Abstract
Glucuronoxylomannan (GXM) is the major capsular polysaccharide of Cryptococcus neoformans. It is essential for fungal virulence and causes a number of deleterious effects to host cells. During the last decades, most of the experimental models designed to study the roles of GXM during cryptococcal infection were based on the stimulation of animal cells. This most commonly involved macrophages or other effector cells, with polysaccharide fractions obtained by precipitation with cationic detergents. More recently, it has been demonstrated that GXM interferes with the physiological state of other target cells, such as the epithelium. In addition, recent studies indicate that the structure of the polysaccharide and, consequently, its functions vary according with the method used for its purification. This raises questions as to what is native GXM and the significance of prior studies. In this paper, we discuss some of the aspects of GXM that are still poorly explored in the current literature, including the relevance of the polysaccharide in the interaction of cryptococci with non-phagocytic cells and the relationship between its structure and biological activity.
Keywords: glucuronoxylomannan, polysaccharide structure, epithelial infection, Cryptococcus neoformans
Cryptococcus and capsular components
Cryptococcus neoformans and C. gattii are pathogenic fungi that infect humans and animals after gaining access to the host through the inhalation of environmental propagules [1]. C. neoformans, but not C. gattii, causes disease primarily in immunocompromised individuals. Inhaled cells of both species are first deposited into the alveolar space, where they are supposed to be confronted by alveolar macrophages and to interact with other such local components as epithelial cells and surfactant proteins. From the alveoli, cryptococci cells migrate to the lung interstitium, where a usually efficient cell-mediated immune response is established [1]. However, in immunocompromised hosts, the pulmonary infection can progress to disseminated disease, allowing the fungus to reach the central nervous system. Dissemination in such cases is thought to occur after primary pulmonary infection or reactivation of a latent infection. Cryptococcal meningitis is usually difficult to treat and is associated with high mortality [2].
Polysaccharide capsules are common features of bacterial pathogens, but in fungi they are exclusively associated with Cryptococcus species [3]. The synthesis and expression of capsular components are essential virulence traits of C. neoformans and C. gattii, as has been extensively reviewed in the literature [4–8]. The cryptococcal capsule is mainly composed of two different polysaccharides. Galactoxylomannan (GalXM), an α1–6 linked galactan containing different types of mannosyl and xylosyl substitutions, is a minor component of the capsule [9,10]. Glucuronoxylomannan (GXM) is a high-molecular weight polysaccharide that accounts for about 90% of the capsular mass [10]. GXM is an α1–3 linked mannan containing β1,2 and β1,4 xylosyl substitutions, as well as β1,2-linked glucuronyl residues [3]. GXM, which is also O-acetylated at the carbon 6 of some of the mannosyl units, forms a heterogeneous macromolecular complex [10] with a molecular mass in the range of 1 to 7×10 7 Daltons. In contrast to capsular components of bacteria, GXM is synthesized in the cytoplasm [11–13] and transferred in vesicles to the extracellular space [13,14], where it is connected to the cell wall and used for distal capsular enlargement [15]. Biosynthesis of GXM involves a number of glycosyltransferases [16], and its assembly at the cell surface apparently requires interactions with cell wall glucan [17,18] and chitin-like molecules [19], as well as cation-mediated self-aggregation [20].
GXM is essential for the virulence of Cryptococcus species [3] in that it provides protection against phagocytosis, modulation of different cytokines and chemokines, and interference with leukocyte migration [4–8]. The mechanisms by which GXM modulates the immune response are multiple and several excellent reviews about the subject are available [4–8]. Remarkably, most of the studies from which the functions of GXM were established used cells from the phagocytic lineage, including macrophages, endothelial cells and neutrophils. However, the impact of GXM production by C. neoformans on non-phagocytic cells has been very poorly explored.
The role of GXM in interaction of Cryptococcus with epithelial cells
It is believed that the alveolar epithelium and macrophages are the first host cells to interact with cryptococci. The role of macrophages in C. neoformans infections is well known, but virtually nothing is known about the bioactivity of epithelial cells in fungal diseases. The epithelium has, in fact, been shown to have a key role in the defense against bacterial pathogens, as for instance, through the extracellular release of proinflammatory cytokines [21] and antimicrobial peptides [22]. As reviewed by Zhang and co-workers [23], the release of proinflammatory chemokines by epithelial cells and alveolar macrophages can be associated with the recruitment of large numbers of polymorphonuclear leukocytes (PMNs) from the pulmonary vasculature into the alveolar space. The recruited PMNs are thought to provide auxiliary phagocytic capacities that are critical for the effective eradication of offending pathogens. However, in mice the PMN influx may be deleterious to the establishment of an effective cellular response [24]. In this context, the interaction of C. neoformans with alveolar epithelial cells could be critical to the control or progress of cryptococcosis.
The interaction of cryptococci with epithelial cells has been explored in a few studies. Previous work demonstrated that adhesins [25], phospholipase B [26] and GXM [27] are important elements influencing the adhesion of C. neoformans to lung epithelial cells. The latter include human type II alveolar cells (A549 cell lineage) [26], immortalized bronchial epithelial cells and primary normal human bronchial epithelium [28]. As detailed below, the outcome of the interaction of C. neoformans with lung epithelial cells includes the induction of the chemokines IL-8 and CXCL1 [28,29], production of the transcription factor CEBP/β[28] and significant reductions in tumor necrosis factor-alpha (TNF-alpha)-induced intercellular adhesion molecule-1 (ICAM-1) expression [30]. Therefore, it is reasonable to expect that epithelial cells contribute to the initial stages of pulmonary host response to C. neoformans.
The first evidence that GXM can mediate the interaction of cryptococci with epithelial cells was provided by Barbosa and co-workers [27]. A monoclonal antibody to GXM inhibited yeast adhesion to a human type II epithelial alveolar cell lineage. Human cells were able to bind GXM, which was deposited at the cell surface after short periods of incubation. After a prolonged exposure, the epithelial cells internalized the polysaccharide [27]. Living C. neoformans cells were also able to reach intracellular compartments of the epithelial cells, which culminated with the death of the host cell. These results, together with the fact that GXM induced IL-8 production by epithelial cells [29], led to the supposition that host epithelial cells express receptors for GXM, which could mediate fungal attachment, invasion and host response. Interestingly, similar experiments using epithelial bronchial cells produced opposite results, i.e., both chemokine activation and cell damage were reduced in the presence of capsular polysaccharides [28]. This result, which suggests an immunosuppressive effect of GXM for bronchial cells, may indicate that the local epithelium-mediated immune response is differentially regulated at distinct host microenvironments.
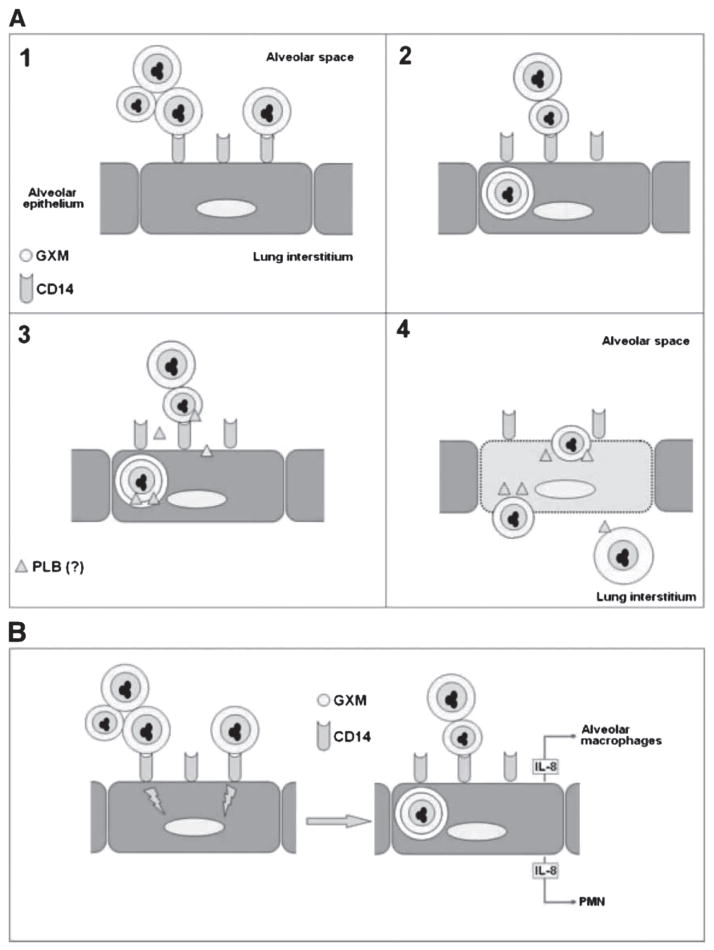
A number of host receptors for GXM have been described in cells of the phagocytic lineage, including members of the TLR family, CD18 and CD14 [5]. However, epithelial receptors for GXM remained unknown until very recently. Following the first study suggesting that alveolar epithelial cells express GXM receptors, Barbosa and co-workers demonstrated that the host receptor used by epithelial alveolar cells for GXM binding is CD14 [29]. The receptor, which was isolated from the epithelial cells in association with GXM, was also involved in the GXM-mediated induction of IL-8. This was the first report on an epithelial receptor for a cryptococcal antigen which was associated to a biological response. These results could have an impact on the understanding, for instance, of how PMNs could be recruited from the pulmonary vasculature into the alveolar space in cryptococcosis. Such mechanism, which could be determinant in disease control, brings to the light the potential role of epithelial respiratory cells in the immunity against C. neoformans. A model to explain the role of capsular GXM in the epithelial infection of the alveoli by cryptococci is proposed in Fig. 1.
Fig. 1.
The putative functions of GXM during the infection of alveolar epithelial cells by Cryptococcus neoformans. (A) Once deposited into the alveolar space, C. neoformans is confronted by alveolar macrophages (not shown) or interact with epithelial cells (Panel 1). The adhesion of cryptococci to type II alveolar epithelial cells occurs via GXM binding to the CD14 receptor [29], in a process that is followed by fungal invasion (Panel 2) by still unknown mechanisms [27]. Phospholipase B (PLB), a membrane-damaging enzyme, is a potential candidate to mediate and/or amplify invasion (Panel 3), since its involvement in the interaction of C. neoformans with epithelial cells has already been demonstrated [26]. Prolonged interactions of Cryptococcus neoformans with the epithelial cells is known to induce host cell damage [27, 28], which may allow cryptococci to cross the epithelial barrier to reach the lung interstitium (Panel 4). B. The GXM-induced release of the proinflammatory chemokines IL-8 by epithelial cells could be associated with the recruitment of polymorphonuclear leukocytes (PMN) from the pulmonary vasculature into the alveolar space, as well as with stimulation of alveolar macrophages.
Purification of native GXM reveals new structural and functional features of the polysaccharide
Capsule size in C. neoformans and C. gattii is regulated by several environmental conditions including; CO2 availability, iron concentration and medium osmolarity [31]. Capsule thickness is also dependent on the nature of the infected tissue [32]. Capsular polysaccharides are continually secreted in vitro and in vivo to the extracellular milieu [9]. Virtually all information about the structure of cryptococcal GXM has originated from studies using exopolysaccharides, which are recovered from culture supernatants [33].
GXM purification and structural determination studies were pioneered in the late 1970s by Bhattacharjee and colleagues [34–37] and further extended by Cherniak and co-workers [33,38,39]. Classically, the protocols for GXM purification involved precipitation of total polysaccharides in culture supernatants with ethanol followed by selective precipitation of GXM by cetyl trimethylammonium bromide (CTAB). CTAB is a cationic detergent which associates to the negatively charged glucuronic acids residues of GXM. Although CTAB purification of GXM has been used by many authors, the method is plagued by problems in removing this detergent from polysaccharide fractions. In this regard, it is not clear if the potential presence of CTAB in ‘purified’ GXM fractions could cause additional structural modifications, changing the polysaccharide properties, and consequently impacting the cellular responses.
We have recently described that C. neoformans produces extracellular vesicles that are potentially involved in the trans-cell wall transport of GXM [14]. The initial protocols of vesicle isolation involved concentration of supernatants in ultrafiltration cells. During this procedure, we have repeatedly observed that thick, viscous films were reproducibly formed over the filtration disc after removal of water from the supernatants. Due to the well-known ability of GXM to self aggregate [10], we hypothesized that the film could contain the polysaccharide in relatively high concentrations. The viscous material was then collected and analyzed by gas chromatography coupled to mass spectrometry and nuclear magnetic resonance. The film was demonstrated to be highly enriched in the building units of GXM [20]. Galactose detection was negative [20] or positive in very low levels [40], indicating that GalXM contamination was minimal.
Divalent cations are known to interact with carboxylic acid groups of acidic polysaccharides, yielding an aggregated structure with increased viscosity [41]. Divalent cations are abundant in the most commonly used chemically defined media for cryptococcal growth [20]. Therefore, it has been speculated that GXM aggregation depended on the interaction of the polysaccharide with divalent cations, which has been in fact confirmed by a number of techniques [20]. Surprisingly, light scattering analysis revealed that the average molecular mass of GXM obtained by cation-dependent aggregation was nine-fold smaller than that obtained by CTAB precipitation [20,40].
The finding that CTAB-purified polysaccharides presented an average molecular mass considerably higher than that of jellified GXM implied that structural changes are induced by detergent binding. Consistent with this notion, circular dichroism spectral analysis revealed that purified GXM to which CTAB was added had larger molar ellipticity signals in the far UV region than the filtered polysaccharide [40]. This result implied that CTAB altered the secondary structure of GXM. The structural differences between the polysaccharide fractions obtained by the two different methods could imply distinct biological activities. Although information on this subject is still limited, two independent studies demonstrated that GXM fractions obtained by filtration or CTAB precipitation are differentially recognized by monoclonal antibodies raised to the polysaccharide [20,40]. These results suggest that studies on the functions of GXM may be influenced by the polysaccharide purification method. Noteworthy, the vast majority of the GXM literature has been based on the functions of the CTAB-precipitated exopolysaccharide.
The aggregation of GXM in ultrafiltration cells through the formation of metal bridges suggested that similar processes could occur at the cryptococcal surface. The structural studies described above were therefore correlated with previous work on the relationship of cations with capsule size in C. neoformans [31]. The parameter used to evaluate GXM aggregation was viscosity as measured by an optical tweezers system [20]. Confirming that divalent metals were responsible for GXM aggregation, a monovalent cation (Na +) and EDTA significantly decreased the solution’s viscosity. Both Na + and EDTA were described to reduce capsule size [20,42] which suggests a connection between GXM aggregation and capsule growth. Interestingly, measurements in the presence of a divalent cation (Ca ++) showed an initial increase of viscosity at low metal concentrations, which then decreased expressively as Ca ++ increased [20]. This effect is in accord with a stoichiometric correlation between the divalent ions and the single negative charges of glucuronic acid. In this regard, the ideal conditions for GXM aggregation would require two glucuronic acids for each atom of Ca ++. In the presence of an enhanced concentration of the divalent ion, the viscosity decreases because of unilateral saturation of glucuronic acid residues with divalent cation, reducing the aggregation of GXM molecules (Fig. 2). Accordingly, chemical reduction of carboxyl groups of glucuronic acid also resulted in GXM solutions with reduced viscosity [20]. Interestingly, the effects of divalent metals on capsule growth followed the same pattern observed in viscosity assays [20].
Fig. 2.
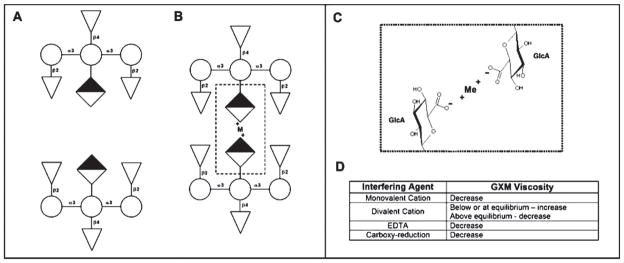
GXM aggregation in the presence of divalent metals. GXM triads, comprising mannose (circules), xylose (triangles) and glucuronic acid (diamonds), are shown in the absence (A) or presence (B) of divalent cations. (C) Two residues of glucuronic acid are connected through one divalent cation. Viscosity, a parameter used to measure GXM aggregation, can be affected by different interfering agents (D).
The basic building units of GXM are always the same, independent of the strain or species (C. neoformans and C. gattii). However, capsule and polysaccharide tridimensional structures are highly variable, depending on genetic background or modulation by environmental components, including host structures during infection [31,32]. For instance, McFadden and coworkers [10] demonstrated that exopolysaccharide fractions of GXM of different serotypes have very distinct molecular masses. Surface associated polysaccharides also appear to be very heterogeneous, as demonstrated by the existence of capsular regions of higher density, usually closer to the cell wall, and the existence of GXM fibers with variable morphologies [9,10,43]. These observations may indicate that secreted and surface-associated polysaccharides show different properties, as detailed below.
GXM can be released from the cell surface by gamma radiation or DMSO extraction [43,44]. When used in sequence, DMSO and gamma radiation efficiently remove the capsular material as evidenced by the fact that they are no longer visualized by Indian ink staining. Interestingly, GXM fractions recovered from culture supernatants, which have historically provided an ample and convenient source of material for structural and immunological studies, significantly differ from capsular material in terms of glycosyl composition, molecular mass, diameter, charge, viscosity, spectral properties and reactivity with monoclonal antibodies [20,40]. This may imply that exopolysaccharides and capsular GXM originate from different synthetic pools. In this regard, the major assumption that the structural features of exopolysaccharide material faithfully mirror those of capsular structures may be not accurate. This supposition certainly opens a new avenue of investigation on the assembly of the cryptococcal capsule and also on the roles of GXM during interaction of C. neoformans with host cells.
Acknowledgments
MLR and LN are supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) and Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ, Brazil). AC is supported by NIH grants AI033142, AI033774, AI052733, and HL059842. We are indebted to Dr Rosana Puccia for helpful discussions and Dr Luiz R. Travassos for constant support and mentorship.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Perfect JR, Casadevall A. Cryptococcosis. Infect Dis Clin North Am. 2002;16:837–874. v–vi. doi: 10.1016/s0891-5520(02)00036-3. [DOI] [PubMed] [Google Scholar]
- 2.Bicanic T, Harrison TS. Cryptococcal meningitis. Br Med Bull. 2004;72:99–118. doi: 10.1093/bmb/ldh043. [DOI] [PubMed] [Google Scholar]
- 3.Doering TL. How does Cryptococcus get its coat? Trends Microbiol. 2000;8:547–553. doi: 10.1016/s0966-842x(00)01890-4. [DOI] [PubMed] [Google Scholar]
- 4.Ellerbroek PM, Walenkamp AM, Hoepelman AI, Coenjaerts FE. Effects of the capsular polysaccharides of Cryptococcus neoformans on phagocyte migration and inflammatory mediators. Curr Med Chem. 2004;11:253–266. doi: 10.2174/0929867043456188. [DOI] [PubMed] [Google Scholar]
- 5.Levitz SM. Receptor-mediated recognition of Cryptococcus neoformans. Nippon Ishinkin Gakkai Zasshi. 2002;43:133–136. doi: 10.3314/jjmm.43.133. [DOI] [PubMed] [Google Scholar]
- 6.Pirofski LA. Polysaccharides, mimotopes and vaccines for fungal and encapsulated pathogens. Trends Microbiol. 2001;9:445–451. doi: 10.1016/s0966-842x(01)02134-5. [DOI] [PubMed] [Google Scholar]
- 7.Vecchiarelli A. The cellular responses induced by the capsular polysaccharide of Cryptococcus neoformans differ depending on the presence or absence of specific protective antibodies. Curr Mol Med. 2005;5:413–420. doi: 10.2174/1566524054022585. [DOI] [PubMed] [Google Scholar]
- 8.Vecchiarelli A. Immunoregulation by capsular components of Cryptococcus neoformans. Med Mycol. 2000;38:407–417. doi: 10.1080/mmy.38.6.407.417. [DOI] [PubMed] [Google Scholar]
- 9.McFadden D, Zaragoza O, Casadevall A. The capsular dynamics of Cryptococcus neoformans. Trends Microbiol. 2006;14:497–505. doi: 10.1016/j.tim.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 10.McFadden DC, De Jesus M, Casadevall A. The physical properties of the capsular polysaccharides from Cryptococcus neoformans suggest features for capsule construction. J Biol Chem. 2006;281:1868–1875. doi: 10.1074/jbc.M509465200. [DOI] [PubMed] [Google Scholar]
- 11.Feldmesser M, Kress Y, Casadevall A. Dynamic changes in the morphology of Cryptococcus neoformans during murine pulmonary infection. Microbiology. 2001;147:2355–2365. doi: 10.1099/00221287-147-8-2355. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Rivera J, Chang YC, Kwon-Chung KJ, Casadevall A. Cryptococcus neoformans CAP59 (or Cap59p) is involved in the extracellular trafficking of capsular glucuronoxylomannan. Eukaryot Cell. 2004;3:385–392. doi: 10.1128/EC.3.2.385-392.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoneda A, Doering TL. A eukaryotic capsular polysaccharide is synthesized intracellularly and secreted via exocytosis. Mol Biol Cell. 2006;17:5131–5140. doi: 10.1091/mbc.E06-08-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodrigues ML, Nimrichter L, Oliveira DL, et al. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot Cell. 2007;6:48–59. doi: 10.1128/EC.00318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaragoza O, Telzak A, Bryan RA, Dadachova E, Casadevall A. The polysaccharide capsule of the pathogenic fungus Cryptococcus neoformans enlarges by distal growth and is rearranged during budding. Mol Microbiol. 2006;59:67–83. doi: 10.1111/j.1365-2958.2005.04928.x. [DOI] [PubMed] [Google Scholar]
- 16.Klutts JS, Yoneda A, Reilly MC, Bose I, Doering TL. Glycosyltransferases and their products: cryptococcal variations on fungal themes. FEMS Yeast Res. 2006;6:499–512. doi: 10.1111/j.1567-1364.2006.00054.x. [DOI] [PubMed] [Google Scholar]
- 17.Reese AJ, Doering TL. Cell wall alpha-1,3-glucan is required to anchor the Cryptococcus neoformans capsule. Mol Microbiol. 2003;50:1401–1409. doi: 10.1046/j.1365-2958.2003.03780.x. [DOI] [PubMed] [Google Scholar]
- 18.Reese AJ, Yoneda A, Breger JA, et al. Loss of cell wall alpha(1–3) glucan affects Cryptococcus neoformans from ultrastructure to virulence. Mol Microbiol. 2007;63:1385–1398. doi: 10.1111/j.1365-2958.2006.05551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodrigues ML, Alvarez M, Fonseca FL, Casadevall A. Binding of the wheat germ lectin to Cryptococcus neoformans suggests an association of chitinlike structures with yeast budding and capsular glucuron-oxylomannan. Eukaryot Cell. 2008;7:602–609. doi: 10.1128/EC.00307-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nimrichter L, Frases S, Cinelli LP, et al. Self-aggregation of Cryptococcus neoformans capsular glucuronoxylomannan is dependent on divalent cations. Eukaryot Cell. 2007;6:1400–1410. doi: 10.1128/EC.00122-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Backhed F, Hornef M. Toll-like receptor 4-mediated signaling by epithelial surfaces: necessity or threat? Microbes Infect. 2003;5:951–959. doi: 10.1016/s1286-4579(03)00189-8. [DOI] [PubMed] [Google Scholar]
- 22.Kumar A, Zhang J, Yu FS. Toll-like receptor 2-mediated expression of beta-defensin-2 in human corneal epithelial cells. Microbes Infect. 2006;8:380–389. doi: 10.1016/j.micinf.2005.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang P, Summer WR, Bagby GJ, Nelson S. Innate immunity and pulmonary host defense. Immunol Rev. 2000;173:39–51. doi: 10.1034/j.1600-065x.2000.917306.x. [DOI] [PubMed] [Google Scholar]
- 24.Mednick AJ, Feldmesser M, Rivera J, Casadevall A. Neutropenia alters lung cytokine production in mice and reduces their susceptibility to pulmonary cryptococcosis. Eur J Immunol. 2003;33:1744–1753. doi: 10.1002/eji.200323626. [DOI] [PubMed] [Google Scholar]
- 25.Merkel GJ, Scofield BA. The in vitro interaction of Cryptococcus neoformans with human lung epithelial cells. FEMS Immunol Med Microbiol. 1997;19:203–213. doi: 10.1111/j.1574-695X.1997.tb01089.x. [DOI] [PubMed] [Google Scholar]
- 26.Ganendren R, Carter E, Sorrell T, Widmer F, Wright L. Phospholipase B activity enhances adhesion of Cryptococcus neoformans to a human lung epithelial cell line. Microbes Infect. 2006;8:1006–1015. doi: 10.1016/j.micinf.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Barbosa FM, Fonseca FL, Holandino C, et al. Glucuronoxylomannan-mediated interaction of Cryptococcus neoformans with human alveolar cells results in fungal internalization and host cell damage. Microbes Infect. 2006;8:493–502. doi: 10.1016/j.micinf.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 28.Guillot L, Carroll SF, Badawy M, Qureshi ST. Cryptococcus neoformans induces IL-8 secretion and CXCL1 expression by human bronchial epithelial cells. Respir Res. 2008;9:9. doi: 10.1186/1465-9921-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbosa FM, Fonseca FL, Figueiredo RT, et al. Binding of glucuron-oxylomannan to the CD14 receptor in human A549 alveolar cells induces interleukin-8 production. Clin Vaccine Immunol. 2007;14:94–98. doi: 10.1128/CVI.00296-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merkel GJ, Scofield BA. The effects of Cryptococcus neoformans-secreted antigens on tumor necrosis factor-alpha-induced intercellular adhesion molecule-1 expression on human lung epithelial cells. FEMS Immunol Med Microbiol. 2000;29:329–332. doi: 10.1111/j.1574-695X.2000.tb01541.x. [DOI] [PubMed] [Google Scholar]
- 31.Zaragoza O, Casadevall A. Experimental modulation of capsule size in Cryptococcus neoformans. Biol Proced Online. 2004;6:10–15. doi: 10.1251/bpo68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivera J, Feldmesser M, Cammer M, Casadevall A. Organ-dependent variation of capsule thickness in Cryptococcus neoformans during experimental murine infection. Infect Immun. 1998;66:5027–5030. doi: 10.1128/iai.66.10.5027-5030.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cherniak R, Morris LC, Anderson BC, Meyer SA. Facilitated isolation, purification, and analysis of glucuronoxylomannan of Cryptococcus neoformans. Infect Immun. 1991;59:59–64. doi: 10.1128/iai.59.1.59-64.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhattacharjee AK, Kwon-Chung KJ, Glaudemans CP. Structural studies on the major, capsular polysaccharide from Cryptococcus bacillisporus serotype B. Carbohydr Res. 1980;82:103–111. doi: 10.1016/s0008-6215(00)85524-x. [DOI] [PubMed] [Google Scholar]
- 35.Bhattacharjee AK, Kwon-Chung KJ, Glaudemans CP. The structure of the capsular polysaccharide from Cryptococcus neoformans serotype D. Carbohydr Res. 1979;73:183–192. doi: 10.1016/s0008-6215(00)85488-9. [DOI] [PubMed] [Google Scholar]
- 36.Bhattacharjee AK, Kwon-Chung KJ, Glaudemans CP. On the structure of the capsular polysaccharide from Cryptococcus neoformans serotype C–II. Mol Immunol. 1979;16:531–532. doi: 10.1016/0161-5890(79)90081-6. [DOI] [PubMed] [Google Scholar]
- 37.Bhattacharjee AK, Kwon-Chung KJ, Glaudemans CP. On the structure of the capsular polysaccharide from Cryptococcus neoformans serotype C. Immunochemistry. 1978;15:673–679. doi: 10.2196/40846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cherniak R, Jones RG, Reiss E. Structure determination of Cryptococcus neoformans serotype A-variant glucuronoxylomannan by 13C-n.m.r. spectroscopy. Carbohydr Res. 1988;172:113–138. doi: 10.1016/s0008-6215(00)90846-2. [DOI] [PubMed] [Google Scholar]
- 39.Cherniak R, Reiss E, Slodki ME, Plattner RD, Blumer SO. Structure and antigenic activity of the capsular polysaccharide of Cryptococcus neoformans serotype A. Mol Immunol. 1980;17:1025–1032. doi: 10.1016/0161-5890(80)90096-6. [DOI] [PubMed] [Google Scholar]
- 40.Frases S, Nimrichter L, Viana NB, Nakouzi A, Casadevall A. Cryptococcus neoformans capsular polysaccharide and exopoly-saccharide fractions manifest physical, chemical, and antigenic differences. Eukaryot Cell. 2008;7:319–327. doi: 10.1128/EC.00378-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutherland IW. Exopolysaccharides in biofilms, flocs and related structures. Water Sci Technol. 2001;43:77–86. [PubMed] [Google Scholar]
- 42.Dykstra MA, Friedman L, Murphy JW. Capsule size of Cryptococcus neoformans: control and relationship to virulence. Infect Immun. 1977;16:129–135. doi: 10.1128/iai.16.1.129-135.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bryan RA, Zaragoza O, Zhang T, et al. Radiological studies reveal radial differences in the architecture of the polysaccharide capsule of Cryptococcus neoformans. Eukaryot Cell. 2005;4:465–475. doi: 10.1128/EC.4.2.465-475.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maxson ME, Cook E, Casadevall A, Zaragoza O. The volume and hydration of the Cryptococcus neoformans polysaccharide capsule. Fungal Genet Biol. 2007;44:180–186. doi: 10.1016/j.fgb.2006.07.010. [DOI] [PubMed] [Google Scholar]