Abstract
Invasive pulmonary aspergillosis (IPA) is a severe fungal infection with a high mortality rate. The incidence of IPA is on the rise due to an increase in the number of patients undergoing transplants and receiving chemotherapy and immunosuppressive therapy. Diagnosis is challenging due to the non-specific nature of symptoms. Voriconazole is the mainstay of therapy. We present a case of an elderly woman presenting with acute bronchitis and asthma exacerbation, who succumbed to overwhelming IPA. It is uncommon for IPA to develop in patients on short-term steroid therapy for asthma exacerbation. The possibility of aspergillosis in immunocompetent patients should be considered in those on systemic steroids and deteriorating pulmonary functions.
Keywords: aspergillus, steroids, asthma exacerbation, vascular invasion, ground glass opacities
Aspergillus is a fungus which is commonly found in the soil, food, plant debris, and indoor environment. The spores are easily aerosolized and inhaled. In the respiratory mucosa, the spores may germinate into hyphae, which in turn can invade the mucosa leading to invasive pulmonary aspergillosis (IPA) (1). Both innate immune responses and inflammatory cells limit fungal growth and prevent disease in the majority of individuals. Depending on host's immune status and specific immune-deficiencies, Aspergillus may lead to different pulmonary manifestations (Table 1).
Table 1.
Clinical course following inhalation of Aspergillus spores
| Normal immune competent host: No infection |
| Previous cavitary lung disease: Aspergilloma |
| Excess Th2 response (allergic response): Allergic broncho pulmonary aspergillosis |
| Mild immune compromised state: Chronic necrotizing aspergillosis |
| Severe immune deficiency: Invasive pulmonary aspergillosis |
IPA usually occurs in severely immunocompromised patients. Classic at-risk patients are those with prolonged neutropenia, either due to chemotherapy or immunosuppressive therapy, post hematopoietic stem cell transplant (SCT), or solid organ transplant (1, 2). Risk factors for IPA are summarized in Table 2.
Table 2.
Risk factors for developing IPA
| Stem cell transplant or solid organ (especially lung) transplant |
| Hematologic malignancies |
| Prolonged neutropenia |
| Critically illness in intensive care unit |
| Steroid use |
| Hemodialysis |
| Liver disease |
| Chronic obstructive pulmonary disease (COPD)/chronic lung diseases |
| Chronic granulomatous diseases |
IPA has rarely been reported in patients on short-course steroid therapy without chronic lung diseases (3, 4). We describe the case of an elderly woman who was found to have extensive IPA, in the absence of classic risk factors.
Case report
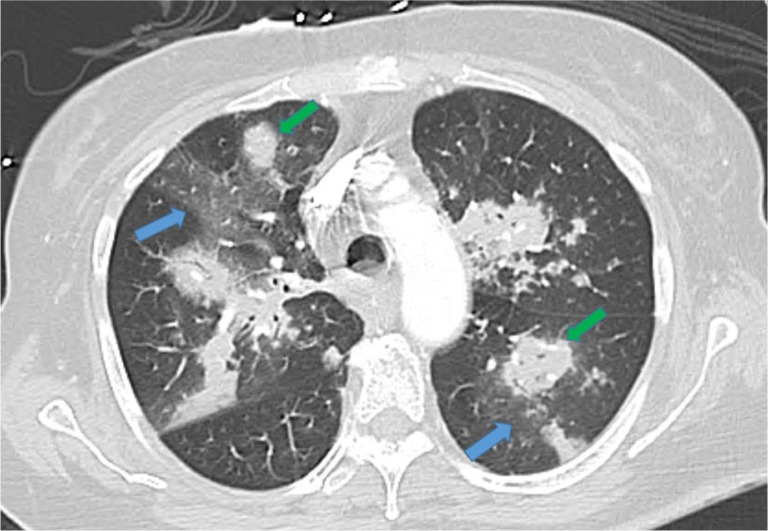
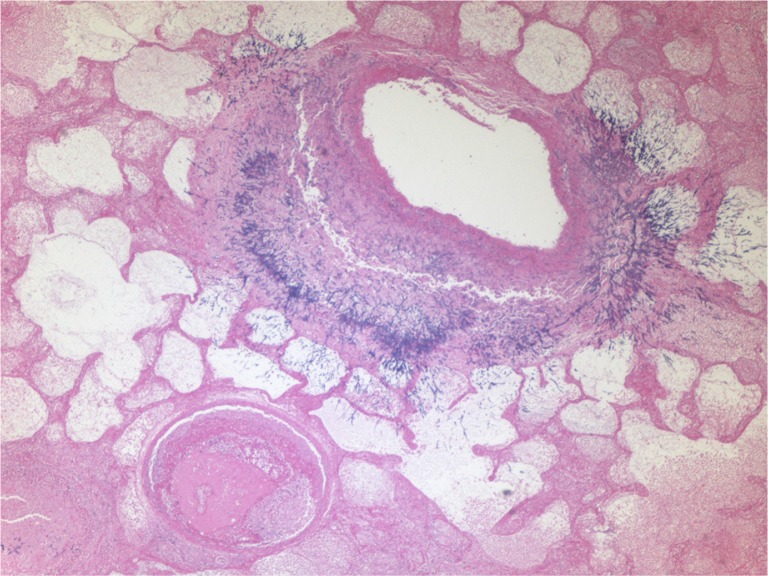
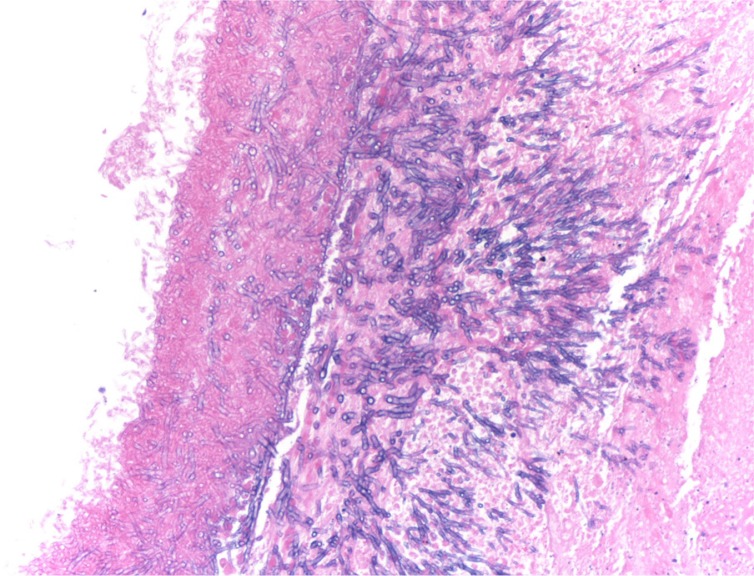
A 73-year-old woman was admitted to our hospital with complaints of shortness of breath and malaise. Nineteen days prior to admission, she was treated with inhaled steroids and cefpodoxime for acute bronchitis. A week before admission, she complained of fever, chills, wheezing, and cough with yellow sputum. She received oral prednisone and levofloxacin. However her symptoms worsened, and she presented to our emergency department. Her medical history included asthma with infrequent exacerbations (three exacerbations in the previous 3 years), hypertension, hyperlipidemia, diabetes mellitus, hypothyroidism, gout, spinal stenosis, and chronic kidney disease. Her home medications included carvedilol, furosemide, atorvastatin, gemfibrozil, sitagliptin, levothyroxine, allopurinol, erythropoietin, montelukast, fluticasone, and albuterol inhalers. Vital signs in the emergency department were temperature 97°F, blood pressure 144/66 mm Hg, heart rate 80/min, respiratory rate 16/min, and oxygen saturation 99% on room air. Examination revealed bilateral diffuse wheezing and pharyngeal wall erythema. Laboratory data showed white cell count 2.8×103 cells/dl with 72% neutrophils, hemoglobin 8.7 g/dl, hematocrit 27.2%, blood urea nitrogen 67 mg/dl, and serum creatinine 2.73 mg/dl. Liver function tests and electrolytes were normal. Electrocardiogram was normal and chest X-ray was without any infiltrate. Peak expiratory flow rate was 200 L/min. Diagnostic impression was asthma exacerbation secondary to acute bronchitis. Intravenous methylprednisolone 40 mg every 8 hours, bronchodilators, and levofloxacin were given. Serum mycoplasma antibody, urine legionella antigen, nasal swab for respiratory syncytial virus and influenza antigen, and rapid streptococcal throat tests were negative. On day 5 of admission, a high-resolution contrast tomographic (HRCT) scanning of the chest revealed bilateral multifocal ground glass opacities. On day 7 of admission, she complained of chest pain and had oxygen saturation of 80% on room air. Cardiac enzymes were undetectable and echocardiogram was normal. Perfusion lung scan showed perfusion defects highly suspicious for pulmonary embolism, and intravenous heparin was started. On day 10 of admission chest X-ray revealed new multifocal bilateral infiltrates, and ceftazidime and azithromycin were added. She was intubated for respiratory failure. Hemodialysis was done on day 11 of admission. Repeat HRCT showed development of new extensive bilateral pulmonary nodular infiltrates with ground glass opacities (Fig. 1). Antibiotics were switched to ertapenem and azithromycin. On day 13 of admission, sputum cytology showed Aspergillus species, and voriconazole was administered. She was extubated on day 14 of admission per the family's wishes and she died later that day. Autopsy revealed Aspergillus hyphae invading through the bronchial mucosa, almost completely obliterating the lumen of mid- to small-sized bronchi (Fig. 2). Vascular invasion by Aspergillus was also evident with concomitant widespread pulmonary arterial thrombi (Fig. 3). Acute tubular necrosis in the kidneys and centrilobular liver necrosis were also reported.
Fig. 1.
High-resolution computed tomographic scan of the chest revealing multiple foci of ground glass opacities (blue arrows) and bilateral pulmonary nodular infiltrates (green arrows).
Fig. 2.
Light microscopic findings of lung specimen (40×) stained with hematoxylin and eosin showing Aspergillus hyphae invading through the bronchial wall. A blood vessel is visible in the lower left field with an occluding thrombus and inflammatory cells in its lumen.
Fig. 3.
Hematoxylin and eosin stain of the lung specimen under a light microscope at 200× showing typical branching, septate Aspergillus hyphae, invading through the mucosa of a distal bronchi.
Discussion
IPA is caused by one of the four species of Aspergillus: A. fumigatus, A. flavus, A. niger, and A. terreus (5). It is increasingly seen due to a rise in the number of patients undergoing SCT and solid organ transplants, and also due to increasing number of patients on chemotherapy (6). Construction work in the vicinity of susceptible patients may increase spore aerosolization and is an independent risk factor (7). Mannose-binding lectin deficiency has also been associated with IPA (8).
Lower respiratory tract symptoms such as cough with or without sputum production, respiratory distress, wheezing, and fever are common. Aspergillus has a predilection for invading vasculature, especially in the neutropenic patients, leading to thrombosis, tissue infarction, and necrosis. In such cases pleuritic chest pain and hemoptysis may be present (2).
The diagnosis of IPA is challenging due to the non-specific nature of the symptoms and is often delayed due to lack of clinical suspicion in patients without classic risk factors. Tissue biopsy with histopathologic demonstration of tissue invasion by fungal hyphae is considered the ‘gold standard’ (9). However, obtaining tissue specimens from critically ill, often intubated, and hemodynamically unstable patients is not always feasible. Identification of Aspergillus species in the sputum could represent colonization, especially in immunocompetent patients (1, 10). On the contrary, isolation of Aspergillus from sputum in patients with leukemia, or in those who have undergone SCT, has a positive predictive value of 80–90% for the presence of IPA (1, 11, 12). With regard to imaging studies, Patterson et al., in their review of 595 cases of IPA, reported that 85% of the cases had computed tomographic (CT) scan findings suggestive of IPA (13). HRCT is recommended in all cases of suspected IPA. Pulmonary nodules are the most commonly seen abnormality whereas the ‘halo sign’ is relatively more specific with a high predictive value for IPA in patients with neutropenic fever after SCT (14). Laboratory tests to detect Aspergillus antigens in body fluids, either serum or bronchoalveolar lavage fluid (BAL), are increasingly helpful in the diagnosis of IPA. Galactomannan (GM) testing or Aspergillus polymerase chain reaction (PCR) assay in BAL has good sensitivity and specificity for IPA (2, 15). Testing for both GM and PCR may increase sensitivity without compromising the specificity (2). BAL GM testing is part of the diagnostic criteria for IPA (16).
Definitions for proven, probable, and possible IPA for the susceptible population of immunocompromised patients with cancer and recipients of SCT have been stated (16). A criterion has also been suggested for the diagnosis of IPA in chronic obstructive pulmonary disease (COPD) patients (17).
The mortality rate of IPA continues to be very high, exceeding 50% in neutropenic patients (14), and 90% in SCT recipients (18). Amphotericin used to be the preferred antifungal, until a large randomized clinical trial comparing amphotericin with voriconazole for primary treatment of IPA showed a better response rate and higher survival (71 vs. 58%) at 12 weeks of therapy in the voriconazole group. Voriconazole also has a better side effect profile and is generally better tolerated. Echinocandins, such as caspofungin, are used as salvage therapy in patients not responding to or not tolerating first-line therapy (1). Due to differences in their mechanisms of action, combination therapy with azoles, trienes, and echinocandins could be a strategy to treat IPA (1). At present, voriconazole is considered the therapy of choice (19).
Prolonged and high-dose corticosteroid therapy is an established risk factor for the development of IPA (1, 2). Steroids inhibit macrophage killing of Aspergillus by non-oxidative mechanisms (20). Steroids may also promote the growth of Aspergillus (21). Although IPA is seen in patients with chronic lung diseases on long-term steroids, it is rarely seen with short use of corticosteroids (1, 2, 22). To the best of our knowledge, diagnosis of IPA after short-course steroid therapy for asthma in a 31-year-old man is the only other case of IPA in a similar clinical scenario (3). Relatively few cases of IPA are observed in truly immunocompetent patients without classic risk factors (3, 4).
Conclusion
Short duration steroid therapy may be a risk factor for IPA in certain individuals. Classic risk factors for IPA were absent in our patient, and thus IPA was not high on the differential diagnosis. In our patient, hypoxia raised the possibility of pulmonary embolism, and perfusion lung scan further supported this diagnosis. Angioinvasive IPA can lead to thrombosis and create perfusion defects (2, 23). Thus, the diagnosis of pulmonary embolism confounded the clinical picture by giving an alternate explanation for the patient's symptoms, preventing further investigations such as bronchoscopy.
Refractoriness to therapy manifested as worsening clinical symptoms, progressive hypoxia, and increasing pulmonary infiltrates despite aggressive antibiotic therapy should raise suspicion for IPA. In our opinion, bronchoscopy should be considered in such patients along with antigen detection with GM and PCR testing. Early treatment with voriconazole is the only hope, and treatment should be initiated as soon as IPA is suspected. Every effort should be made to taper off steroids in these patients.
Acknowledgements
Dr Giulia Orsatti, MD (Pathology), provided the light microscopic images of lung (Figs. 2 and 3).
Conflict of interests and funding
The authors have not received any funding or benefits from the industry or elsewhere to conduct this study.
References
- 1.Zmeili OS, Soubani AO. Pulmonary aspergillosis: A clinical update. QJM. 2007;100(6):317–34. doi: 10.1093/qjmed/hcm035. [DOI] [PubMed] [Google Scholar]
- 2.Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2014 Oct 29; doi: 10.1136/thoraxjnl-2014-206291. pii: thoraxjnl-2014-206291. [DOI] [PubMed] [Google Scholar]
- 3.Lake KB, Browne PM, Van Dyke JJ, Ayers L. Fatal disseminated aspergillosis in an asthmatic patient treated with corticosteroids. Chest. 1983;83(1):138–9. doi: 10.1378/chest.83.1.138. [DOI] [PubMed] [Google Scholar]
- 4.Choi YR, Kim JT, Kim JE, Jung HW, Choe KH, Lee KM, et al. Invasive aspergillosis involving the lungs and brain after short period of steroid injection: A case report. Tuberc Respir Dis (Seoul) 2012;72(5):448–51. doi: 10.4046/trd.2012.72.5.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinbach WJ, Marr KA, Anaissie EJ, Azie N, Quan SP, Meier-Kriesche HU, et al. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J Infect. 2012;65(5):453–64. doi: 10.1016/j.jinf.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Chandrasekar P. Invasive mold infections: Recent advances in management approaches. Leuk Lymphoma. 2009;50(5):703–15. doi: 10.1080/10428190902777434. [DOI] [PubMed] [Google Scholar]
- 7.Pini G, Faggi E, Donato R, Sacco C, Fanci R. Invasive pulmonary aspergillosis in neutropenic patients and the influence of hospital renovation. Mycoses. 2008;51(2):117–22. doi: 10.1111/j.1439-0507.2007.01453.x. [DOI] [PubMed] [Google Scholar]
- 8.Lambourne J, Agranoff D, Herbrecht R, Troke PF, Buchbinder A, Willis F, et al. Association of mannose-binding lectin deficiency with acute invasive aspergillosis in immunocompromised patients. Clin Infect Dis. 2009;49(10):1486–91. doi: 10.1086/644619. [DOI] [PubMed] [Google Scholar]
- 9.Shelhamer JH, Gill VJ, Quinn TC, Crawford SW, Kovacs JA, Masur H, et al. The laboratory evaluation of opportunistic pulmonary infections. Ann Intern Med. 1996;124(6):585–99. doi: 10.7326/0003-4819-124-6-199603150-00008. [DOI] [PubMed] [Google Scholar]
- 10.Soubani AO, Khanchandani G, Ahmed HP. Clinical significance of lower respiratory tract Aspergillus culture in elderly hospitalized patients. Eur J Clin Microbiol Infect Dis. 2004;23(6):491–4. doi: 10.1007/s10096-004-1137-1. [DOI] [PubMed] [Google Scholar]
- 11.Horvath JA, Dummer S. The use of respiratory-tract cultures in the diagnosis of invasive pulmonary aspergillosis. Am J Med. 1996;100(2):171–8. doi: 10.1016/s0002-9343(97)89455-7. [DOI] [PubMed] [Google Scholar]
- 12.Yu VL, Muder RR, Poorsattar A. Significance of isolation of Aspergillus from the respiratory tract in diagnosis of invasive pulmonary aspergillosis. Results from a three-year prospective study. Am J Med. 1986;81:249–54. doi: 10.1016/0002-9343(86)90259-7. [DOI] [PubMed] [Google Scholar]
- 13.Patterson TF, Kirkpatrick WR, White M, Hiemenz JW, Wingard JR, Dupont B, et al. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. I3 Aspergillus Study Group. Medicine (Baltimore) 2000;79(4):250–60. doi: 10.1097/00005792-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Yeghen T, Kibbler CC, Prentice HG, Berger LA, Wallesby RK, McWhinney PH, et al. Management of invasive pulmonary aspergillosis in hematology patients: A review of 87 consecutive cases at a single institution. Clin Infect Dis. 2000;31(4):859–68. doi: 10.1086/318133. [DOI] [PubMed] [Google Scholar]
- 15.Hoenigl M, Prattes J, Spiess B, Wagner J, Prueller F, Raggam RB, et al. Performance of galactomannan, beta-d-glucan, Aspergillus lateral-flow device, conventional culture, and PCR tests with bronchoalveolar lavage fluid for diagnosis of invasive pulmonary aspergillosis. J Clin Microbiol. 2014;52(6):2039–45. doi: 10.1128/JCM.00467-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bulpa P, Dive A, Sibille Y. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease. Eur Respir J. 2007;30(4):782–800. doi: 10.1183/09031936.00062206. [DOI] [PubMed] [Google Scholar]
- 18.Fukuda T, Boeckh M, Carter RA, Sandmaier BM, Maris MB, Maloney DG, et al. Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning. Blood. 2003;102(3):827–33. doi: 10.1182/blood-2003-02-0456. [DOI] [PubMed] [Google Scholar]
- 19.Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, et al. Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347(6):408–15. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 20.Schaffner A, Schaffner T. Glucocorticoid-induced impairment of macrophage antimicrobial activity: Mechanisms and dependence on the state of activation. Rev Infect Dis. 1987;9(Suppl 5):S620–9. doi: 10.1093/clinids/9.supplement_5.s620. [DOI] [PubMed] [Google Scholar]
- 21.Ng TT, Robson GD, Denning DW. Hydrocortisone-enhanced growth of Aspergillus spp.: Implications for pathogenesis. Microbiology. 1994;140(Pt 9):2475–9. doi: 10.1099/13500872-140-9-2475. [DOI] [PubMed] [Google Scholar]
- 22.Cornet M, Mallat H, Somme D, Guérot E, Kac G, Mainardi JL, et al. Fulminant invasive pulmonary aspergillosis in immunocompetent patients – A two-case report. Clin Microbiol Infect. 2003;9(12):1224–7. doi: 10.1111/j.1469-0691.2003.00792.x. [DOI] [PubMed] [Google Scholar]
- 23.Lopes Bezerra LM, Filler SG. Interactions of Aspergillus fumigatus with endothelial cells: Internalization, injury, and stimulation of tissue factor activity. Blood. 2004;103(6):2143–9. doi: 10.1182/blood-2003-06-2186. [DOI] [PubMed] [Google Scholar]