Abstract
Objectives:
In older patients, bone mineral density (BMD) diminishes with age, increasing susceptibility to femoral neck fractures. Evidence has emerged that patients who should have dual x-ray absorptiometry scans to evaluate their bone health are not doing so. Because computed tomography (CT) attenuation has now been correlated with BMD thresholds relating to osteoporosis, virtually any existing CT scan that includes the L1 vertebra can be used to assess BMD. This study evaluates the utility of CT attenuation in characterizing BMD in patients after femoral neck fractures.
Methods:
The electronic medical records of adults who presented to a level I trauma center with hip fractures were evaluated for eligibility. Those with a CT scan of the abdomen or other CT scan with a complete view of the L1 vertebra were included. To measure attenuation, a region of interest was selected to include the body of the L1 vertebra in the axial plane and exclude the cortices and posterior venous complex.
Results:
Of the 589 patients reviewed, 217 met inclusion criteria; 112 were aged 18 to 64, while 105 were ≥65. Eight (7.1%) patients in the younger cohort had a mean CT attenuation below the 110-HU threshold set for 90% specificity, whereas 31 (29.5%) patients in the older cohort had a mean CT attenuation below this threshold. Using the 160-HU threshold set for 90% sensitivity, 39 (34.8%) patients of the younger cohort and 74 (70%) patients of the older cohort were osteoporotic; all differences in CT attenuation by age were strongly significant (P < .0001).
Conclusions:
A significantly larger proportion of older patients with hip fractures had osteoporosis, helping validate the utility of CT attenuation in this context. In addition, a large proportion of these patients already had these images available, thus potentially helping limit cost and unnecessary medical investigations.
Introduction
Hip fractures are a significant cause of morbidity in older patients,1,2 becoming more frequent as the population ages. The estimated number of hip fractures worldwide is projected to increase from 1.7 million in 1990 to 6.3 million in 2050.1 Although other fracture sites can result from osteoporosis, fractures at the femoral neck, vertebrae, and distal radius have been most strongly and consistently associated with osteoporosis among all risk groups.3,4
Osteoporosis can be characterized as high turnover or low turnover; in the former, osteoclast activity is increased and resorption lacunae are deeper and more numerous; in the latter, osteoblasts primarily fail to form bone during normal bone turnover. The main manifestation of osteoporosis, the high-turnover variant, occurs in menopausal women.5,6 Osteoporosis, which is considered a “silent disease” until a fracture occurs, is associated with impaired bone strength,7,8 which is influenced by a variety of factors9 but strongly correlates with bone mineral density (BMD).10-15
It is well established that structurally insufficient osteoporotic bone is predisposed to fracture16-18 and that BMD can affect fracture stability after osteoporotic fractures. Patients with osteoporosis have an increased frequency of early instability, malunion, and late carpal malalignment compared to those with normal BMD.19 Biomechanical studies have also established that osteoporosis reduces the strength of fixation of implants,20,21 while cadaveric studies have highlighted an inverse relationship between BMD and fracture magnitude.22 Although BMD testing is recommended for patients at risk of fractures,23,24 it often does not happen.24 We know that we can use opportunistic use of computed tomography (CT) attenuation from scans ordered for other purposes to generate good estimates of BMD.25 This project thus aims to elucidate the utility of opportunistic CT in detecting osteoporosis in patients with femoral neck fractures, enumerate the frequency with which osteoporosis occurs in these patients, and determine whether it is affected by age.
Methods
After institutional review board approval, we reviewed the records of patients who presented to our level I trauma center from June 2010 to December 2012. Adults (≥18) with an acute hip fracture were eligible for the study if they had a CT scan of their abdomen (or other CT scan with a view of L1) in the 6 months before or after their fracture. Patients were excluded if they were minors (<18), had a fracture through the L1 vertebra, or had a CT scan that was performed outside of the prescribed period. Images were acquired on General Electric scanners (VCT 64 slice or LightSpeed 16 slice; GE Healthcare, Waukesha, WI). The CT scanners used for acquiring images were calibrated daily during the period in which all initial images were taken.
Using a picture archiving and communications system, regions of interest (ROIs) were selected. The method was similar to that of Pickhardt and colleagues.25 The L1 vertebra was identified in the axial plane and viewed in bone and soft tissue windows. We chose the largest elliptical area over the body of L1 that could be selected without including the cortex. We also ensured that specific areas with hardware, focal heterogeneity, and the posterior venous complex were excluded from the ROI. For each patient, we measured mean, minimum, and maximum CT attenuation in HU.25
Bone mineral density was evaluated by comparing values for CT attenuation in HU with previously published thresholds.25 We evaluated the following thresholds: (1) ≤110 HU or >110 HU (to achieve about 90% specificity in differentiating osteoporosis from nonosteoporosis), (2) ≤135 HU or >135 HU (to achieve a balanced specificity and sensitivity in differentiating between osteoporosis and nonosteoporosis), and (3) ≤160 HU or >160 HU (to achieve a sensitivity of about 90% in differentiating osteoporosis from nonosteoporosis and a balanced sensitivity and specificity in differentiating normal and low BMD). The patient cohort was stratified by age into 2 groups (<65 and ≥65 years). The mean CT attenuation in HU was compared using Student t-test. For each threshold value, we compared the proportion of each age-stratified group above or below the threshold using Fisher exact test.
Results
Of 589 patients, 217 met inclusion criteria; 112 were 18 to 64, while 105 were ≥65. Age-related differences in the mechanism of fractures were significant, with the high-velocity mechanism accounting for 96 (86%) of the 112 younger patients and 10 (9.5%) of the 105 patients 65 and older (P = .00049). In all, 8 (7.1%) patients in the younger cohort had a mean CT attenuation below the 110-HU threshold set for 90% specificity, and 31 (29.5%) patients in the older cohort were below that threshold. Using the 160-HU threshold, set for 90% sensitivity, 39 (34.8%) patients in the younger cohort and 74 (70%) patients in the older cohort had osteoporosis; all differences in CT attenuation by age were strongly significant (P < .0001; Table 1). Finally, using the 135-HU threshold for balanced sensitivity and specificity, 18 (16%) of the 112 patients younger than 65 had BMD below this, while 56 (53%) of the 105 patients 65 and older had BMD below this threshold.
Table 1.
Comparison of Mean Computed Tomography Attenuation in the 2 Age Cohorts at Different Thresholds.
| <65 years (n = 112), % | ≥65 years (n = 105), % | P value | |
|---|---|---|---|
| ≤110 HU (90% specificity) | 7 (7.1) | 31 (29.5) | 1.6 × 10−5 |
| ≤135 HU (balanced sensitivity and specificity) | 18 (16.1) | 56 (53.3) | 6.2 × 10−9 |
| ≤160 HU (90% sensitivity) | 39 (34.8) | 74 (70.5) | 7.4 × 10−9 |
In the younger cohort, 7 (6%) of the 112 patients previously had at least 1 dual x-ray absorptiometry (DXA) scan. All but 2 of the patients were female, but the 2 male patients in this group had BMD of 207 and 230 HU, respectively—above all thresholds for differentiating osteoporosis from nonosteoporosis. In contrast, the 5 female patients who had a previous DXA scans were 50 to 64 years old and had mean CT attenuation of 79 to 125 HU (Table 2).
Table 2.
Characteristics of Patients Younger Than 65 With Dual X-Ray Absorptiometry and Computed Tomography Attenuation Data.
| Age | Sex | Mechanism of injury (high/low velocity) | Classification | DXA (yes/no) | Smoking | Surgery (yes/no) | Type of surgery | Previous fragility fracture | Mean CT attenuation |
|---|---|---|---|---|---|---|---|---|---|
| 37 | M | Fall, high | Right intertrochanteric hip | Yes | Yes | Yes | Intramedullary nail | No | 230 |
| 50 | F | Fall, low | Right femoral neck | Yes | No | Yes | Right total hip arthroplasty | No | 103 |
| 55 | M | Fall, high | Yes | Yes | No | 207 | |||
| 58 | F | Pathologic sacral, low | Yes | Yes | No | 79 | |||
| 59 | F | Pathologic, low | Right femoral head | Yes | No | Yes | Right total hip arthroplasty | No | 156 |
| 62 | F | Fall, low | Right femoral neck | Yes | Former | Yes | Right total hip arthroplasty | No | 125 |
| 63 | F | MVA, high | Petrochanteric left femur | Yes | No | Yes | Intramedullary nail | No | 92 |
| 64 | F | Fall, low | Left intertrochanteric femur | Yes | Former | Yes | Intramedullary nailing, left hip | No | 122 |
| 64 | F | Fall, low | Right intertrochanteric, subtrochanteric femur | Yes | Former | Yes | Intramedullary nail, intertrochanteric and subtrochanteric femur | No | 122 |
Abbreviations: CT, computed tomography; DXA, dual x-ray absorptiometry; F, female; M, male; MVA, motor vehicle accident.
In the older cohort, 27 (26%) of the 105 patients had undergone at least 1 DXA scan; of these, 19 were females and 8 were males. Overall, the values for CT attenuation were 34 to 312 HU. Of the older patients with hip fractures, 10 sustained these fractures, despite having BMD that was nonosteoporotic even at the highest threshold (160 HU; Table 3). The regression generated to evaluate age versus BMD showed a correlation, with R 2 of .369 (Figure 1).
Table 3.
Characteristics of Patients Aged 65 and Older With Dual X-Ray Absorptiometry and Computed Tomography Attenuation Data.
| Age | Sex | Mechanism of injury (high/low velocity) | Classification | DXA (yes/no) | smoking | Surgery (yes/no) | Type of surgery | Previous fragility fracture | Mean CT attenuation |
|---|---|---|---|---|---|---|---|---|---|
| 65 | F | MVA, high | Yes | No | No | 144 | |||
| 67 | F | Fall, low | Right femoral neck | Yes | Former | Yes | ORIF right femoral neck, capsulotomy right hip | No | 131 |
| 67 | F | Fall, low | Yes | Yes | No | No | 190 | ||
| 68 | F | Fall, low | Left intertrochanteric hip | Yes | No | Yes | ORIF left intertrochanteric hip | No | 92 |
| 68 | F | Fall, low | Right femoral neck | Yes | Former | Yes | ORIF right femur | No | 173 |
| 69 | F | Twist, low | Yes | No | No | 203 | |||
| 72 | F | Fall, low | Right intertrochanteric | Yes | Former | Yes | Right sliding hip screw | No | 111 |
| 73 | M | Fall, low | Right subcapital displaced femoral neck | Yes | Former | Yes | Right hemiarthroplasty | No | 186 |
| 74 | M | MVA, high | Left intertrochanteric femur | Yes | No | Yes | Cephalamedullary nailing left femur | No | 167 |
| 76 | F | Fall, low | Left femoral neck | Yes | No | Yes | Left hip hemiarthroplasty | No | 71 |
| 77 | M | Fall, low | Left basicervical femoral neck | Yes | Former | Yes | Left hip bipolar hemiarthroplasty | No | 105 |
| 77 | M | Fall, low | Yes | No | No | 118 | |||
| 78 | F | Fall, low | Right hip greater trochanteric | Yes | No | No | 102 | ||
| 78 | F | Fall, low | Left femoral neck | Yes | No | Yes | Left hip hemiarthroplasty | No | 93 |
| 79 | M | Fall, low | Left hip, greater trochanter | Yes | Former | No | Previous left femoral neck | 111 | |
| 80 | F | Fall, low | Right femoral neck | Yes | Former | Yes | ORIF right femoral neck | No | 271 |
| 81 | M | Fall, high | Left intertrochanteric hip | Yes | Former | Yes | Intermedullary nail left intertrochanteric hip | No | 160 |
| 81 | F | Fall, low | Left intertrochanteric | Yes | No | Yes | Left intermedullary nailing | No | 78 |
| 81 | F | Fall, low | Right femoral neck | Yes | Former | Yes | Right hip hemiarthroplasty | Previous fracture | 312 |
| 82 | F | Fall, low | Yes | no | No | 130 | |||
| 83 | F | Fall, low | Greater trochanteric (incomplete) | Yes | No | No | 183 | ||
| 86 | M | Fall, low | Closed fracture of hip | Yes | No | Yes | Right hemi joint hip replacement | 241 | |
| 87 | F | Fall, low | Yes | Former | No | 151 | |||
| 87 | M | Fall, low | Left intertrochanteric, closed hip | Yes | Former | No | 87 | ||
| 87 | F | Fall, low | Left intertrochanteric | Yes | Former | Yes | Left intertrochanteric | No | 34 |
| 88 | F | Fall, low | Right femoral neck | Yes | Former | Yes | Right total hip arthroplasty | No | 147 |
| 88 | F | Fall, low | Right intertrochanteric proximal femur | Yes | No | Yes | Cephalamedullary nailing | No | 104 |
Abbreviations: CT, computed tomography; DXA, dual x-ray absorptiometry; F, female; M, male; MVA, motor vehicle accident; ORIF, open reduction and internal fixation.
Figure 1.
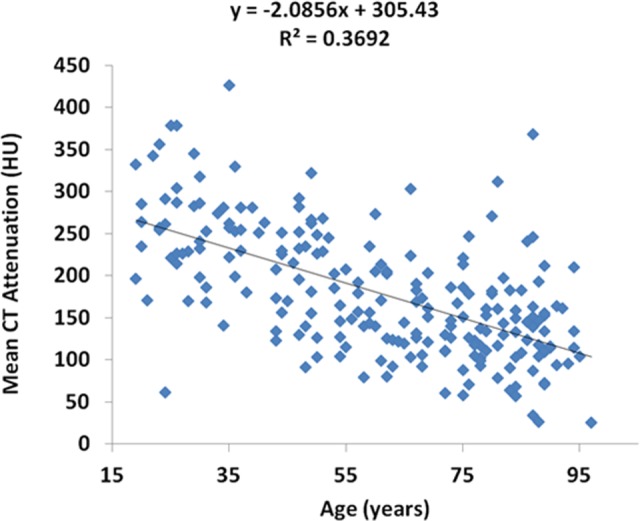
A regression outlining the association between age and bone mineral density (BMD), using computed tomography (CT) attenuation.
Discussion
Hip fractures are a significant cause of morbidity in older patients,1 with a projected increase associated with an aging population.1 Age-related osteoporosis underlies many of these fractures arising from minimal trauma.3 With this in mind, modalities that give patients and their clinicians accessible ways to monitor the health of their bones are desirable. This is broadly in keeping with the aims of the Own the Bone initiative.26 We found that CT images from which estimates of BMD could be derived were readily available in nearly 40% of patients with hip fracture who were included in this study, whereas only 34 (around 6%) patients of the same cohort had previously undergone DXA scans. These suggest that data from CT scans may be more widely available than that of DXA scans, although the CT scans were ordered for reasons other than assessment of BMD. That this can be done retrospectively is attractive as are the greater sensitivity and specificity of CT attenuation, the potential for cost savings, and the potential to reduce the number of medical procedures to which our patients are exposed.
Using opportunistic CT, a sizeable proportion of fractures were found to be associated with osteoporosis, especially in the elderly patients. Because osteoporosis is associated with negative clinical outcomes,27 a window of opportunity using this approach allows early diagnosis and preemptive treatment, which may in turn slow, halt, or even reverse the hitherto silent bone loss. For patients treated surgically, the surgeon must be aware of the quality of the patient’s bone to help ensure best results when considering which procedure or implant to use.20 After a fracture, the patient is predisposed to subsequent fractures,28,29 which may be associated with even greater risks of morbidity, mortality, and resource use than that of the index fracture. If our data represent broad clinical demographics, just under half of the patients who fracture their hips may be able to have their BMD assessed without the need for further investigation.
Osteoporosis is a disease in which screening of asymptomatic individuals may be beneficial because it has a long preclinical course before fracture, and a reliable test to establish the diagnosis and treatments has been shown to reduce the risk. Early diagnosis may afford the opportunity to use lower cost treatment to slow osteoporosis progression. Although it has been established that osteoporosis screening with BMD measurements should be individualized, how this approach to screening should occur remains controversial.
Although the association between age and BMD is somewhat intuitive, it is important to be able to demonstrate this relationship in a patient population known to have a strong association with osteoporosis. Patients with hip fracture are one such group; this population’s appropriateness is further confirmed by the strength of the correlation demonstrated when a regression is generated. Because many patients who should have DXA scans do not, it would seem that a large proportion of patients become aware of their condition only after a fracture. Given the relatively ubiquitous nature of abdominal CT scans in US healthcare24—especially among older patients—this technique could forestall future fractures.
Although overtesting of BMD (eg, in premenopausal women with low fracture risk) is limited, the fundamental problem with osteoporosis is thought to be underdiagnosis due to undertesting30-32; less than 33% of older US women and 5% of older US men have had a BMD.31 Even after a low-velocity fracture, only 10% to 20% of older patients are treated for osteoporosis.32 Clinical anecdotes, especially in view of our data, suggest that many of these patients have a CT scan that could be mined for CT attenuation-derived BMD. The CT-based assessment of BMD has a higher sensitivity and specificity than DXA; the clinical implication was illustrated in the index publication,25 which identified a group of patients whose BMD was shown to be normal by DXA scanning but went on to sustain a fragility fracture. Reevaluation using CT attenuation for most of these patients showed a diminished BMD.
The retrospective nature of this study is a limiting factor, although the parameter measured would largely be unchanged if collected prospectively. It should also be noted that opportunistic CT attenuation is, as yet, not sufficiently developed to be used to predict the risk of future fractures. Also, because our study was set in an urban level I trauma center, there may be a preponderance of existing CT scans because they may be incorporated into trauma protocols, which may be activated more often in larger centers. Finally, beyond using age 65 as a threshold to distinguish between old and young, no other stratification was used; this is particularly germane to the female cohort because of the onset of menopause about 12 to 14 years before the threshold. This was particularly apparent in that the female patients in the younger cohort with DXA scans were virtually of ages 50 to 64. Notwithstanding, as a proof of concept, it achieves its aim, especially in being able to identify patients with osteoporosis that had otherwise not been identified—an important benchmark for any new technique.
Overall, the opportunistic use of CT attenuation to evaluate BMD may provide a high-sensitivity and high-specificity approach that may provide cost savings, both from additional investigations and from the cost of managing future fractures. Further studies are needed, ideally, prospectively, to confirm the frequency with which additional imaging is available, what patient subgroups may most often benefit, and what an appropriate patient-flow algorithm would look like if CT attenuation were more widely adopted.
Acknowledgments
The authors thank Mary Van Beusekom, ELS (HealthPartners), for editing this article.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Chen CW, Huang TL, Su LT, et al. Incidence of subsequent hip fractures is significantly increased within the first month after distal radius fracture in patients older than 60 years. J Trauma Acute Care Surg. 2013;74 (1):317–321. [DOI] [PubMed] [Google Scholar]
- 2. Owen RA, Melton LJ, III, Ilstrup DM, Johnson KA, Riggs BL. Colles’ fracture and subsequent hip fracture risk. Clin Orthop Relat Res. 1982;(171):37–43. [PubMed] [Google Scholar]
- 3. Lips P, van Schoor NM. Quality of life in patients with osteoporosis. Osteoporos Int. 2005;16 (5):447–455. [DOI] [PubMed] [Google Scholar]
- 4. Warriner AH, Patkar NM, Curtis JR, et al. Which fractures are most attributable to osteoporosis? J Clin Epidemiol. 2011;64 (1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rosier RBS. Bone metabolism and metabolic bone diseases. In: Koval K, ed. Orthopaedic Knowledge Update 7. Rosemontm, IL: American Academy of Orthopaedic Surgeons, 2002. [Google Scholar]
- 6. Gardner MJ, Demetrakopoulos D, Shindle MK, Griffith MH, Lane JM. Osteoporosis and skeletal fractures. HSS J. 2006;2 (1):62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gass M, Dawson-Hughes B. Preventing osteoporosis-related fractures: an overview. Am J Med. 2006;119 (4 suppl 1):S3–S11. [DOI] [PubMed] [Google Scholar]
- 8. Cummings S, Melton L. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359 (9319):1761–1767. [DOI] [PubMed] [Google Scholar]
- 9. Melton LJ, III, Thamer M, Ray NF, et al. Fractures attributable to osteoporosis: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12 (1):16–23. [DOI] [PubMed] [Google Scholar]
- 10. Melton LJ, III, Chao EYS, Lane J. Biomechanical aspects of fractures. In: Riggs BL, Melton LJ, III, eds. Osteoporosis: Etiology, Diagnosis, and Management. New York, NY: Raven Press; 1988:111–131. [Google Scholar]
- 11. Hansson TH, Keller TS, Panjabi MM. A study of the compressive properties of lumbar vertebral trabeculae: effects of tissue characteristics. Spine. 1987;12 (1):56–62. [DOI] [PubMed] [Google Scholar]
- 12. Lang SM, Moyle DD, Berg EW, et al. Correlation of mechanical properties of vertebral trabecular bone with equivalent mineral density as measured by computed tomography. J Bone Joint Surg. 1988;70 (10):1531–1538. [PubMed] [Google Scholar]
- 13. Myers BS, Arbogast KB, Lobaugh B, Harper KD, Richardson WJ, Drezner MK. Improved assessment of lumbar vertebral body strength using supine lateral dual-energy X-ray absorptiometry. J Bone Miner Res. 1994;9 (5):687–693. [DOI] [PubMed] [Google Scholar]
- 14. Beck TJ, Ruff CB, Scott WW, Jr, Plato CC, Tobin JD, Quan CA. Sex differences in geometry of the femoral neck with aging: a structural analysis of bone mineral data. Calcif Tissue Int. 1992;50 (1):24–29. [DOI] [PubMed] [Google Scholar]
- 15. Courtney AC, Wachtel EF, Myers ER, Hayes WC. Effects of loading rate on strength of the proximal femur. Calcif Tissue Int. 1994;55 (1):53–58. [DOI] [PubMed] [Google Scholar]
- 16. Quek ST, Peh WC. Radiology of osteoporosis. Sem Musculoskel Radiol. 2002;6 (3):197–206. [DOI] [PubMed] [Google Scholar]
- 17. Seeman E, Delmas PD. Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354 (21):2250–2261. [DOI] [PubMed] [Google Scholar]
- 18. Fitzpatrick SK, Casemyr NE, Zurakowski D, Day CS, Rozental TD. The effect of osteoporosis on outcomes of operatively treated distal radius fractures. J Hand Surg Am. 2012;37 (10):2027–2034. [DOI] [PubMed] [Google Scholar]
- 19. Clayton RA, Gaston MS, Ralston SH, Court-Brown CM, McQueen MM. Association between decreased bone mineral density and severity of distal radial fractures. J Bone Joint Surg. 2009;91 (3):613–619. [DOI] [PubMed] [Google Scholar]
- 20. Bonnaire F, Zenker H, Lill C, Weber AT, Linke B. Treatment strategies for proximal femur fractures in osteoporotic patients. Osteoporos Int. 2005;16 (suppl 2):S93–S102. [DOI] [PubMed] [Google Scholar]
- 21. Seebeck J, Goldhahn J, Stadele H, Messmer P, Morlock MM, Schneider E. Effect of cortical thickness and cancellous bone density on the holding strength of internal fixator screws. J Orthop Res. 2004;22 (6):1237–1242. [DOI] [PubMed] [Google Scholar]
- 22. Lill CA, Goldhahn J, Albrecht A, Eckstein F, Gatzka C, Schneider E. Impact of bone density on distal radius fracture patterns and comparison between five different fracture classifications. J Orthop Trauma. 2003;17 (4):271–278. [DOI] [PubMed] [Google Scholar]
- 23. US Preventive Services Task Force. Screening for osteoporosis: U.S. preventive services task force recommendation statement. Ann Intern Med. 2011;154 (5):356–364. [DOI] [PubMed] [Google Scholar]
- 24. Majumdar SR, Leslie WD. Conventional computed tomography imaging and bone mineral density: opportunistic screening or “incidentaloporosis”? Ann Intern Med. 2013;158 (8):630–631. [DOI] [PubMed] [Google Scholar]
- 25. Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med. 2013;158 (8):588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. American Orthopaedic Association. Own the Bone. Web site http://www.ownthebone.org/ Accessed June 11, 2014.
- 27. Akan K, Cift H, Ozkan K, Eceviz E, Tasyikan L, Eren A. Effect of osteoporosis on clinical outcomes in intertrochanteric hip fractures treated with a proximal femoral nail. J Int Med Res. 2011;39 (3):857–865. [DOI] [PubMed] [Google Scholar]
- 28. Hodsman AB, Leslie WD, Tsang JF, Gamble GD. 10-Year probability of recurrent fractures following wrist and other osteoporotic fractures in a large clinical cohort: an analysis from the Manitoba Bone Density Program. Arch Intern Med. 2008;168 (20):2261–2267. [DOI] [PubMed] [Google Scholar]
- 29. Lyles KW, Schenck AP, Colón-Emeric CS. Hip and other osteoporotic fractures increase the risk of subsequent fractures in nursing home residents. Osteoporos Int. 2008;19 (8):1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mazanec D. Osteoporosis screening: time to take responsibility [editorial]. Arch Intern Med. 2004;164 (10):1047–1048. [DOI] [PubMed] [Google Scholar]
- 31. Curtis JR, Carbone L, Cheng H, et al. Longitudinal trends in use of bone mass measurement among older Americans, 1999-2005. J Bone Miner Res. 2008;23 (7):1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Majumdar SR, McAlister FA, Johnson JA, et al. Interventions to increase osteoporosis treatment in patients with ‘incidentally’ detected vertebral fractures. Am J Med. 2012;125 (9):929–936. [DOI] [PubMed] [Google Scholar]


