Abstract
Compression or irritation of the sciatic nerve and its branches, the common fibular and tibial nerves, causes sciatica which is a common syndrome characterized most often by radiating pain from the lower back down the legs and also manifesting as sensory and motor deficits. Sciatica is a common presentation of lumbosacral disc prolapse and degenerative disease of the lumbar spine in ambulatory settings. Schwannomas rarely cause sciatica; hence, it is seldom considered in evaluation of a patient with radiculopathy. Our patient presented with lumbar radiculopathy, mild degenerative changes on lumbar magnetic resonance imaging (MRI) scan, and failed conservative treatment. Myelopathy was confirmed with electromyogram (EMG). Thoracolumbar spine MRI revealed the schwannoma in the thoracic region. He recovered neurologic function after tumor excision. This case highlights the diagnostic challenge that may arise in evaluating a patient with lumbar radiculopathy, negative lumbosacral spine imaging, and failure of conservative therapy.
Keywords: lumbar radiculopathy, sciatica, paraspinal tumors, schwannoma
Schwannomas are benign tumors of peripheral nerves affecting the nerve sheath. They are frequently asymptomatic but may become symptomatic depending on location. When occurring around the spinal cord, schwannomas are a common cause of extramedullary, intradural tumors. Paraspinal schwannomas arise from the dorsal nerve root, thereby causing sensory symptomatology. Large paraspinal lesions may also eventually cause compressive myelopathy. Treatment consists of surgical excision with generally excellent outcomes.
Case presentation
A 37-year-old man initially presented with subacute paresthesias characterized by numbness, tingling, and abnormal cold sensation. Physical examination at the time revealed reduced sensation in his right lateral foot. He was treated conservatively for presumed peroneal nerve palsy from compression phenomenon associated with crossing his legs. One week later, the symptoms progressed proximally to his knees. He had hyperreflexia at both his ankles and knees, worse on the right. At this time, the presumptive diagnosis was broad-based disk bulge causing lumbar radiculopathy. He was treated conservatively with analgesics and stretching exercises. His symptoms, however, progressively worsened. Two weeks later, he returned noting abnormal sensation involving the right lower leg and the anterior and medial lower halves of the thigh, with associated right ankle weakness, hypoesthesia in the left first interdigital space, and decreased control of his right leg leading to a clumsy gait with several falls. He denied associated back pain, abnormal bladder or bowel sphincter control. There was no vertigo, tinnitus, or hearing or cognitive impairment. Family and past medical history was negative for osseous, neurologic, or dermatologic diseases.
Physical examination at this time revealed right-sided foot drop, asymmetric hyper-reflexia on both posterior tibialis and Achilles’ tendons, more pronounced on the right, and bilateral ankle clonus. He also had decreased sensation to light touch in lower extremities medially. Gait was abnormal; he favored plantar flexion and inversion of right foot but not the left. Toe walking was normal but he was unable to heel walk or squat. Romberg sign was positive with decreased balance on right leg standing more than the left. Joint position sense on right big toe was impaired. There was no pain or spinal tenderness. The rest of his physical examination was normal.
Blood tests revealed normal hematologic, kidney, liver, and thyroid functions. Vitamin B12 level was normal and Rapid Plasma Reagent (RPR) was negative for syphilis. Lumbosacral spine magnetic resonance imaging (MRI) did not show any nerve root compression. Nerve conduction and electromyogram (EMG) demonstrated an abnormality in nerve conduction in the spinal region. Lumbo-sacral plexus and peripheral nerve conduction were normal. Given the predominantly L4 to L5 deficits, and the knowledge that lumbar spinal cord segments are in the thoracic vertebral segments, a thoracic MRI was performed (Fig. 1). This revealed a large extramedullary mass at T7–T8 with severe compression of the spine and deviation of the spinal cord to the right. He underwent urgent decompressive laminectomy. Intraoperative findings were an extradural tumor arising from the left T7 dorsal root. Pathologic examination revealed a schwannoma. Postoperatively, radicular symptoms resolved, but paresthesias persisted in the lateral right thigh. Follow-up MRI showed return of the cord to its position in the spinal canal. Annual MRI scans of his spine for the subsequent 3 years have not shown tumor recurrence.
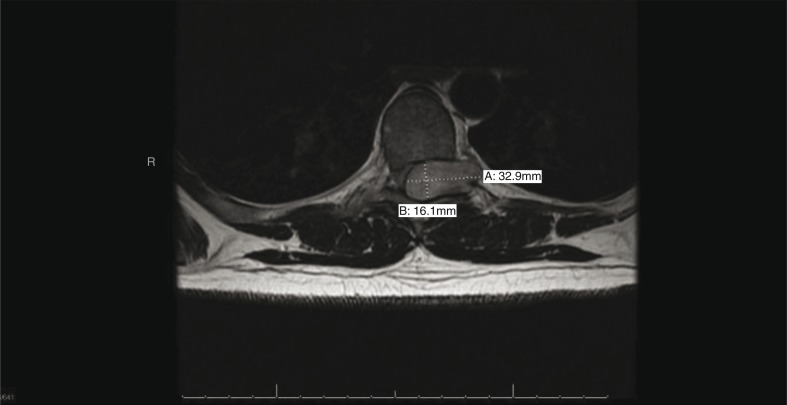
Fig. 1.
T1-weighted axial MRI thoracic spine, showing a dumbbell-shaped tumor arising from the dorsal nerve root, with mass effect on normal spinal cord.
Discussion
Schwannomas, also known as neurilemoma, are rare, benign tumors of the nerve sheath of peripheral nerves. Schwannomas have an incidence of 3% of all spinal tumors. In a paraspinal location, they are the commonest cause of intradural extramedullary tumors but may also be extradural or extramedullary (1). Schwannomas can affect any peripheral nerve in the body. They most commonly involve nerves in the head and neck. Up to 2.7% of schwannomas are located in the retroperitoneal region (2). Paraspinal schwannomas involve the dorsal nerve roots, affecting people in the fourth and fifth decades of life (3). Paraspinal schwannomas are frequently asymptomatic and diagnosed incidentally on imaging of the spine (3). Symptoms may consist of lancinating pain and/or paresthesias. Non-specific abdominal or back pain may occur (4). Motor and sensory deficits, as seen in our patient, rarely occur (3). Schwannomas can develop anywhere along the course of the sciatic nerve causing radiculopathy (4, 5). These tumors may be sporadic and solitary or multiple, as in neurofibromatosis type 1 (NF1).
Schwannomas are associated with 60% of NF1; the tumors occur at an early age, are multiple, and are prone to recurrence and malignant transformation. Patients with schwannoma may be screened for NF based on clinical features, including six or more light brown spots on the skin (‘café-au-lait’ spots), measuring 5 mm or more in children or more than 15 mm in adolescents and adults; two or more neurofibromas, or one plexiform neurofibroma, freckling in the area of the armpit or the groin; Lisch nodules or iris hamartomas; an optic nerve glioma, sphenoid dysplasia, pseudoarthosis or kyphoscoliosis, or the tibia (one of the long bones of the shin). The presence of tumors in any of the afore-mentioned features is characteristic of NF1. A parent, sibling, or child with NF is an additional clue, although 30–50% of NF cases may follow spontaneous mutation. Paraspinal schwannomas are frequently asymptomatic and are diagnosed incidentally on imaging of the spine (3). Symptoms may consist of lancinating pain and/or paresthesias. Non-specific abdominal or back pain may occur (4). Motor and sensory deficits, as seen in our patient, rarely occur (3). Schwannomas can occur anywhere along the course of the sciatic nerve causing radiculopathy (4, 5). Interestingly, in our patient, the lumbar radiculopathy was caused by a schwannoma at the T7 dorsal nerve root tumor as it exits through its foramen, compressing the adjacent lumbar spinal cord and lumbar nerve roots as they course down to exit through the lumbar foramen. Of note, there was an absence of T7 radicular symptoms. The lumbar nerve roots were normal intraoperatively. The spinal cord levels do not directly correlate with the vertebral spinal levels. Therefore, the lumbar spinal cord may be located in the area of the thoracic region of the vertebra (6). This explains why the lumbosacral spine MRI did not reveal the cause of lumbar radiculopathy symptoms, since the lesion was not at the neuroforamen. The progression from lumbar radiculopathy to myelopathy was due to severe spinal compression from the nerve root tumor. This is shown on the MRI (Figs. 1 and 2).
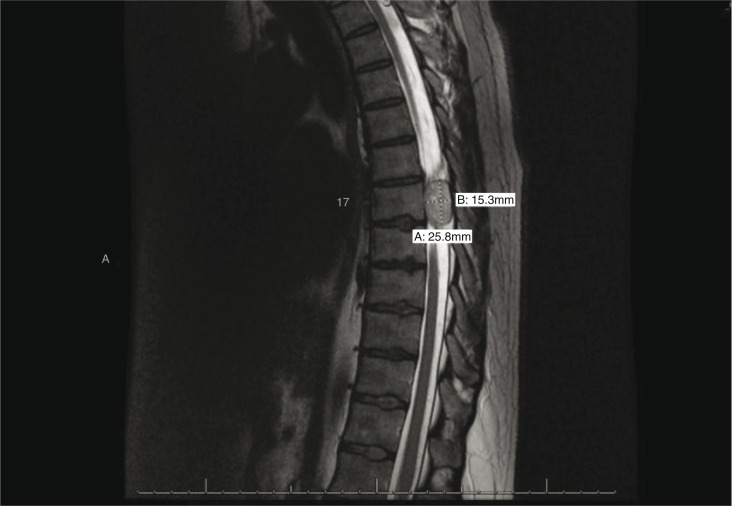
Fig. 2.
T1-weighted sagittal MRI thoracic spine showing sharply defined margins of the schwannoma, which arises separately from the neural tissue.
Paraspinal schwannomas may be localized on CT scan or MRI of the spine. CT scan will show ill-defined mass in the affected area. On MRI, schwannomas are clearly seen as solid tumors arising from the dorsal nerve root. They may be sausage, round, or dumbbell shaped. They are hypointense to isointense on T1-weighted images and hyperintense on T2-weighted images (1, 3). Contrast enhancement varies depending on cystic or solid components of the tumor. The mass may cause compression and deviation of the spinal cord, seen as in the MRI of this patient (3). Hemorrhagic, cystic, or fatty degeneration may also occur, thus, distinguishing it from other paraspinal tumors (1). However, pathologic examination is needed for definitive diagnosis.
Surgical resection is the treatment of choice (1, 3). Unlike neurofibromas, schwannomas do not arise from the nerve fibers and so the tumor is easily separated from nerve fibers without neurologic compromise. In the rare case that this is impossible, the remnant tumor may be followed up radiographically if it is histologically benign. Malignant schwannomas are treated with adjuvant radiation therapy. Postoperative transient neurologic deficit from surgical dissection and trauma may occur.
In conclusion, patients with lumbar radicular pain may rarely have tumors along the course of the spinal nerve. In patients with equivocal clinical examination, EMG may help localize the lesion along the very long course of the lumbar nerve fibers from the lumbar segment within the thoracic vertebrae to the nerve exit through the lumbar foramen to the supplied organs. The prognosis of schwannoma is excellent with surgical excision. Patients with schwannoma should be screened for NF based on clinical evaluation.
References
- 1.Wein S, Gaillard F. Intradural spinal tumours and their mimics: A review of radiographic features. Postgrad Med J. 2013;89(1054):457–69. doi: 10.1136/postgradmedj-2012-131503. [DOI] [PubMed] [Google Scholar]
- 2.Cury J, Coelho RF, Srougi M. Retroperitoneal schwannoma: Case series and literature review. Clin São Paulo Braz. 2007;62(3):359–62. doi: 10.1590/s1807-59322007000300024. [DOI] [PubMed] [Google Scholar]
- 3.Chamberlain MC, Tredway TL. Adult primary intradural spinal cord tumors: A review. Curr Neurol Neurosci Rep. 2011;11(3):320–8. doi: 10.1007/s11910-011-0190-2. [DOI] [PubMed] [Google Scholar]
- 4.Rustagi T, Badve S, Parekh AN. Sciatica from a foraminal lumbar root schwannoma: Case report and review of literature. Case Rep Orthop. 2012;2012:142–3. doi: 10.1155/2012/142143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prusick VR, Herkowitz HN, Davidson DD, Stambough JL, Rothman RH. Sciatica from a sciatic neurilemoma. A case report and review of the literature. J Bone Joint Surg Am. 1986;68(9):1456–7. [PubMed] [Google Scholar]
- 6.Goshgarian H. In: Spinal cord medicine: Principles and practice. Lin VW, Cardenas DD, Cutter NC, et al., editors. Chapter 2. New York, NY: Demos Medical Publishing; 2003. pp. 15–18. [Google Scholar]