia progenitor cell
INTRODUCTION
Allogeneic stem cell transplantation (allo-SCT) has been used as a highly effective curative treatment for chronic myeloid leukemia (CML) for over 30 years, in large part because of the susceptibility of this disease, particularly in its chronic phase (CP), to the graft versus leukemia (GVL) effect of the incoming donor immune system.1-5 As a consequence many thousands of patients with CML have received allo-SCT worldwide and are now long term survivors. The introduction of imatinib and other tyrosine kinase inhibitors (TKI) relegated the place of allo-SCT in CML to second or third line therapy in many patients, causing significant reduction in transplants for CML.6-12 Most CML patients who are candidates for allo-SCT in this century are patients refractory or intolerant to TKI, or those with CML more advanced than first CP.13-15 Relapse of CML after allo-SCT remains a current issue because relapse can be delayed, occurring even in the second decade after allo-SCT, and patients transplanted in the era of TKI treatment have a higher chance of relapse because they were transplanted for advanced or refractory disease.16-18 Management of relapsed CML may include DLI, TKI, chemotherapy or second allo-SCT.6-11, 19 With the availability of TKI and improved transplant management, the factors determining outcome after post allo-SCT relapse today are likely to differ from earlier observations. Furthermore many questions remain unanswered about the biological basis of relapse in CML – particularly the nature of the minimal residual disease (MRD) in the progenitor cell compartment responsible for relapse20 and its relationship with treatment and post-relapse outcome. Recent studies showed that with advance to blastic phase the predominant leukemia initiating population in CML shifts from a lineage−CD34+CD38− progenitor to a more differentiated granulocyte-macrophage progenitor.21 To better define factors affecting outcome after relapse in CML and to characterize the CML progenitor populations at relapse, we studied 59 patients relapsing post allo-SCT at our institute between 1993 and 2008. In 22 relapsed patients, we correlated the pattern of BCR-ABL expression in defined subpopulations of hematopoietic stem and progenitor cells with treatment response and outcome. Here we present clinical and biological parameters that were associated with post relapse outcome of CML following allo-SCT.
METHODS
Study Population
Between September 1993 and August 2008, 109 consecutive patients with Philadelphia chromosome positive CML underwent allo-SCT from a 6/6 HLA identical sibling in successive National Heart, Lung and Blood Institute (NHLBI) institutional review board-approved protocols (93-H-0212, 97-H-0202, 97-H-0099, 00-H-0001, 01-H-0010, 02-H-0111, 03-H-0192, 04-H-0112, 06-H-0248, 07-H-0136, 09-H-0062). Written informed consent, consistent with the Helsinki Declaration, was obtained from all patients and donors.
Transplant conditioning regimen and ex-vivo T cell depletion
Ninety six patients (88%) received a myeloablative conditioning regimen with cyclophosphamide 120mg/kg +/− fludarabine 125mg/m2 and total body irradiation (1200 to 1360 cGy). Thirteen patients (12%) received a non-myeloablative conditioning regimen consisting of cyclophosphamide 120mg/kg and fludarabine 125mg/m2. Twenty seven patients (25%) received bone marrow and 82 patients (75%) received G-CSF-mobilized peripheral blood as the stem cell source. Thirteen patients (12%) given nonmyeloablative conditioning received a T-lymphocyte replete graft and 96 (88%) received a 4-log10 ex-vivo T-lymphocyte depleted graft. T cell depletion was achieved using the CellPro system (CellPro Inc., Bothel, WA) before 1999, Isolex 300i (Nexell Therapeutics Inc, Irvine, CA) from 1999 to 2006 and thereafter positive selection of CD34+ cells using the MiltenyiCliniMacs system (MiltenyiBiotec Inc., Auburn, CA). Cyclosporine (CSA) was used throughout for GVHD prophylaxis, with varied dose according to protocol.22 In addition to CSA, methotrexate and/or mycophenolate mofetil were used in T-replete graft protocols.
Clinical BCR-ABL monitoring
Patients were monitored for molecular evidence of leukemia by PCR- based amplification of b2a2 and b3a2 transcripts encoding the p210 BCR-ABL fusion product. Blood samples were collected at least every three months up to 1 year post transplant, then at least annually thereafter. In all cases, RNA was extracted from peripheral blood leukocytes after ammonium chloride lysis of red cells, and cDNA was produced by reverse transcription using random hexamer primers. Before 2003, samples were assayed qualitatively using double-nested PCR to amplify BCRABL.23 After June 2003, a commercial real-time PCR kit (Roche, Indianapolis, IN, USA) replaced as the method to detect BCR-ABL, with G6PDH as the control gene as described previously.24 After June 2009, the ipsogen BCR-ABL1 Mbcr Kit (Qiagen, Valencia, CA) was used to measure BCR-ABL and the control gene ABL1. The double-nested assay and real-time BCR-ABL assays were all qualitatively comparable in sensitivity detecting BCR-ABL transcripts in K562 RNA diluted to 1: 100,000-1,000,000 in control RNA. To facilitate serial monitoring over time and to reconcile the different techniques used for BCR-ABL measurement, positive values obtained with the older assay were mathematically “converted” to units of BCRABL: ABL1 based on a good linear log-log relationship (r2 0.883, p<0.001) between values obtained in parallel measurements of 50 positive clinical samples with both assays. In this study, we defined a sample positive for BCR-ABL if a detectable band was noted in a double-nested assay, or if either of two duplicate samples generated a measurable crossing point in the real-time PCR assay. Samples were considered negative, if no BCR-ABL band or crossing point was detected and the housekeeper gene expression assay confirmed good control gene expression.25
Definition and management of relapse
Chronic phase (CP) is defined as first chronic phase, while blast crisis, accelerated phase, and second chronic phase were categorized as advanced-phase (AP) disease. Molecular relapse was defined as a detectable BCR-ABL transcript level by PCR on more than 2 consecutive occasions, requiring salvage treatments. Hematological relapse followed the Center for International Bone and Marrow Transplantation Registry (CIBMTR) criteria26. For relapsed patients, the BCR-ABL patterns were further classified into two categories. Patients who had consecutively positive BCR-ABL post relapse were categorized as persistently positive. Patients who remained BCRABL negative for two or more consecutive readings after initial positive readings post-transplant were categorized as persistently negative. Molecular relapse was treated with DLI with or without imatinib 400-600 mg/day. Hematological relapse was treated at the investigator's discretion with imatinib, other TKI, interferonα or second SCT as previously described.19, 27 Concurrent TKI and DLI was defined as the initiation of both treatments within 6 months of each other.
Measurement of BCR-ABL in the hematopoietic progenitor cell compartment
Cell samples
Mononuclear cells from peripheral blood or bone marrow were isolated by density gradient centrifugation (OrganonTeknika, Durham, NC) and cryopreserved in RPMI1640 supplemented with 20% fetal calf serum and 10% dimethyl sulfoxide. After thawing, CD34+ cells were selected by immunomagnetic beads (MiniMACS, Miltenyi Biotech, Auburn, CA). Sample date varied between the subjects, but all samples from relapsed patients were collected when the concurrent peripheral blood showed either positive BCR-ABL or hematological relapse after the diagnosis of relapse (median time from diagnosis of relapse to sample collection was 386 days). Six patients who were on TKI alone and never received allo-SCT, served as controls (Supplementary Table 1).
Flow cytometry
Fluorescence-activated cell sorting (FACS) was performed with the FACSAria sorter (BD Biosciences, San Jose, CA). Monoclonal antibodies for cell surface markers were FITC-CD38 (Beckman Coulter, Fullerton, CA), PE- lineage (Lin) markers28, PE-Cy5- IL3Rα, APC-CD90, APC-Cy7- or PE-Cy7-CD34 (BD Biosciences, San Jose, CA) and PE-Texas Red-CD45RA (Beckman-Coulter). CD38+ and CD38− cells were gated off the total CD34+ population. Lin-CD34+CD38− CD90+hematopoietic stem cells (HSC)21, common myeloid progenitors (CMP) Lin−CD34+CD38+ IL3Rα+CD45RA− , and granulocyte-macrophage progenitors (GMP) Lin-CD34+CD38+ IL3Rα+CD45RA+ were collected (Supplementary Figure 1).29
Real-time quantitative polymerase chain reaction (RQ-PCR)
Total RNA was extracted from sorted CD34+ subpopulations using the RNeasy kit (Qiagen, Valencia, CA), and cDNA synthesized using the Advantage RT-for-PCR kit (Clontech, Mountain View, CA). ABL1 expression was used as the endogenous cDNA control.30 BCR-ABL transcripts were assessed according to an MRD detection assay using recommended standardized probe and primers.31 All reactions by RQ-PCR using the ABI PRISM 7900 system (Applied Biosystems, Foster City, CA, USA) were performed in triplicate. BCR-ABL RQ-PCR reactions consistently detected one leukemic cell in 1,000,000 non-leukemic cells.
Statistical methods
Patient characteristics were described by the frequencies and sample proportions and compared by the Fisher's exact test between subgroups. Kaplan-Meier estimates were used to estimate time-to-event distributions of overall survival (OS) post-transplant, and post relapse survival (PRS), with PRS defined as the time from first relapse until last follow up. Log-rank tests were used to compare the survival curves. For the analysis of progenitor cell compartment BCR-ABL measurement, since the timing of sample collection is not always identical to the diagnosis of relapse, we analyzed the data according to the disease status at transplant rather than the type of relapse. Also, time from sample collection to last follow up was used as a surrogate end point for PRS in this subgroup analysis. Cox proportional hazards regression models were used to examine the prognostic factors associated with PRS. Backward stepwise selection was used to determine the parsimonious multivariate models. The proportional hazards assumption is tested using the scaled Schoenfeld residuals and there was no evidence for non-proportionality in any of the models presented. All tests were two-sided and a P-value <0.05 was used for statistical significance. Analysis was performed using the SAS statistical software (version 9.3, SAS Institute Inc., Cary, NC, USA). Graphs were created using Prism 5.03 (GraphPad Software, Inc. La Jolla, CA, USA).
RESULTS
Characteristics of relapsed patients
One hundred and nine patients received allo-SCT between 1993 and 2008. Eighty three patients (76%) were in CP at transplant and ninety-three patients (84.5%) had never received TKI prior to transplantation. Fifty-nine patients (54%) relapsed (37 molecular and 22 hematological) and the median time from transplant to relapse was 1 year (363 days, range: 16 to 3773 days). Twenty (34%) patients died, 16 (80%) following relapse, and 4 (20%) from non-relapse causes. Ten-year overall survival post-transplant was 65% in relapsed patients. The characteristics of relapsed are described in Table 1. Forty-seven (80%) patients with relapse were TKI naïve. Eighteen (30.5%) had AP at the time of transplant while 41(69.5%) were in CP.
Table 1.
Characteristics of CML patients with relapse after allo-SCT
| n, (%) | ||
|---|---|---|
| Sex | Females | 21 (36%) |
| Males | 38 (64%) | |
| Age>40 | Yes | 25 (42%) |
| No | 34 (58%) | |
| Ethnicity | Blacks | 4(7%) |
| Whites | 13(22%) | |
| Asian | 11(19%) | |
| Hispanics | 30(51%) | |
| Unknown | 1(1%) | |
| Disease status | AP | 18(30%) |
| CP | 41(70%) | |
| Pre-transplant TKI use | Yes | 12 (20%) |
| No | 47 (80%) | |
| Graft source | BM | 9 (15%) |
| PBSC | 50 (85%) | |
| Female to male | Yes | 15 (75%) |
| No | 44 (25%) | |
| Donor CMV | Negative | 12 (20%) |
| Positive | 47 (80%) | |
| Recipient CMV | Negative | 9 (15%) |
| Positive | 50 (85%) | |
| aGVHD | Yes | 25 (42.4%) |
| No | 34 (57.6%) | |
| aGVHD grade II-IV | Yes | 17 (28.8%) |
| No | 42 (71.2%) | |
| cGVHD | Yes | 36 (61%) |
| No | 23 (39%) | |
| Grade of cGVHD | Extensive | 22 (37%) |
| Limited | 14 (23%) | |
| None | 23 (40%) | |
| IST>3 years | Yes | 14 (24%) |
| No | 43 (73%) | |
| Not available | 2 (3%) | |
| Outcome | Death | 20 (34%) |
| Alive | 39 (66%) | |
| Median time to death | Days (range) | 671 (88-2908) |
| Cause of death | Infection | 3 (15%) |
| Relapse | 16 (80%) | |
| Respiratory failure | 1 (5%) |
Abbreviations: AP: Advanced phase; CP: Chronic phase; CMV: Cytomegalovirus BM: bone marrow; PBSC, peripheral blood stem cell; aGVHD: acute graft versus host disease; cGVHD: chronic graft versus host disease; IST: immunosuppressive therapy; TKI: tyrosine kinase inhibitor
Post relapse outcome
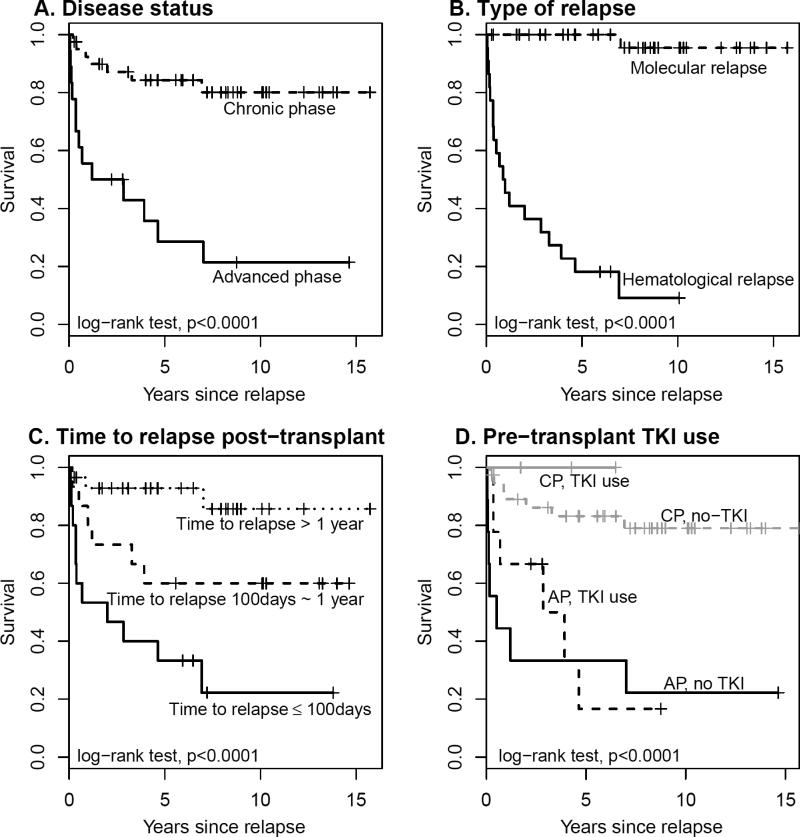
The median follow-up from relapse was 7.9 years (2899 days: range 98-5744). After salvage therapy for relapse, 26 (44.1%) patients became persistently negative for BCR-ABL, while 33 (55.9%) never achieved a permanent negative BCR-ABL pattern (persistently positive). Patient characteristics of persistently negative vs. persistently positive individuals are shown in Table 2. After relapse 34 patients (57.6%) received TKI; 42 (71.2%) received DLI; 15 (25.4%) received concurrent DLI and TKI. The 5-year post relapse survival (PRS) survival was 68%. In univariate analysis of the relationship of patient characteristics and PRS, CP disease status at first transplant, and molecular relapse, favorably influence the PRS (Figure 1 A&B). Earlier relapse post-transplant was associated with poor PRS (P<0.001), with 5-year PRS rate of 33%, 67% and 93% for patients with diagnosis of relapse within 100 days, between 100 days to 1 years, and after one year from transplant, respectively (Figure 1C). In contrast, age at transplant, age at relapse, gender, female to male transplant, graft source, recipient CMV serostatus, donor CMV serostatus, acute GvHD, pre-transplant TKI use, DLI, or concurrent DLI with TKI had no impact on post relapse survival. Pre-transplant TKI use showed a favorable trend on PRS after stratifying by the disease status (Figure 1D). In addition, patients with a persistently positive BCR-ABL pattern had a 5-year PRS of 41% compared to 100% for persistently negative pattern (P<0.001). In a univariate Cox model with a time-dependent covariate, the post-relapse TKI use had no effect on PRS. Multivariate analyses were performed to adjust for potential confounding factors. In the Cox regression models with backward elimination, significant factors associated with PRS were disease status, pre-transplant TKI use and time to diagnosis of relapse from transplant. These factors remained significant after controlling for age at relapse, gender, female to male transplant and graft source. Because 19/20 deaths were observed in the subgroups of patients with hematological relapse (N= 22) or persistent positive BCR-ABL (N=33), the Cox models were also performed separately for these subgroups and similar results were obtained (Table 3). Interestingly, pre-transplant TKI was found to be a protective factor for longer PRS after adjusting for the disease status and time to diagnosis of relapse from transplant, especially for high risk patients with hematological relapse or persistent positive BCR-ABL.
Table 2.
Characteristics of relapsed CML patients after allo-SCT based on BCR-ABL transcript pattern
| Persistently Negative (N= 26) | Persistently Positive (N= 33) | P value | ||
|---|---|---|---|---|
| n, (%) | n, (%) | |||
| Disease status | AP | 5 ( 19%) | 13 (39%) | 0.15 |
| CP | 21 (81%) | 20 (61%) | ||
| Pre-transplant TKI use | Yes | 3 (12%) | 9 (27%) | 0.20 |
| No | 23 (88%) | 24 (73%) | ||
| Post relapse TKI | Yes | 17 (65%) | 17 (52%) | 0.30 |
| No | 9 (35%) | 16 (48%) | ||
| Graft source | BM | 5 (19%) | 4 (12%) | 0.49 |
| PBSC | 21 (81%) | 29 (88%) | ||
| aGVHD grade II-IV | Yes | 9 (35%) | 8 (24%) | 0.40 |
| No | 17 (65%) | 25 (76%) | ||
| cGVHD grade | Extensive | 11 (42%) | 11 (33%) | 0.22 |
| Limited | 8 (31%) | 6 (18%) | ||
| No | 7 (27%) | 16 (49%) | ||
| IST>3years | Yes | 9 (35%) | 5 (15%) | 0.13 |
| No | 17 (65%) | 26 (85%) | ||
| Relapse type | Hematological | 1 (4%) | 21 (64%) | <0.001 |
| Molecular | 25 (96%) | 12 (36%) | ||
| Outcome | Death | 1 (4%) (1-AP) | 19 (58%) (12- AP; 7 CP) | <0.0001 |
| Alive | 25 (96%) | 14(42%) | ||
| Cause of death | Relapse | 0 (0%) | 16 (84%) (11-AP; 5CP) | 0.051 |
| Infection | 1 (100%) | 2 (11%) (2-CP) | ||
| Respiratory failure | 0 (0%) | 1 (5%) (1-AP) | ||
| Median post relapse survival | 3060 days | 814 days | <0.0001 (log-rank test) | |
Abbreviations: AP: Advanced phase; CP: Chronic phase; BM: bone marrow; PBSC, peripheral blood stem cell; aGVHD: acute graft versus host disease; cGVHD: chronic graft versus host disease; IST, immunosuppressive therapy; TKI: tyrosine kinase inhibitor
Figure 1.
Post relapse survival curve dichotomized by significantly associated factors A) disease status, B) type of relapse, C) time to diagnosis of relapse post-transplant, and D) pre-transplant TKI use.
Table 3.
Multivariate analysis for post-relapse survival risk factors.
| Factors | Model 1 (N=59 relapsed patients) | Model 2 (N=33 patients with persistent positive BCR-ABL) | Model 3 (N=22 patients with hematological relapse) | |||
|---|---|---|---|---|---|---|
| Adjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value | |
| Disease status at transplant (AP) | 15.9 (4.7–53.8) | <0.001 | 26.0 (6.3–107.7) | <0.0001 | 11.8 (2.8 – 50.3) | <0.001 |
| Time to relapse <100 days | 10.2 (3.2-32.8) | <0.001 | 5.3 (1.7– 16.6) | 0.004 | 2.5 (0.77 – 7.9) | 0.13 |
| Pre-transplant TKI use | 0.17 (0.04–0.67) | 0.011 | 0.08 (0.02 – 0.34) | <0.001 | 0.14 (0.03 – 0.66) | 0.012 |
Abbreviations: AP: Advanced phase; HR: hazard ratio; TKI: tyrosine kinase inhibitor
BCR-ABL expression in progenitor cells
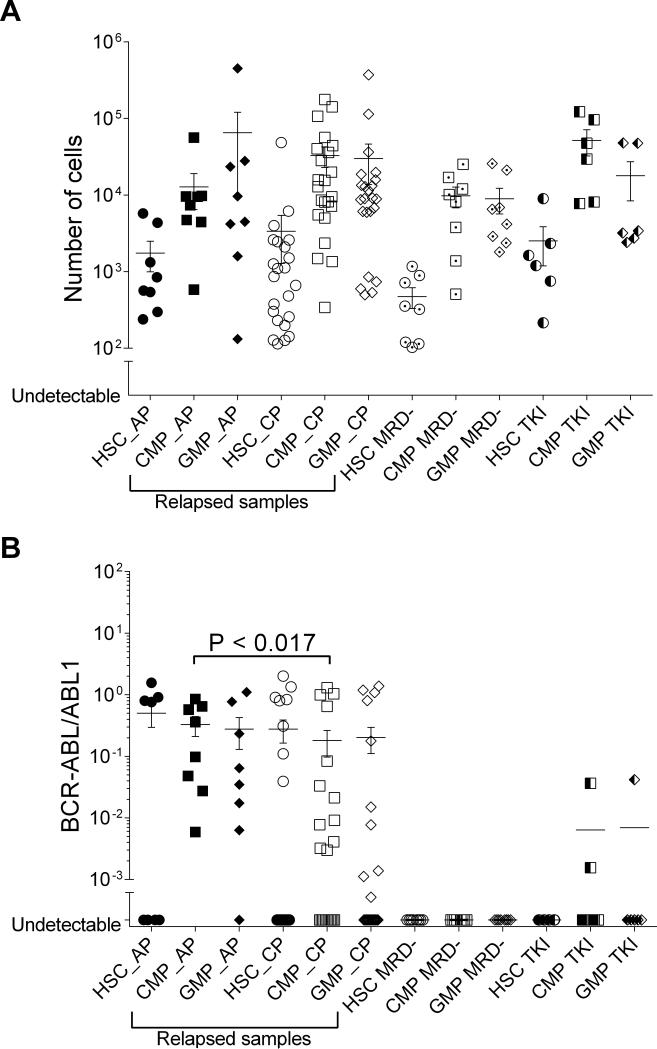
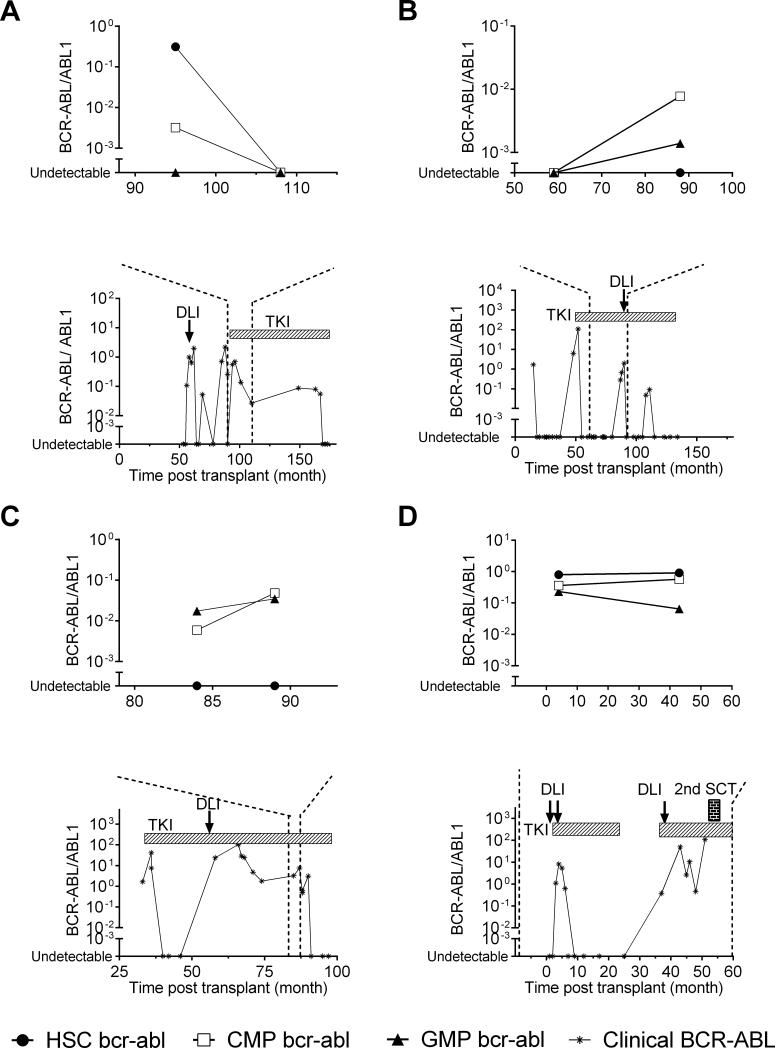
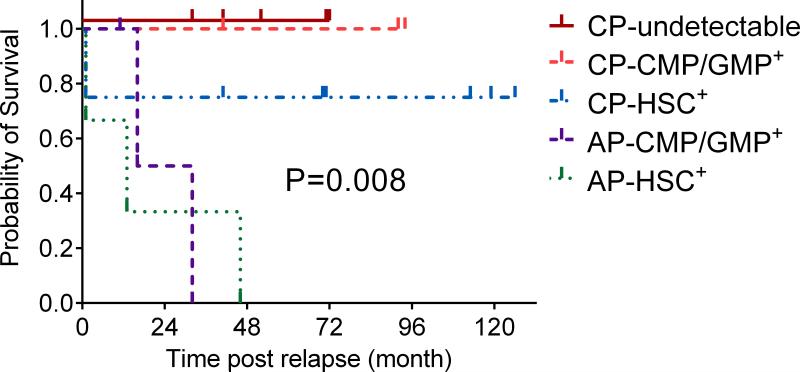
To evaluate biological characteristics of CML relapse after allo-SCT, we performed BCR-ABL analysis of the hematopoietic progenitor compartment in 36 patients (22 relapsed, 8 non-relapsed patients, and 6 TKI only controls). The characteristics of these 22 relapsed patients were not significantly different from the entire relapsed cohort (Supplementary Table 2). The distribution pattern of absolute numbers of progenitors sorted in the three compartments did not differ between relapsed and non-relapsed samples (Figure 2A). Seventy-seven percent of relapsed samples harbored BCR-ABL transcript in at least one of the progenitor cell compartments (HSC, CMP, or GMP), indicating relapse could first be detected as an expansion in any of the three progenitor compartments (Figure 2B). BCR-ABL transcripts were generally more detectable in the CMP/GMP compartment compared with the HSC group, irrespective of the time to diagnosis of relapse (Supplementary Figure 2) In AP, significantly higher BCRABL transcripts were detected in the CMP compartment (P< 0.017) compared to that in CP. BCR-ABL transcripts in HSC and GMP compartments were not significantly different between AP and CP. The distribution of BCR-ABL transcript in progenitor cell compartment in each individual sample was heterogeneous according to the disease status. In 8 CP, the BCR-ABL transcript levels were detected predominantly in the HSC compartment (Figure 3A), while in 3 CP, they were detected predominantly in the CMP/GMP compartment (Figure 3B); and 5 had no detectable BCR-ABL transcripts. In contrast in 3 AP, BCR-ABL transcript levels were detected predominantly in the CMP/GMP compartment (Figure 3C), while in the other 3AP, the BCRABL transcript levels were detected predominantly in the HSC compartment (Figure 3D). Therefore, we categorized the BCR-ABL distribution pattern into either 1) non-detectable BCRABL in the progenitor cells 2) HSC predominant or 3) CMP/GMP predominant. The BCR-ABL distribution pattern in progenitor cell compartment was highly correlated with PRS (P=0.008: Figure 4).
Figure 2.
Hematopoietic progenitor cells numbers assessed and BCR-ABL transcript in hematopoietic progenitor cell compartment based on disease status of 45 time points from 36 patients. A) Distribution of absolute number of hematopoietic progenitor cells B) Distribution of BCR-ABL transcript in hematopoietic progenitor cell compartments.
Abbreviations: HSC: hematopoietic stem cells; CMP: common myeloid progenitors, GMP: granulocyte- macrophage progenitors; CP: chronic phase; AP: advanced phase; MRD- : no minimal residual disease; TKI: control patients on TKI, who never received allo-SCT
Figure 3.
Representative data of BCR-ABL transcript levels in progenitor cell compartment and clinically monitored BCR-ABL values. A) CP with BCR-ABL transcript observed predominantly in the CMP or GMP compartment, B) CP with BCR-ABL transcript observed predominantly in the HSC compartment, C) AP with BCR-ABL transcript observed predominantly the CMP or GMP compartment, and D) AP with BCR-ABL transcript observed predominantly the HSC compartment. Abbreviations: HSC: HSC: hematopoietic stem cells; CMP: common myeloid progenitors, GMP: granulocyte- macrophage progenitors; DLI: donor lymphocyte infusion; TKI: tyrosine kinase inhibitors; 2nd SCT: second allogeneic stem cell transplantation
Figure 4.
Kaplan Meier survival plot comparing post relapse survival in AP vs. CP according to the distribution of BCR-ABL transcript pattern in the progenitor cell compartment.
Abbreviations: CP- undetectable: chronic phase patients with no detectable BCR-ABL transcript pattern; CP-HSC+ : chronic phase patients with BCR-ABL transcript pattern detected predominantly in hematopoietic stem cells; CP- CMP/GMP+ : chronic phase patients with BCRABL transcript pattern detected predominantly in common myeloid progenitors or granulocyte-macrophage progenitors; AP – CMP/GMP+ : advanced phase patients with BCR-ABL transcript pattern detected predominantly in common myeloid progenitors or granulocyte-macrophage progenitors; AP-HSC+ : advanced phase patients with BCR-ABL transcript pattern detected predominantly in hematopoietic stem cells.
DISCUSSION
In this study, we explored the features that affected the outcome of 59 patients relapsing after SCT for CML. This is the first detailed clinical and biological analysis of post relapse outcome in CML in the modern therapeutic era. Unlike acute myeloid leukemia which rarely recurs after 3 years post-transplant, late relapses at prolonged intervals from SCT occur in CML.16-18 However little is known about the mechanisms underlying relapse of CML after transplant. It is suggested that long lived leukemia initiating progenitors of CML persist in a dormant state.32, 33 In this model, the GVL effect is constantly needed to hold leukemia initiating cells from causing a relapse.34
The characteristics of CML relapse after allo-SCT may vary depending on the status of the disease at transplant and exposure to TKI pre transplant. Of the 20 patients who died after relapse in this series, all but one died directly of leukemia. We therefore have used survival post relapse as a surrogate for treatment success. Treatment for relapse included DLI, TKI, combinations of both with or without interferon, or second transplant. We previously reported the synergy between TKI and DLI in a smaller subset of this series with a shorter follow up.19 While there was an early survival advantage for use of DLI in addition to TKI, this significance is lost after longer follow-up. As is well established, we found that disease status at transplant was the major factor determining survival after relapse.35 We also found earlier relapse from transplant, and the absence of pre-transplant TKI exposure were significantly associated with poor survival after relapse. In addition, post-relapse BCR-ABL pattern was highly correlated with PRS, with patients who had persistently negative BCR-ABL surviving longer than those persistently positive as reported previously.32 The post-hoc power analysis showed that our study has 80% power to detect a hazard ratio of 4 (or 0.25) with a two-sided significance level of 0.05, thus our study would have limited power to identify risk factors with smaller effect. Nevertheless, all factors associated with poor post relapse outcomes may be explained by the persistence of MRD. Therefore, we speculate that leukemic HSC population might be the source of MRD and the last cell population to be eradicated by GVL.
Analysis of BCR-ABL in the progenitor cell compartments permitted a further categorization of post-relapse outcome. In CP, we found that the presence of BCR-ABL expression in HSC compartment was associated with poorer outcome compared to the group with BCR-ABL expression either predominant in the GMP/CMP compartment or undetectable in any progenitor cell compartment. In AP, all patients had BCR-ABL transcript in the CMP/GMP compartment as described previously21, however those with the worst prognosis had a predominance of BCRABL in the HSC compartment. The underlying biology of the leukemia stem cell in AP may affect immunogenicity, rendering them more resistant to GVL in comparison to CP, thus accounting for the poorer outcome in patients who are transplanted in AP, and poorer response clinically to DLI. Alternatively, leukemia HSC susceptibility to immune attack may be similar between CP and AP. Relapse therefore arises due to the persistence of a critical number of such cells, which in our study rendered them detectable. This critical number of leukemic stem cells would, stochastically, result in a greater probability of the emergence of a cell which has developed immunological36 or pharmacological resistance,37 or both, and intrinsically also has the ability to propagate leukemia. In our study, the sample size available for the analysis of progenitor cell compartment is too small to make a conclusion, however we hope our data may encourage further research to answer these hypothesis.
In summary, while relapse of CML post allo-SCT is often considered a relatively easily treated complication with a favorable outcome, this is only true for molecular relapse in patients with CP. Our study draws attention to a significant overall mortality following relapse of CML post-transplant (5-year mortality 30%). Therefore newer agents are required to manage post-transplant relapse in CML patients especially targeting the HSC compartment, particularly in AP.
Supplementary Material
Acknowledgments
This paper is dedicated to the memory of John Michael Goldman who inspired us with his insightful observations on the definition and nature of cure of CML
We thank N Hensel, S Miner, M Franco Colon, and F Chinian (Hematology Branch, NHLBI, NIH, USA) for their technical support. We also thank all patients who participated in this study.
Funding:
This research was supported by the Intramural Research Program of the National Institutes of Health, at the National Heart, Lung, and Blood Institute.
Footnotes
Author contributions:
N.A.J and S.I have equal authorship contributions.
Study concept and design (N.A.J., S.I., A.J.B. A.S.Y.): In vitro experiment and data collection (K.K., A.S.Y.): Analysis and interpretation of data (N.A.J., S.I., R.K., M.B., P.M., A.J.B., A.S.Y.): Drafting of the manuscript (N.A.J., S.I., R.K., M.B., C.H., P.M., A.J.B., A.S.Y.) : Statistical analysis (N.A.J., S.I., X.T.): Subject enrollment and clinical data collection (M.B., K.L., B.N.S., V.M, K.R., R.Q.L., A.S., E.K., J.S., A.J.B., A.S.Y) : Obtaining bone marrow samples from controls (V.M) : Obtained funding and study supervision (A.J.B.):
Competing interests:
All authors declare no conflict of interest.
Supplementary information is available at BMT's website
REFERENCES
- 1.Barrett AJ, van Rhee F. Graft-versus-leukaemia. Baillieres Clin Haematol. 1997;10(2):337–55. doi: 10.1016/s0950-3536(97)80011-x. [DOI] [PubMed] [Google Scholar]
- 2.Ringden O, Pavletic SZ, Anasetti C, Barrett AJ, Wang T, Wang D, et al. The graft-versus-leukemia effect using matched unrelated donors is not superior to HLA-identical siblings for hematopoietic stem cell transplantation. Blood. 2009;113(13):3110–8. doi: 10.1182/blood-2008-07-163212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clift RA, Buckner CD, Thomas ED, Doney K, Fefer A, Neiman PE, et al. Treatment of chronic granulocytic leukaemia in chronic phase by allogeneic marrow transplantation. Lancet. 1982;2(8299):621–3. doi: 10.1016/s0140-6736(82)92735-0. [DOI] [PubMed] [Google Scholar]
- 4.Goldman JM, Apperley JF, Jones L, Marcus R, Goolden AW, Batchelor R, et al. Bone marrow transplantation for patients with chronic myeloid leukemia. The New England journal of medicine. 1986;314(4):202–7. doi: 10.1056/NEJM198601233140403. [DOI] [PubMed] [Google Scholar]
- 5.Speck B, Bortin MM, Champlin R, Goldman JM, Herzig RH, McGlave PB, et al. Allogeneic bone-marrow transplantation for chronic myelogenous leukaemia. Lancet. 1984;1(8378):665–8. doi: 10.1016/s0140-6736(84)92179-2. [DOI] [PubMed] [Google Scholar]
- 6.Olavarria E, Ottmann OG, Deininger M, Clark RE, Bandini G, Byrne J, et al. Response to imatinib in patients who relapse after allogeneic stem cell transplantation for chronic myeloid leukemia. Leukemia. 2003;17(9):1707–12. doi: 10.1038/sj.leu.2403068. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian HM, O'Brien S, Cortes JE, Giralt SA, Rios MB, Shan J, et al. Imatinib mesylate therapy for relapse after allogeneic stem cell transplantation for chronic myelogenous leukemia. Blood. 2002;100(5):1590–5. [PubMed] [Google Scholar]
- 8.Ullmann AJ, Hess G, Kolbe K, Friedrich-Freksa A, Meyer RG, Gschaidmeier H, et al. Current results on the use of imatinib mesylate in patients with relapsed Philadelphia chromosome positive leukemia after allogeneic or syngeneic hematopoietic stem cell transplantation. Keio J Med. 2003;52(3):182–8. doi: 10.2302/kjm.52.182. [DOI] [PubMed] [Google Scholar]
- 9.Kim YJ, Kim DW, Lee S, Kim YL, Hwang JY, Park YH, et al. Cytogenetic clonal evolution alone in CML relapse post-transplantation does not adversely affect response to imatinib mesylate treatment. Bone marrow transplantation. 2004;33(2):237–42. doi: 10.1038/sj.bmt.1704332. [DOI] [PubMed] [Google Scholar]
- 10.DeAngelo DJ, Hochberg EP, Alyea EP, Longtine J, Lee S, Galinsky I, et al. Extended follow-up of patients treated with imatinib mesylate (gleevec) for chronic myelogenous leukemia relapse after allogeneic transplantation: durable cytogenetic remission and conversion to complete donor chimerism without graft-versus-host disease. Clin Cancer Res. 2004;10(15):5065–71. doi: 10.1158/1078-0432.CCR-03-0580. [DOI] [PubMed] [Google Scholar]
- 11.Goldman JM, Melo JV. Chronic myeloid leukemia--advances in biology and new approaches to treatment. The New England journal of medicine. 2003;349(15):1451–64. doi: 10.1056/NEJMra020777. [DOI] [PubMed] [Google Scholar]
- 12.Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108(6):1809–20. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 13.Jabbour E, Cortes J, Kantarjian HM, Giralt S, Jones D, Jones R, et al. Allogeneic stem cell transplantation for patients with chronic myeloid leukemia and acute lymphocytic leukemia after Bcr-Abl kinase mutation-related imatinib failure. Blood. 2006;108(4):1421–3. doi: 10.1182/blood-2006-02-001933. [DOI] [PubMed] [Google Scholar]
- 14.Pavlu J, Szydlo RM, Goldman JM, Apperley JF. Three decades of transplantation for chronic myeloid leukemia: what have we learned? Blood. 2011;117(3):755–63. doi: 10.1182/blood-2010-08-301341. [DOI] [PubMed] [Google Scholar]
- 15.Silver RT. The blast phase of chronic myeloid leukaemia. Best practice & research. Clinical haematology. 2009;22(3):387–94. doi: 10.1016/j.beha.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 16.van Rhee F, Lin F, Cullis JO, Spencer A, Cross NC, Chase A, et al. Relapse of chronic myeloid leukemia after allogeneic bone marrow transplant: the case for giving donor leukocyte transfusions before the onset of hematologic relapse. Blood. 1994;83(11):3377–83. [PubMed] [Google Scholar]
- 17.Goldman JM, Majhail NS, Klein JP, Wang Z, Sobocinski KA, Arora M, et al. Relapse and late mortality in 5-year survivors of myeloablative allogeneic hematopoietic cell transplantation for chronic myeloid leukemia in first chronic phase. J Clin Oncol. 2010;28(11):1888–95. doi: 10.1200/JCO.2009.26.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yong AS, Goldman JM. Relapse of chronic myeloid leukaemia 14 years after allogeneic bone marrow transplantation. Bone marrow transplantation. 1999;23(8):827–8. doi: 10.1038/sj.bmt.1701729. [DOI] [PubMed] [Google Scholar]
- 19.Savani BN, Montero A, Kurlander R, Childs R, Hensel N, Barrett AJ. Imatinib synergizes with donor lymphocyte infusions to achieve rapid molecular remission of CML relapsing after allogeneic stem cell transplantation. Bone marrow transplantation. 2005;36(11):1009–15. doi: 10.1038/sj.bmt.1705167. [DOI] [PubMed] [Google Scholar]
- 20.Goldman JM. The significance of BCR-ABL transcripts after allogeneic stem cell transplantation for chronic myeloid leukemia. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013;19(5):679–80. doi: 10.1016/j.bbmt.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. The New England journal of medicine. 2004;351(7):657–67. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 22.Solomon SR, Nakamura R, Read EJ, Leitman SF, Carter C, Childs R, et al. Cyclosporine is required to prevent severe acute GVHD following T-cell-depleted peripheral blood stem cell transplantation. Bone Marrow Transplant. 2003;31(9):783–8. doi: 10.1038/sj.bmt.1703928. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal R, Doren S, Hicks B, Dunbar CE. Long-term culture of chronic myelogenous leukemia marrow cells on stem cell factor-deficient stroma favors benign progenitors. Blood. 1995;85(5):1306–12. [PubMed] [Google Scholar]
- 24.Mensink E, van de Locht A, Schattenberg A, Linders E, Schaap N, Geurts van Kessel A. Quantitation of minimal residual disease in Philadelphia chromosome positive chronic myeloid leukaemia patients using real-time quantitative RT-PCR. British journal of haematology. 1998;102(3):768–74. doi: 10.1046/j.1365-2141.1998.00823.x. [DOI] [PubMed] [Google Scholar]
- 25.Savani BN, Rezvani K, Mielke S, Montero A, Kurlander R, Carter CS, et al. Factors associated with early molecular remission after T cell-depleted allogeneic stem cell transplantation for chronic myelogenous leukemia. Blood. 2006;107(4):1688–95. doi: 10.1182/blood-2005-05-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khoury HJ, Kukreja M, Goldman JM, Wang T, Halter J, Arora M, et al. Prognostic factors for outcomes in allogeneic transplantation for CML in the imatinib era: a CIBMTR analysis. Bone marrow transplantation. 2012;47(6):810–6. doi: 10.1038/bmt.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito S, Pophali P, Co W, Koklanaris EK, Superata J, Fahle GA, et al. CMV reactivation is associated with a lower incidence of relapse after allo-SCT for CML. Bone marrow transplantation. 2013 doi: 10.1038/bmt.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yong AS, Szydlo RM, Goldman JM, Apperley JF, Melo JV. Molecular profiling of CD34+ cells identifies low expression of CD7, along with high expression of proteinase 3 or elastase, as predictors of longer survival in patients with CML. Blood. 2006;107(1):205–12. doi: 10.1182/blood-2005-05-2155. [DOI] [PubMed] [Google Scholar]
- 29.Yong AS, Keyvanfar K, Eniafe R, Savani BN, Rezvani K, Sloand EM, et al. Hematopoietic stem cells and progenitors of chronic myeloid leukemia express leukemia-associated antigens: implications for the graft-versus-leukemia effect and peptide vaccine-based immunotherapy. Leukemia. 2008;22(9):1721–7. doi: 10.1038/leu.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beillard E, Pallisgaard N, van der Velden VH, Bi W, Dee R, van der Schoot E, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using ‘real-time’ quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR) - a Europe against cancer program. Leukemia. 2003;17(12):2474–86. doi: 10.1038/sj.leu.2403136. [DOI] [PubMed] [Google Scholar]
- 31.Gabert J, Beillard E, van der Velden VH, Bi W, Grimwade D, Pallisgaard N, et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia - a Europe Against Cancer program. Leukemia. 2003;17(12):2318–57. doi: 10.1038/sj.leu.2403135. [DOI] [PubMed] [Google Scholar]
- 32.Kaeda J, O'Shea D, Szydlo RM, Olavarria E, Dazzi F, Marin D, et al. Serial measurement of BCR-ABL transcripts in the peripheral blood after allogeneic stem cell transplantation for chronic myeloid leukemia: an attempt to define patients who may not require further therapy. Blood. 2006;107(10):4171–6. doi: 10.1182/blood-2005-08-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sobrinho-Simoes M, Wilczek V, Score J, Cross NC, Apperley JF, Melo JV. In search of the original leukemic clone in chronic myeloid leukemia patients in complete molecular remission after stem cell transplantation or imatinib. Blood. 2010;116(8):1329–35. doi: 10.1182/blood-2009-11-255109. [DOI] [PubMed] [Google Scholar]
- 34.Barrett AJ. Understanding and harnessing the graft-versus-leukaemia effect. British journal of haematology. 2008;142(6):877–88. doi: 10.1111/j.1365-2141.2008.07260.x. [DOI] [PubMed] [Google Scholar]
- 35.Boehm A, Walcherberger B, Sperr WR, Wohrer S, Dieckmann K, Rosenmayr A, et al. Improved outcome in patients with chronic myelogenous leukemia after allogeneic hematopoietic stem cell transplantation over the past 25 years: a single-center experience. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(1):133–40. doi: 10.1016/j.bbmt.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 36.Eisterer W, Jiang X, Christ O, Glimm H, Lee KH, Pang E, et al. Different subsets of primary chronic myeloid leukemia stem cells engraft immunodeficient mice and produce a model of the human disease. Leukemia. 2005;19(3):435–41. doi: 10.1038/sj.leu.2403649. [DOI] [PubMed] [Google Scholar]
- 37.Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest. 2011;121(1):396–409. doi: 10.1172/JCI35721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.