Abstract
Background
Physical activity (PA) and healthy eating (HE) are important behaviors to encourage in breast cancer survivors (BCS). We examined associations between various factors and barriers to PA (BPA) and barriers to HE (BHE), as well as relationships between barriers and body mass index (BMI) in younger BCS.
Methods
Self-reported data from 162 BCS (mean age 48 years) were used. BPA were assessed with a 21-item scale and BHE with a 19-item scale. Participants were classified as high or low on each scale. Sociodemographic, medical, and psychosocial characteristics were compared by high/low barriers. Correlates of continuous BPA and BHE were assessed as were associations between BHE, BPA and BMI.
Results
61% of participants were characterized as having low BHE and low BPA; 12% were high for both. High BHE/High BPA participants had the least favorable scores for depression, perceived stress, social support, fatigue, bladder control, and weight problems. Factors associated with BHE were lower education, higher perceived stress, and more severe weight problems. Factors associated with BPA were more severe bladder control problems and lower physical well-being. Higher BHE and BPA were significantly and uniquely associated with higher BMI, controlling for covariates.
Conclusions
Several biopsychosocial factors (e.g., depression, stress, fatigue) characterize young BCS who experience barriers to both HE and PA. The correlates of BHE and BPA are distinct. Both BHE and BPA are associated with BMI. These results should be considered in designing interventions for younger women with breast cancer.
Keywords: breast cancer, survivorship, diet, physical activity, obesity
INTRODUCTION
Breast cancer survivors (BCS) constitute the largest segment of female cancer survivors [1]. Most early stage breast cancer patients have a life expectancy similar to age-matched women [2], and there is need to reduce their risk for comorbid conditions and secondary cancers. This is particularly true for younger BCS (i.e., 50 years or younger), for whom several decades of additional survival is anticipated. In a recent systematic review, we identified substantial rates of anxiety and depressive symptoms among younger BCS, along with fertility concerns, menopausal symptoms, and weight gain [3].
Weight gain is of particular concern for BCS, in that excess body weight is a risk factor for cancer recurrence [4]. Younger women may be at increased risk for weight gain as they are more likely to experience premature menopause, induced by adjuvant chemotherapy [5]. In addition, some evidence shows that the association between weight gain after diagnosis and breast cancer survival is greater in pre-menopausal women than in post-menopausal survivors [6].
A recent meta-analysis demonstrated that higher physical activity (PA) was associated with reduced breast cancer-specific mortality as well as overall mortality in BCS [7]. In a previous study in young BCS [8], we found an association between higher levels of PA and lower BMI and blood pressure, as well as higher physical functioning and energy levels. However, participants reported lower levels of PA than was shown in a similar sample of women without cancer, suggesting a need to help young survivors increase PA. In our systematic review [3], we also found that lack of PA and weight gain are common in young BCS. Research linking dietary intake to improved outcomes in survivors is less clear, though there is some evidence to show that reducing fat and alcohol consumption as well as increasing intake of fruits, vegetables, and other sources of dietary fiber such as whole grains may be beneficial [9,10].
Despite the importance of weight and PA as factors influencing mortality after breast cancer, little is known about the barriers to maintaining normal weight and increasing PA in this setting. We initiated the After Breast Cancer (ABC) study to identify behavioral and lifestyle risk factors for obesity and physical inactivity in younger BCS that would be relevant for future intervention development. This paper presents the results of a cross-sectional survey that examined a variety of domains (health-related quality of life, medical and treatment variables, weight and health behaviors), in addition to perceived barriers to PA and healthy eating (HE). The specific questions addressed in this paper are: 1) What are the perceived barriers to HE and PA in young BCS and how do women vary by barrier status?; 2) How do the demographic, medical, and psychosocial factors associated with barriers for PA and HE differ?; and 3) Do the perceived barriers to HE and PA contribute to higher body mass index (BMI) independent of other factors related to high BMI in this population?
METHODS
Participants and Recruitment
Study recruitment began in 2009, using the UCLA Health System tumor registry to identify potentially eligible breast cancer patients diagnosed between 2003-2007. Eligibility criteria were: stage 1, 2, or 3 breast cancer diagnosed at age ≤ 50 years; currently alive and disease free; > 1 year post-initial cancer diagnosis; > 6 months after cancer treatment (i.e., completed chemotherapy and/or radiation, but could be receiving endocrine therapy); agreed to complete survey; ability to read and write English; female; provides informed consent.
Invitation letters were mailed to potential subjects, who were asked to return a mailed response form indicating their interest in participating. Trained research staff screened potential participants via telephone. Eligible participants were mailed consent forms and questionnaire packets to complete and return in postage paid envelopes, and reminder calls were made to return questionnaires. Non-respondents received a second mailing and additional contact by phone to explain the study and screen for eligibility. The study was approved by the UCLA Institutional Review Board and written consent was obtained from each participant.
Measures
Demographic and Medical Characteristics
were assessed with questions used in prior studies [11,12] (see Table 1 for all variables). Current chronic conditions were assessed using a checklist of 13 conditions. Current height (in feet and inches) as well as current weight and weight (lbs) at diagnosis were assessed via self-report. BMI was calculated in kg/m2. Menstrual history was measured via a series of questions used previously [11].
Table 1.
Descriptive characteristics of participants by responses to the Barriers to Healthy Eating (HE) and Barriers to Physical Activity (PA) scales1.
| DEMOGRAPHIC CHARACTERISTICS | ||||||
|---|---|---|---|---|---|---|
| Total Sample n=162 | Low Barriers PA, Low Barriers HE (n=99) | High Barriers PA, Low Barriers HE (n=29) | Low Barriers PA, High Barriers HE (n=15) | High Barriers PA, High Barriers HE (n=19) | P-value 2 | |
| Age (years) | 47.6 (5.6) | 47.9 (5.7) | 47.8 (6.0) | 46.5 (5.2) | 46.8 (4.7) | 0.73 |
| Ethnicity (n=162) | ||||||
| White, non-Hispanic | 111(68.5%) | 70 (70.7%) | 19 (65.5%) | 7 (46.7%) | 15 (78.9%) | 0.07 |
| Hispanic | 16 (9.9%) | 7 (6.9%) | 2 (6.9%) | 6 (40.0%) | 1 (5.3%) | |
| Black, non-Hispanic | 9 (5.6%) | 5 (5.1%) | 3 (10.3%) | 1 (5.3%) | ||
| Asian | 23 (14.2%) | 15 (15.2%) | 4 (13.8%) | 2 (13.3%) | 2 (10.5%) | |
| Other | 3 (1.9%) | 2 (2.0%) | 1 (3.4%) | |||
| Marital Status | ||||||
| Married/living as married vs. single/divorced | 122 (75.3%) | 76 (76.8%) | 22 (75.9%) | 9 (60.0%) | 15 (78.9%) | 0.54 |
| Lives Alone (percent yes) | 24 (14.8%) | 12 (12.1%) | 5 (17.2%) | 4 (26.7%) | 3 (15.8%) | 0.50 |
| Has Children (percent yes) | 109 (67.3%) | 66 (66.7%) | 20 (69.0%) | 8 (53.3%) | 15 (78.9%) | 0.47 |
| Educational Attainment (n=162) | 0.35 | |||||
| High school grad, GED, or vocational or training school | 9 (5.6%) | 2 (2.0%) | 4 (13.8%) | 1 (6.7°%) | 2 (10.5%) | |
| Some college or AA | 41 (25.3%) | 23 (23.2%) | 6 (20.7%) | 6 (40.0%) | 6 (31.6%) | |
| 4 year college graduate | 47 (29.0%) | 32 (32.2%) | 7 (24.1%) | 3 (20.0%) | 5 (26.3%) | |
| Some graduate school | 24 (14.8%) | 16 (16.2%) | 5 (17.2%) | -- | 3 (15.8%) | |
| Completed graduate school | 41 (25.3%) | 26 (26.3%) | 7 (24.1%) | 5 (33.3%) | 3 (15.8%) | |
| Current Employment Status | 0.92 | |||||
| Full-time | 86 (53.1%) | 51 (51.5%) | 16 (55.2%) | 10 (66.7%) | 9 (47.4%) | |
| Part-time | 28 (17.3%) | 18 (18.2%) | 4 (13.8%) | 1 (6.7°%) | 5 (26.3%) | |
| Full time homemaker, full or part time volunteer, student, or retired | 31 (19.1%) | 19 (19.2%) | 6 (20.7%) | 2 (13.3%) | 4 (21.1%) | |
| Unemployed, on temporary medical leave, or permanently disabled | 17 (10.5%) | 11 (11.1%) | 3 (10.3%) | 2 (13.3%) | 1 (5.3%) | |
| Total Family Income (n=157) | 0.24 | |||||
| Under $60,000 | 27 (17.2%) | 17 (17.7%) | 4 (13.8%) | 2 (14.3%) | 4 (22.2%) | |
| $60,001-$100,000 | 56 (35.7%) | 27 (28.1%) | 14 (48.3%) | 8 (57.1%) | 7 (38.9%) | |
| Over $100,000 | 74 (47.1%) | 52 (54.2%) | 11 (37.9%) | 4 (28.6%) | 7 (38.9%) |
| MEDICAL CHARACTERISTICS | P-value2 | |||||
|---|---|---|---|---|---|---|
| Total Sample n=162 | Low Barriers PA, Low Barriers HE (n=99) | High Barriers PA, Low Barriers HE (n=29) | Low Barriers PA, High Barriers HE (n=15) | High Barriers PA, High Barriers HE (n=19) | ||
| Height (inches) | 64.8 (2.8) | 64.7 (2.8) | 64.5 (2.9) | 65.3 (2.6) | 65.7 (2.2) | 0.39 |
| Current Weight (lbs) | 151.0 (38.3) | 143.3a (30.5) | 161.7 (47.7) | 149.1 (30.1) | 176.3a (50.9) | 0.002 |
| Current BMI | 25.1 (5.5) | 24.0b,c (4.3) | 27.0b (6.6) | 24.5 (4.6) | 28.6c (7.8) | 0.001 |
| BMI at Diagnosis (n=158) | 24.5 (5.5) | 23.4d,e (4.4) | 26.4d (7.2) | 24.5 (4.7) | 28.1e (6.9) | 0.002 |
| Menopausal Status | 0.031 | |||||
| Pre | 54 (33.3%) | 31 (31.3%) | 8 (27.6%) | 10 (66.7%) | 5 (26.3%) | |
| Post | 93 (57.4%) | 60 (60.6%) | 16 (55.2%) | 4 (26.7%) | 13 (68.4%) | |
| Unknown -- Hysterectomy | 4 (2.5%) | -- | 2 (6.9%) | 1 (6.7%) | 1 (5.3%) | |
| Unknown -Treatment-related Amenorrhea | 11 (6.8%) | 8 (8.1%) | 3 (10.3%) | |||
| Count of co-morbidities (0 to 13, n=161) | 1.2 (1.1) | 1.1 (0.9) | 1.2 (1.3) | 0.9 (0.9) | 1.8 (1.7) | 0.07 |
| Years since diagnosis | 3.4 (1.5) | 3.3 (1.5) | 3.5 (1.5) | 3.1 (1.5) | 3.7 (1.4) | 0.57 |
| Type of surgery (n=161) | 0.16 | |||||
| Mastectomy only | 100 (62.1%) | 68 (68.7%) | 14 (48.3%) | 7 (50.0%) | 11 (57.9%) | |
| Lumpectomy only | 61 (37.9%) | 31 (31.3%) | 15 (51.7%) | 7 (50.0%) | 8 (42.1%) | |
| Chemotherapy and/or Radiation | 0.14 | |||||
| Had neither chemotherapy nor radiation | 21 (13.0%) | 15 (15.2%) | 2 (6.9%) | 2 (13.3%) | 2 (10.5%) | |
| Had Chemotherapy only (percent yes) | 40 (24.7%) | 29 (29.3%) | 3 (10.3%) | 5 (33.3%) | 3 (15.8%) | |
| Had Radiation only (percent yes) | 15 (9.3%) | 9 (9.1%) | 2 (6.9%) | 3 (20.0%) | 1 (5.3%) | |
| Had both chemotherapy and radiation (percent yes) | 86 (53.1%) | 46 (46.5%) | 22 (75.9%) | 5 (33.3%) | 13 (68.4%) | |
| Received Herceptin or other biotherapy (percent yes, n=157) | 36 (22.9%) | 22 (22.9%) | 7 (25.0%) | 3 (21.4%) | 4 (21.1%) | 0.99 |
| Currently receiving endocrine therapy | 98 (60.5%) | 61 (61.6%) | 19 (65.5%) | 8 (53.3%) | 10 (52.6%) | 0.76 |
| QUALITY OF LIFE AND SYMPTOMS | ||||||
|---|---|---|---|---|---|---|
| Total Sample n=162 | Low Barriers PA, Low Barriers HE (n=99) | High Barriers PA, Low Barriers HE (n=29) | Low Barriers PA, High Barriers HE (n=15) | High Barriers PA, High Barriers HE (n=19) | P-value | |
| CESD | 14.1 (10.1) | 12.2a (8.7) | 15.0 (12.4) | 18.3 (11.9) | 19.5 a (9.4) | 0.008 |
| PSS ( n=161) | 16.7 (6.7) | 15.2b (6.1) | 17.9 (6.9) | 19.5 (7.0) | 20.7 b (7.2) | 0.002 |
| MOS emotional social support (n=162) | 75.1 (22.5) | 79.6c (19.5) | 68.3 (26.1) | 71.3 (22.6) | 64.8 c (26.1) | 0.01 |
| MOS instrumental social support (n=161) | 73.8 (27.0) | 79.7d (23.0) | 65.3 (31.8) | 65.8 (27.3) | 62.5 d (31.7) | 0.006 |
| FSI Level of fatigue on the day felt most fatigued during the last week | 6.3 (2.6) | 5.8e (2.7) | 6.8 (2.5) | 7.0 (2.4) | 7.6 e (1.7) | 0.01 |
| SF-12 Physical Component (n=159) | 47.9 (9.6) | 49.5f,g (8.7) | 43.6f,h (10.1) | 51.7h,i (8.0) | 43.0g,i (10.9) | 0.001 |
| SF-12 Mental Component (n=159) | 47.1 (10.8) | 49.1j (9.4) | 46.2 (13.5) | 41.2j (9.6) | 42.8 (11.6) | 0.01 |
| BCPT Symptom Scales | ||||||
| Hot flashes | 1.4 (1.3) | 1.4 (1.3) | 1.4 (1.2) | 1.5 (1.3) | 1.4 (1.1) | 1.0 |
| Nausea | 0.2 (0.4) | 0.2 (0.4) | 0.3 (0.4) | 0.1 (0.2) | 0.3 (0.5) | 0.53 |
| Bladder Control | 0.5 (0.8) | 0.4k (0.7) | 0.7 (0.8) | 0.7 (1.1) | 1.0k (1.0) | 0.003 |
| Vaginal Problems | 1.4 (1.4) | 1.6 (1.4) | 1.3 (1.4) | 1.3 (1.3) | 1.1 (1.4) | 0.60 |
| Musculoskeletal pain | 1.6 (1.2) | 1.4 (1.2) | 1.9 (1.1) | 1.3 (0.9) | 1.9 (1.1) | 0.13 |
| Cognitive problems | 1.5 (1.1) | 1.4l (1.1) | 1.4 (0.9) | 2.3l (1.3) | 1.7 (1.1) | 0.049 |
| Weight problems | 1.7 (1.2) | 1.4m (1.2) | 1.7 (1.2) | 1.9 (1.3) | 2.6m (1.1) | 0.002 |
| Arm Problems | 0.6 (0.8) | 0.4n (0.7) | 1.2n (1.1) | 0.7 (0.9) | 0.7 (0.8) | <0.001 |
Superscripts a-n indicate pairs which differ significantly
For menopausal status, the Low PA/High HE group differs significantly from all 3 other groups
1Barrier groups were created based on mean responses to each of the two scales. Participants were considered to have low barriers for each of the scales if they reported a mean response of less than 2.5 and to have high barriers if they reported a mean response of 2.5 or higher. Both BHE and BPA scale response options ranged from 1 to 5 with 1 representing “Never” and 5 representing “Very Often.”
2Analysis of Variance (ANOVA) used to compare participants by barrier quadrants for continuous variables and chi-square tests for categorical variables.
Additional Scale and item descriptions: Currently receiving endocrine therapy: e.g. Tamoxifen, Femara, Aromasin, Arimidex, Lupron or Zoladex (percent yes); Depression: Center for Epidemiologic Studies Depression Scale, CES-D, higher=more depressed); Stress: (Perceived Stress Scale, PSS, higher=more perceived stress, n=161; Social support: MOS emotional social support, higher=more support, n=162; MOS instrumental social support, higher=more support, n=161; Well-being: Short Form Health Survey (SF-12): Physical Component, higher =higher functioning, Short Form Health Survey (SF-12): Mental Component, higher=higher functioning; Breast Cancer-Related Symptoms (Breast Cancer Prevention Trial Symptom Checklist) ,BCPT Symptom Scales, Scales range from 0-4 with higher indicating more symptoms.
Quality of Life and Symptoms
Depressive symptoms over the last week were assessed using the Center for Epidemiologic Studies Depression (CES-D) Scale [13]. Perceived stress over the last month was measured with the Perceived Stress Scale (PSS) 10-item version [14]. An 8-item version of the MOS Social Support Survey [15] was used to assess social support. Fatigue severity over the past week was measured with the Fatigue Symptom Inventory (FSI), which was developed for and validated in cancer patients [16-18]. Health-related quality of life (HRQL) over the past month was assessed with the MOS 12-Item Health Survey Short Form (SF-12)[19] yielding two subscales: Physical Component Summary (PCS) and Mental Component Summary (MCS), with normative data available for the general population, and individuals with chronic conditions. These scales have been widely used in studies of BCS [3,8,11,12]. Breast cancer-related symptoms were measured with the Breast Cancer Prevention Trial (BCPT) Symptom Scales [20].
Barriers to Physical Activity and Healthy Eating
Perceived barriers to PA (BPA) were measured by a 21-item scale adapted and used by Rogers et al. in both breast cancer patients [21] and survivors [22]. Participants rated how often a list of barriers “interfered with your plan to exercise in the past month” and responses were: 1=Never, 2=Rarely, 3=Sometimes, 4= Often, or 5=Very Often. The individual items are listed in Table 2. A mean score was calculated by dividing the overall sum by the number of items, with a higher score indicating higher perceived BPA. Cronbach's alpha for the scale was 0.91.
Table 2.
Frequency of responses to Barriers to Healthy Eating and Barriers to Physical Activity Scales
| Barrier | Percent Response | Mean score | SD | ||||
|---|---|---|---|---|---|---|---|
| Healthy Eating | Never | Rarely | Sometimes | Often | Very Often | ||
| Holidays and special occasions are a problem | 16.6 | 18.4 | 32.5 | 25.8 | 6.7 | 2.9 | 1.2 |
| I feel like eating whatever I want | 14.1 | 19.6 | 35.6 | 20.9 | 9.8 | 2.9 | 1.2 |
| High fat foods taste better | 16.6 | 20.9 | 33.7 | 17.8 | 11.0 | 2.9 | 1.2 |
| I eat a lot of meals away from home | 20.2 | 24.5 | 30.7 | 15.3 | 9.2 | 2.7 | 1.2 |
| It's easier to grab another type of snack and eat it in my car | 29.4 | 22.7 | 31.3 | 9.8 | 6.7 | 2.4 | 1.2 |
| It takes too much planning to eat a healthier diet | 36.8 | 14.7 | 33.1 | 9.8 | 5.5 | 2.3 | 1.2 |
| High fat foods are a traditional part of my culture | 35.0 | 31.3 | 17.2 | 13.5 | 3.1 | 2.2 | 1.1 |
| Healthier foods are too expensive | 45.4 | 20.9 | 24.5 | 4.9 | 4.3 | 2 | 1.1 |
| There are no healthy food options at sporting events | 53.4 | 16.0 | 16.6 | 8.6 | 5.5 | 2 | 1.2 |
| I can't keep track of what I need to eat | 43.6 | 30.1 | 21.5 | 3.1 | 1.8 | 1.9 | 1.0 |
| Fruits and vegetables don't fill me up | 44.2 | 28.8 | 21.5 | 1.8 | 3.7 | 1.9 | 1.0 |
| Fruits and vegetables take too long to prepare | 54.0 | 20.9 | 21.5 | 2.5 | 1.2 | 1.8 | 1.0 |
| I don't know how to cook healthier meals | 60.1 | 19.0 | 11.0 | 5.5 | 4.3 | 1.8 | 1.1 |
| There are no healthier foods in vending machines | 61.7 | 15.4 | 9.3 | 6.8 | 6.8 | 1.8 | 1.3 |
| I don't like the taste of healthier foods | 55.2 | 27.6 | 12.9 | 3.7 | 0.6 | 1.7 | 0.9 |
| My family doesn't support me for eating more healthfully | 66.9 | 20.2 | 9.2 | 1.8 | 1.8 | 1.5 | 0.9 |
| I don't like the taste of fruits and vegetables | 69.1 | 19.8 | 9.9 | 0.6 | 0.6 | 1.4 | 0.7 |
| I don't know how to cook vegetables | 71.8 | 19.0 | 6.1 | 1.8 | 1.2 | 1.4 | 0.8 |
| I don't know where to find low fat foods | 77.3 | 19.0 | 3.7 | 0.0 | 0.0 | 1.3 | 0.5 |
| Physical Activity | |||||||
| Lack of time | 11.0 | 18.4 | 30.1 | 19.0 | 21.5 | 3.2 | 1.3 |
| Lack of self-discipline | 10.4 | 18.4 | 39.9 | 17.8 | 13.5 | 3.1 | 1.2 |
| Fatigue (or lack of energy) | 15.3 | 20.2 | 31.3 | 16.6 | 16.6 | 3.0 | 1.3 |
| Procrastination | 19.6 | 17.8 | 34.4 | 13.5 | 14.7 | 2.9 | 1.3 |
| Lack of interest in exercise | 16.6 | 25.8 | 35.0 | 11.0 | 11.7 | 2.8 | 1.2 |
| Family responsibilities | 24.5 | 20.2 | 25.2 | 19.0 | 11.0 | 2.7 | 1.3 |
| Exercise not in routine | 31.3 | 17.2 | 20.2 | 14.1 | 17.2 | 2.7 | 1.5 |
| Pain or discomfort | 33.7 | 20.2 | 25.2 | 9.2 | 11.7 | 2.5 | 1.3 |
| Lack of enjoyment from exercise | 32.5 | 25.8 | 22.1 | 9.2 | 10.4 | 2.4 | 1.3 |
| Exercise is not a priority | 36.2 | 23.3 | 23.3 | 11.0 | 6.1 | 2.3 | 1.2 |
| Exercise is boring | 43.2 | 25.9 | 15.4 | 8.6 | 6.8 | 2.1 | 1.2 |
| Lack of company | 46.6 | 25.2 | 14.7 | 7.4 | 6.1 | 2.0 | 1.2 |
| Inconvenient exercise schedule | 51.5 | 14.7 | 19.6 | 7.4 | 6.7 | 2.0 | 1.3 |
| Weather | 46.6 | 27.6 | 18.4 | 3.7 | 3.7 | 1.9 | 1.1 |
| Lack of equipment | 63.8 | 20.9 | 5.5 | 4.3 | 5.5 | 1.7 | 1.1 |
| Cost of exercising | 71.8 | 12.3 | 8.6 | 3.7 | 3.7 | 1.6 | 1.0 |
| Lack of skills | 71.8 | 16.6 | 7.4 | 3.1 | 1.2 | 1.5 | 0.9 |
| No facilities or space to exercise | 77.3 | 8.6 | 8.0 | 2.5 | 3.7 | 1.5 | 1.0 |
| Fear of injury | 69.3 | 17.2 | 9.2 | 2.5 | 1.8 | 1.5 | 0.9 |
| Feeling nauseated | 78.5 | 12.9 | 8.6 | 0.0 | 0.0 | 1.3 | 0.6 |
| Lack of knowledgeable exercise staff | 77.9 | 12.3 | 8.0 | 1.2 | 0.6 | 1.3 | 0.7 |
Note: Participants rated how often a list of barriers “interfered with your plan to exercise in the past month” and responses were: 1=Never, 2=Rarely, 3=Sometimes, 4= Often, or 5=Very Often.
Perceived barriers to HE (BHE) were assessed by a scale developed for this study to parallel the Rogers scale. A list of 19 barriers to HE were adapted from an existing intervention checklist [23], and used the same Likert scale format as the BPA scale, with the same instructions. The individual items are listed in Table 2. A mean score was calculated by dividing the overall sum by the number of items, with a higher score indicating higher BHE. Cronbach's alpha for the scale was 0.86.
Data analysis
Descriptive Comparisons
With the goal of distinguishing between women for whom BHE and BPA were largely absent from those who experienced barriers with some regularity, we categorized participants as having “low” barriers if their mean response was 2.49 or lower (out of 5) on each scale, corresponding to a response of “never” or “rarely.” Conversely, “high” barriers were identified as a mean response of 2.5 or higher, corresponding to “sometimes,” “often,” or “very often.” Participants were then further grouped as: 1) Low BPA, Low BHE (or Low/Low), 2) High BPA, Low BHE, 3) Low BPA, High BHE, and 4) High BPA, High BHE (or High/High), and examined for relationships with medical, demographic or psychosocial characteristics. Participant characteristics were compared by barrier groupings using analysis of variance (ANOVA) for continuous variables with Tukey's test for post-hoc comparisons. Chi-square tests were conducted for categorical variables and post-hoc comparisons were explored for significant variables using chi-square tests comparing groups pairwise.
Multivariable Modeling
Correlates of BPA and BHE
Psychosocial and HRQL measures were included in the model based on their bivariate relationships with either the BPA or BHE scale score. Pearson correlations were used for continuous variables and chi-square tests were used for categorical variables with the low vs. high categorizations of the 2 barrier scales. Independent variables were selected for inclusion in multivariate regression models if the Pearson correlation exceeded 0.30 or the chi-square test p-value was less than 0.10. Relevant medical and demographic covariates were selected as control variables in the models and included current age, ethnicity (white vs. not white), has children (yes vs. no), married or living as married (yes vs. no), four year college graduate or more (yes vs. no), cormorbid conditions (yes vs. no), had radiation therapy only (yes vs. no), had chemotherapy only (yes vs. no), had both radiation and chemotherapy (yes vs. no), and currently receiving endocrine therapy (yes vs. no). Multivariable models were built for BPA and BHE. If an independent variable was significantly associated with either BPA or BHE bivariately, it was included in the models for both BPA and BHE, so that potential predictors of the two scales could be compared.
The second set of multivariable models was created to assess whether BPA and/or BHE were associated with BMI. The same medical and demographic variables were included in the BMI models, and identical criteria were used for selection of potential psychosocial and quality life variables for inclusion in the models. For chi-square tests of bivariate associations, a categorization of normal weight (BMI<25) vs. overweight/obese (BMI≥25) participants was used. Three separate multivariable models with BMI as the dependent variable were fitted: 1) BMI was regressed on BHE, controlling for relevant covariates; 2) BMI was regressed on BPA in a similar fashion; and 3) BMI was regressed on both BHE and BPA, and an F test was employed to test for the joint significance of including both BHE and BPA in the same model.
Statistical analyses were conducted using SPSS version 20 (IBM SPSS Statistics, Chicago: IBM Corporation).
RESULTS
Recruitment Results and Patient Characteristics
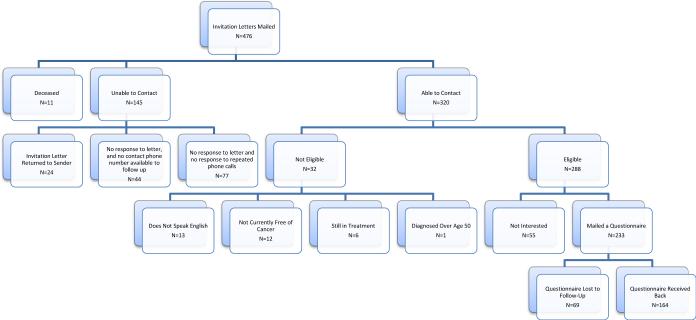
Study recruitment results are presented in Figure 1. Invitation letters were mailed to 476 potential participants, with contact available among 320 (67% of total), and 288 of 320 being eligible (90%). Among the eligible women, 233 (81%) were interested in participating and they were mailed the study questionnaire. 164 completed the questionnaires (57% of the eligible women), which is similar to response rates in previous studies [3,24]. There were no significant differences in current age, race/ethnicity, stage at diagnosis, type of surgery, or tumor characteristics between the responders (n=164) and non-responders (n=312) (data not shown). Of the 164 participants, 162 had complete responses for both the BHE and BPA scales and were included in analyses.
Figure 1.
ABC Study Recruitment Flow Chart
Table 1 provides demographic and medical characteristics of the study participants. The average age was 48 years (range 28-56), and most were white (69%). The average time since diagnosis was 3.4 years. Over half of the women received both chemotherapy and radiation, and 61% were receiving endocrine therapy at the time of survey. The majority were post-menopausal at survey and about half reported that they had become menopausal during the course of their cancer treatment. Nearly 40% were categorized as overweight or obese based on their current BMI.
Barriers to Physical Activity and Barriers to Healthy Eating
Table 2 shows the individual items from each of the barrier scales. The frequency with which each item was endorsed is displayed in each row. The three most highly endorsed BHEs were “Holidays and special occasions are a problem,” “I feel like eating whatever I want,” and “High fat foods taste better.” These three barriers each had a mean score of 2.9 (out of 5). The three BPAs with the highest mean scores were “Lack of time” (3.2), “Lack of self-discipline (3.1), and “Fatigue (or lack of energy)” (3.0).
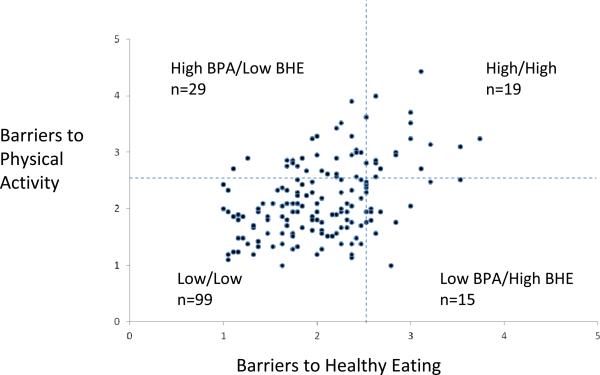
The mean sum of response on the BPA scale was 45.7 (SD 14.5) on the 21 items (minimum 21, max 93) corresponding to an average response of 2.2 per item. The mean sum of response on the BHE scale was 38.7 (SD 10.8) on the 19 items, (minimum 19, maximum 71), corresponding to a mean response of 2.0 per item. Sixty-one percent of the participants were classified as low BPA and low BHE, 18% as high BPA and low BHE, 9% as low BPA and high BHE, and 12% as high BPA and high BHE. The distribution of these groupings is graphically displayed in Figure 2. There was a strong correlation between BPA and BHE (r=0.44, p<0.0001).
Figure 2.
Subgroups of young breast cancer survivors categorized by level of barriers to healthy eating and barriers to physical activity. Participants (n=162) are graphed according to their mean response on the barriers to physical activity scale (BPA) and the barriers to healthy eating scale (BHE). For each scale, the following categories apply: 1=never, 2=rarely, 3=sometimes, 4=often, 5=very often. A mean response of 2.5 or higher for each scale was classified as “high” whereas a response of less than 2.5 was classified as “low.”
Because little was known about which women might be most likely to report BPA or BHE, we first examined the relationship of key characteristics to BPA and BHE scores in the four groups (Table 1). No demographic differences were noted among the groups; however, women in the High/High group were more likely to be currently heavier (p=0.001), and were heavier at diagnosis (p=0.002). There was also a significant difference in menopausal status with the Low PA/High HE group having the highest percentage of pre-menopausal women.
Several of the HRQL and symptom scales were significantly associated with the barrier groupings including depression (p=0.008) perceived stress (p=0.002), emotional social support (p=0.01), instrumental social support (p=0.006), fatigue (p=0.01), PCS (p=0.001), MCS (p=0.01), and symptoms related to bladder control (p=0.003), cognitive problems (p=0.049), weight problems (p=0.002), and arm problems (p<0.001). Post-hoc tests revealed that the majority of the significant differences were between the Low/Low group and the High/High group (p<0.05, Table 1). The High/High group had the least favorable scores for the majority of these variables. The Low BPA/High BHE group had the least favorable scores for the MCS and cognitive symptoms, and their scores for these variables were significantly different from the Low/Low group (p<0.05). The Low BPA/High BHE group also was more likely to be premenopausal than the other 3 groups (p<0.05). The High BPA/Low BHE group reported more arm problems, higher current BMI and higher BMI at diagnosis than the Low/Low group and also had lower PCS scores than the both the Low/Low group and the Low PA/High BHE group.
Regression Analyses
Table 3 shows the regressions of BPA and BHE on demographic characteristics, HRQL, symptoms, and cancer treatment. Model 1 shows the correlates of BHE (R2=0.21). There was a significant inverse relationship between BHE and having a four year college degree or more (p=0.02) as well as a positive relationship between BHE and perceived stress (p=0.03) and perceived weight problems (p=0.002). Model 2 shows the correlates of BPA (R2=0.34). There was a significant positive relationship between BPA and experiencing bladder control issues (p=0.01), as well as an inverse relationship between BPA and the PCS (p=0.003). The bladder control difficulties were only associated with BPA in the bivariate analyses, and thus not included in the BHE model.
Table 3.
Multivariate regression of Barriers to Healthy Eating and Barriers to Physical Activity on Demographics, Quality of Life, Symptoms, and Cancer Treatment
| Predictors | Model 1: BHE | Model 2: BPA | ||
|---|---|---|---|---|
| R2=0.21 | R2=0.34 | |||
| Coef (SE) | P-value | Coef (SE) | P-value | |
| Current Age | −0.004 | 0.61 | −0.01 | 0.26 |
| Ethnicity (white vs. not white) | 0.008 | 0.94 | 0.10 | 0.36 |
| Has Children (yes vs. no) | 0.03 | 0.79 | 0.12 | 0.30 |
| Married or living as married (yes vs. no) | 0.001 | 0.99 | 0.11 | 0.33 |
| Four year college grad or more (yes vs. no) | −0.24 | 0.02 | −0.09 | 0.38 |
| Comorbid conditions (yes vs. no) | −0.05 | 0.60 | −0.15 | 0.18 |
| Had radiation only (yes vs. no) | −0.01 | 0.94 | 0.28 | 0.19 |
| Had chemotherapy only (yes vs. no) | −0.25 | 0.10 | −0.14 | 0.40 |
| Had both radiation and chemotherapy (yes vs. no) | −0.09 | 0.53 | 0.21 | 0.16 |
| Currently receiving endocrine therapy (yes vs. no) | −0.007 | 0.94 | 0.07 | 0.47 |
| Perceived Stress | 0.02 | 0.03 | 0.01 | 0.15 |
| Level of fatigue on the day most fatigue of past week | 0.02 | 0.34 | 0.02 | 0.30 |
| BCPT Bladder Control | 0.17 | 0.01 | ||
| BCPT Weight Problems | 0.13 | 0.002 | 0.07 | 0.15 |
| Physical Component Well-Being | 0.004 | 0.44 | −0.02 | 0.003 |
Additional Scale and item descriptions: Currently receiving endocrine therapy: e.g. Tamoxifen, Femara, Aromasin, Arimidex, Lupron or Zoladex; Stress: Perceived Stress Scale; Physical Component Well-Being: Short Form Health Survey (SF-12); Level of Fatigue: FSI; Breast Cancer-Related Symptoms: Breast Cancer Prevention Trial Symptom Checklist ,BCPT Symptom Scales
Table 4 shows results of multivariate regression of BMI on BHE and BPA. Before BHE and BPA were included in the same model, BMI was regressed on BHE and BPA separately (data not shown). BHE was significantly positively associated with BMI (p=0.001, R2=0.22). In addition, BPA was significantly positively associated with BMI (p=0.001; R2=0.22). When both BPA and BHE were included together (Table 4), the F test showed joint significance (p=0.001), indicating that the two variables together accounted for significant variation in BMI, after adjusting for the other variables in the model; furthermore, BHE and BPA were each independently associated with BMI when controlling for the other (both p<0.05, R2=0.24).
Table 4.
Multivariate Regression of BMI on BHE and BPA
| Predictors | BMI on BHE and BPA | |
|---|---|---|
| R2=0.24 | ||
| Coef (SE) | P-value | |
| Current Age | 0.19 | 0.01 |
| Ethnicity (white vs. not white) | 1.0 | 0.25 |
| Has Children (yes vs. no) | −0.48 | 0.61 |
| Married or living as married (yes vs. no) | 0.69 | 0.49 |
| Four year college grad or more (yes vs. no) | −1.97 | 0.03 |
| Comorbid conditions (yes vs. no) | 2.13 | 0.02 |
| Had radiation only (yes vs. no) | 0.08 | 0.96 |
| Had chemotherapy only (yes vs. no) | 0.65 | 0.63 |
| Had both radiation and chemotherapy (yes vs. no) | 1.40 | 0.27 |
| Currently receiving endocrine therapy (yes vs. no) | −0.90 | 0.28 |
| Barriers to Healthy Eating (BHE) | 1.72 | 0.03 |
| Barriers to Physical Activity (BPA) | 1.40 | 0.046 |
*P-value for test of joint significance=0.001
Additional Scale and item descriptions: Currently receiving endocrine therapy: e.g. Tamoxifen, Femara, Aromasin, Arimidex, Lupron or Zoladex; BPA: adapted from Rogers, BHE: developed by study team.
P≤0.05 was deemed significant and is shown in bold.
DISCUSSION
In this study sample, the most frequently reported barrier to PA was “lack of time” and the most frequently reported barriers to HE were “Holidays and special occasions are a problem,” “I feel like eating whatever I want,” and “High fat foods taste better.” Our analyses suggest that the correlates of BHE and BPA are distinct. Namely, the factors associated with higher BHE were being less educated, having higher perceived stress, and increased perceived severity of weight problems. In comparison, the factors associated with BPA were increased severity of bladder control problems and lower physical well-being. Several of the variables associated with higher BHE or higher BPA are amenable to intervention, such as management of perceived stress or improving physical functioning.
The regression model for BMI and BHE demonstrated that a one unit increase in barrier severity on the BHE scale corresponded to a 2.4 unit increase in BMI unadjusted for BPA and 1.7 unit increase adjusted for BPA. This one unit increase could be viewed as the difference between a participant responding “sometimes” (on average) vs. responding “often” to the set of barriers. Similarly, our results suggest that each 1 unit increase in response on the BPA scale corresponds to an increase of 2.1 units in BMI unadjusted for BHE and 1.4 adjusted for BHE. Considering that BMI had a standard deviation of 5.5 in this sample, such differences in BMI could be considered clinically significant. Finally, the two barrier scales are independently related to BMI when included in the same regression model.
To put our results into perspective, it is important to note that the levels of reported BPA and BHE were fairly low in the sample, with most women (61%) falling into the Low/Low category, indicating that they were most likely to respond that they “never” or “rarely” experienced the various barriers. This finding is consistent with a previous study by Rogers et al. (22) with breast cancer patients. Despite the overall low level of barriers reported, a small group of women (12%) reported relatively high barriers on both scales. Women in the High/High group were the most overweight and had more symptoms such as depression, perceived stress, fatigue, and lower physical functioning, all of which could be potentially modified with targeted interventions. Women in this group may need interventions that include treatment for depressive symptoms.
To our knowledge, our study is the first to examine correlates of barriers to PA as well as barriers to HE, and the intersection of the two sets of barriers. We identified 6 studies [22,25-29] that investigated barriers to PA in BCS, as well as 3 studies that evaluated barriers to PA in breast cancer patients [21,30,31]. No studies were found that explored barriers to HE in BCS or patients. An additional 6 studies [32-37] reported on barriers to PA in other cancer patient or survivor populations, 3 of which [32,36,37] also reported on barriers to HE, but did not systematically explore correlates of perceived barriers or associations between perceived barriers and BMI.
Of the 6 studies that focused on barriers to PA in BCS, two were conducted with a group of survivors who had a mean age of 50 years or less [25,26]. One of these studies examined 64 BCS with a mean age of 43 years. The most influential barriers were “lack of time,” “inertia,” and “not in routine,” and an index developed to measure the barriers was accurate at predicting reported levels of PA [25]. Predictors of the barriers or associations with adiposity were not evaluated. In another study of 51 survivors ages 33 to 63, the authors report that lack of time was the main barrier to PA [26]. The remaining publications, conducted with older samples of women, focused on describing the most common barriers in survivors and/or evaluating whether the perceived barriers were associated with reported PA or self-efficacy for PA.
Our findings provide some insight into potentially modifiable risk factors that could be targeted for lifestyle interventions in younger BCS. The BHE scale was positively associated with perceived stress as well as weight problems (bothered by “weight gain” of “being unhappy with body appearance”). Interventions could target self-acceptance/self-esteem and perceived stress. Stress may contribute to BHE by prompting emotional eating or creating the perception of not enough time available to cook/eat healthfully. These factors may interrelate with some of the other psychosocial and physical concerns that are common in younger survivors [3]. Finally, given that educational attainment was inversely associated with BHE, interventions for women in this group may be valuable.
The correlates of BPA were concentrated in the physical domain. Specifically, interventions that help survivors to manage and improve their bladder control and overall physical functioning may be useful in reducing their barriers to PA. Though we found that the correlates of BHE and BPA are distinct, our results show that both sets of barriers are independently and positively related to BMI.
Our study findings are limited by the cross-sectional design, as well as the use of self-reported height and weight; however, we have previously demonstrated high concordance between self-report and measured height and weight in a similar population of young BCS [8]. We also do not have measures of actual dietary intake to correlate with perceived BHE; however, we did collect a self-report measure of PA (the Godin-leisure time physical activity scale), and found a significant inverse correlation with BPA (r=-0.36, p<0.0001). Future studies should include more rigorous, objective measures of PA as well as measures of actual dietary intake.
In conclusion, this study describes the correlates of perceived barriers to both PA and HE in young BCS and identifies potential targets for future interventions. Although most younger BCS did not report substantial barriers to either PA or HE, an important minority did and they would likely benefit from interventions designed to improve PA due to its benefit in reduction of breast cancer events and overall mortality [38,39]. Identifying women who report perceived barriers to PA may be a first step in increasing PA.
ACKNOWLEDGMENTS
We thank the participants in this study for contributing to this research and increasing our knowledge about the experiences of younger women with breast cancer. We also want to acknowledge the support of the research team members, including Barbara Kahn-Mills, Sasha Sobolevsky, and Patricia Voege.
Funding: This project was supported by funding from the Jonsson Cancer Center Foundation to Dr. Patricia Ganz. Additional funding from NIH R25 CA 87949 to Drs. Ventura, Stanton, and Ganz and NIH CA16024 to Dr. Crespi.
Footnotes
Conflicts of interest: the authors have no conflicts of interest to declare Word count: 3483
REFERENCES
- 1.American Cancer Society Cancer Treatment & Suvivorship: Facts and Figures 2012-2013. 2012 [Google Scholar]
- 2.American Cancer Society . Breast Cancer Facts and Figures 2011-2012. American Cancer Society, Inc.; Atlanta: 2012. [Google Scholar]
- 3.Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(5):386–405. doi: 10.1093/jnci/djr541. [DOI] [PubMed] [Google Scholar]
- 4.Ewertz M, Jensen MB, Gunnarsdóttir K, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29(1):25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 5.Goodwin PJ, Ennis M, Pritchard KI, et al. Adjuvant treatment and onset of menopause predict weight gain after breast cancer diagnosis. J Clin Oncol. 1999;17(1):120–129. doi: 10.1200/JCO.1999.17.1.120. [DOI] [PubMed] [Google Scholar]
- 6.Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23(7):1370–1378. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 7.Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical Activity, Biomarkers, and Disease Outcomes in Cancer Survivors: A Systematic Review. J Natl Cancer Inst. 2012;104(11):815–40. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herman DR, Ganz PA, Petersen L, Greendale GA. Obesity and cardiovascular risk factors in younger breast cancer survivors: The Cancer and Menopause Study (CAMS). Breast Cancer Res Treat. 2005;93(1):13–23. doi: 10.1007/s10549-005-2418-9. [DOI] [PubMed] [Google Scholar]
- 9.Davies NJ, Batehup L, Thomas R. The role of diet and physical activity in breast, colorectal, and prostate cancer survivorship: a review of the literature. Br J Cancer. 2011;105(Suppl 1):S52–73. doi: 10.1038/bjc.2011.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–74. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 11.Ganz PA, Greendale GA, Petersen L, Kahn B, Bower JE. Breast cancer in younger women: reproductive and late health effects of treatment. J Clin Oncol. 2003;21(22):4184–4193. doi: 10.1200/JCO.2003.04.196. [DOI] [PubMed] [Google Scholar]
- 12.Ganz PA, Kwan L, Stanton AL, Bower JE, Belin TR. Physical and psychosocial recovery in the year after primary treatment of breast cancer. J Clin Oncol. 2011;29(9):1101–1109. doi: 10.1200/JCO.2010.28.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Measure. 1977;1:385–401. [Google Scholar]
- 14.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 15.Ganz PA, Guadagnoli E, Landrum MB, Lash TL, Rakowski W, Silliman RA. Breast cancer in older women: quality of life and psychosocial adjustment in the 15 months after diagnosis. J Clin Oncol. 2003;21(21):4027–4033. doi: 10.1200/JCO.2003.08.097. [DOI] [PubMed] [Google Scholar]
- 16.Hann DM, Jacobsen PB, Azzarello LM, et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7(4):301–310. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- 17.Hann DM, Denniston MM, Baker F. Measurement of fatigue in cancer patients: further validation of the Fatigue Symptom Inventory. Qual Life Res. 2000;9(7):847–854. doi: 10.1023/a:1008900413113. [DOI] [PubMed] [Google Scholar]
- 18.Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract. 1998;6(3):143–152. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- 19.Ware J, Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Stanton AL, Bernaards CA, Ganz PA. The BCPT symptom scales: a measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst. 2005;97(6):448–456. doi: 10.1093/jnci/dji069. [DOI] [PubMed] [Google Scholar]
- 21.Rogers LQ, Shah P, Dunnington G, et al. Social cognitive theory and physical activity during breast cancer treatment. Oncol Nurs Forum. 2005;32(4):807–815. doi: 10.1188/05.ONF.807-815. [DOI] [PubMed] [Google Scholar]
- 22.Rogers LQ, McAuley E, Courneya KS, Verhulst SJ. Correlates of physical activity self-efficacy among breast cancer survivors. Am J Health Behav. 2008;32(6):594–603. doi: 10.5555/ajhb.2008.32.6.594. [DOI] [PubMed] [Google Scholar]
- 23.Calfas KJ, Patrick K, Hagler A, Norman GJ, Zabinski MF, JF S. Twelve-month dietary and physical activity outcomes in ‘PACEi-Women in Balance’: A primary-care and web-based intervention. Annals of Behavioral Medicine. 2006;31(suppl):S184. [Google Scholar]
- 24.Ganz PA, Rowland JH, Desmond K, Meyerowitz BE, Wyatt GE. Life after breast cancer: understanding women's health-related quality of life and sexual functioning. J Clin Oncol. 1998;16(2):501–514. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- 25.Leddy SK. Incentives and barriers to exercise in women with a history of breast cancer. Oncol Nurs Forum. 1997;24(5):885–890. [PubMed] [Google Scholar]
- 26.Loh SY, Chew SL, Lee SY. Barriers to exercise: perspectives from multiethnic cancer survivors in Malaysia. Asian Pac J Cancer Prev. 2011;12(6):1483–1488. [PubMed] [Google Scholar]
- 27.Ottenbacher AJ, Day RS, Taylor WC, et al. Exercise among breast and prostate cancer survivors--what are their barriers? J Cancer Surviv. 2011;5(4):413–419. doi: 10.1007/s11764-011-0184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers LQ, Vicari S, Courneya KS. Lessons learned in the trenches: facilitating exercise adherence among breast cancer survivors in a group setting. Cancer Nurs. 2010;33(6):E10–17. doi: 10.1097/NCC.0b013e3181db699d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sander AP, Wilson J, Izzo N, Mountford SA, Hayes KW. Factors that affect decisions about physical activity and exercise in survivors of breast cancer: a qualitative study. Phys Ther. 2012;92(4):525–536. doi: 10.2522/ptj.20110115. [DOI] [PubMed] [Google Scholar]
- 30.Rogers LQ, Courneya KS, Verhulst S, Markwell S, Lanzotti V, Shah P. Exercise barrier and task self-efficacy in breast cancer patients during treatment. Support Care Cancer. 2006;14(1):84–90. doi: 10.1007/s00520-005-0851-2. [DOI] [PubMed] [Google Scholar]
- 31.Gho SA, Steele JR, Munro BJ. Is bra discomfort a barrier to exercise for breast cancer patients? Support Care Cancer. 2010;18(6):735–741. doi: 10.1007/s00520-009-0707-2. [DOI] [PubMed] [Google Scholar]
- 32.Anderson AS, Caswell S, Wells M, Steele RJ, Macaskill S. “It makes you feel so full of life” LiveWell, a feasibility study of a personalised lifestyle programme for colorectal cancer survivors. Support Care Cancer. 2010;18(4):409–415. doi: 10.1007/s00520-009-0677-4. [DOI] [PubMed] [Google Scholar]
- 33.Clark MM, Vickers KS, Hathaway JC, et al. Physical activity in patients with advanced-stage cancer actively receiving chemotherapy. J Support Oncol. 2007;5(10):487–493. [PubMed] [Google Scholar]
- 34.Courneya KS, Friedenreich CM, Quinney HA, et al. A longitudinal study of exercise barriers in colorectal cancer survivors participating in a randomized controlled trial. Ann Behav Med. 2005;29(2):147–153. doi: 10.1207/s15324796abm2902_9. [DOI] [PubMed] [Google Scholar]
- 35.Rogers LQ, Courneya KS, Robbins KT, et al. Physical activity correlates and barriers in head and neck cancer patients. Support Care Cancer. 2008;16(1):19–27. doi: 10.1007/s00520-007-0293-0. [DOI] [PubMed] [Google Scholar]
- 36.Satia JA, Campbell MK, Galanko JA, James A, Carr C, Sandler RS. Longitudinal changes in lifestyle behaviors and health status in colon cancer survivors. Cancer Epidemiol Biomarkers Prev 4. 2004;13(6):1022–1031. [PubMed] [Google Scholar]
- 37.Satia JA, Walsh JF, Pruthi RS. Health behavior changes in white and African American prostate cancer survivors. Cancer Nurs. 2009;32(2):107–117. doi: 10.1097/NCC.0b013e3181982d4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paxton RJ, Phillips KL, Jones LA, et al. Associations among physical activity, body mass index, and health-related quality of life by race/ethnicity in a diverse sample of breast cancer survivors. Cancer. 2012;118(16):4024–4031. doi: 10.1002/cncr.27389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bertram LA, Stefanick ML, Saquib N, et al. Physical activity, additional breast cancer events, and mortality among early-stage breast cancer survivors: findings from the WHEL Study. Cancer Causes Control. 2011;22(3):427–435. doi: 10.1007/s10552-010-9714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]