Abstract
Patients with congenital heart disease (CHD) are at risk for neurocognitive impairments. Little is known about the impact of CHD on the organization of large-scale brain networks. We applied graph analysis techniques to diffusion tensor imaging (DTI) data obtained from 49 adolescents with dextro-transposition of the great arteries (d-TGA) repaired with the arterial switch operation in early infancy and 29 healthy referent adolescents. We examined whether differences in neurocognitive functioning were related to white matter network topology. We developed mediation models revealing the respective contributions of peri-operative variables and network topology on cognitive outcome. Adolescents with d-TGA had reduced global efficiency at a trend level (p = 0.061), increased modularity (p = 0.012), and increased small-worldness (p = 0.026) as compared to controls. Moreover, these network properties mediated neurocognitive differences between the d-TGA and referent adolescents across every domain assessed. Finally, structural network topology mediated the neuroprotective effect of longer duration of core cooling during reparative neonatal cardiac surgery, as well as the detrimental effects of prolonged hospitalization. Taken together, worse neurocognitive function in adolescents with d-TGA is mediated by global differences in white matter network topology, suggesting that disruption of this configuration of large-scale networks drives neurocognitive dysfunction. These data provide new insights into the interplay between perioperative factors, brain organization, and cognition in patients with complex CHD.
Keywords: Brain, d-TGA, Fractional anisotropy, MRI, Neurodevelopment
Highlights
-
•
Network topology mediates neurocognitive outcomes in congenital heart disease.
-
•
Network topology mediates the neuroprotective effect of hypothermia on cognition.
-
•
Network topology mediates the effect of prolonged hospitalization on cognition.
1. Introduction
Congenital heart disease (CHD) constitutes the most common congenital defect in children (Van Der Linde et al., 2011). As greater numbers of children have survived to adulthood in recent years, neurodevelopmental disabilities have emerged as the most common – and potentially most disabling – long-term complication of CHD and its treatment (Donofrio et al., 2011; Olsen et al., 2011; Tabbutt et al., 2012). Neurocognitive deficits in survivors of CHD involve multiple domains including executive function, attention, visual-spatial skills, and memory (Bellinger et al., 2011a; Rollins et al., 2014; Schaefer et al., 2013; Von Rhein et al., 2014). Neuroimaging studies have documented a variety of brain abnormalities starting in utero and extending well into the post-operative period (Donofrio et al., 2011; Limperopoulos et al., 2010; Miller et al., 2007; Beca et al., 2013; Bertholdt et al., 2014; Lynch et al., 2014; Clouchoux et al., 2013). These findings support the hypothesis that neurocognitive deficits in children with CHD are related to both abnormal brain development and brain injury, stemming from a complex interaction between patient-specific factors and peri-operative/medical factors, recently captured in the term “encephalopathy of CHD” (Volpe, 2014).
Yet the relationship between brain structural abnormality and neuropsychological outcome remains poorly defined in individuals with subtypes of CHD such as dextro-transposition of the great arteries (d-TGA). While minor structural abnormalities such as T2 hyperintensities or Chiari 1 malformations visible on anatomical MRI are more prevalent in adolescents with d-TGA (Bellinger et al., 2011a), these abnormalities were not associated with neurocognitive deficits. On the other hand, white matter microstructural characteristics of this cohort found using diffusion tensor imaging (DTI) have also been recently described (Rollins et al., 2014; Rivkin et al., 2013). Reduced fractional anisotropy (FA), a marker of white matter fiber organization, was found in multiple white matter regions, and FA values in these regions correlated with numerous measures of neurocognitive function including mathematical, mnemonic and executive performance, visual spatial processing and position on an autism screening scale.
Recently, the application of graph analysis techniques to human neuroimaging data has opened the door to a systems-level approach in neurology, modeling connectivity of vital neural systems within the overall framework of the human brain — the “connectome” (Filippi et al., 2013; Sporns, 2013; Bassett and Bullmore, 2006; Park, and Friston, 2013; Wang et al., 2014; Meunier et al., 2010; Power et al., 2011; Kaiser, 2013; Fornito et al., 2013). This approach has broken new ground in understanding the neural substrates of complex neuropsychiatric and neurodevelopmental disorders, including schizophrenia, autism, and attention deficit hyperactivity disorder (ADHD) (Bullmore and Sporns, 2009; Griffa et al., 2013; Sporns, 2013; He et al., 2012; Micheloyannis et al., 2006; Wen et al., 2011; Yu et al., 2011; Li et al., 2014; Goch et al. 2014a, 2014b). Graph analysis characterizes network topology in regard to three overarching properties: integration, segregation, and small-worldness (the balance between integration and segregation) (Fair et al., 2007). Supporting higher-order cognition, segregation allows for specialized processing to occur within densely interconnected subnetworks, while integration allows for the rapid combination of specialized information mapped across these distributed neural networks. Accordingly, reduced structural network integration has been shown to correlate with reduced cognitive abilities in normative aging and neurological disease populations (Wen et al., 2011; Vlooswijk et al., 2011), while alterations in structural network segregation have been attributed to cognitive dysfunction in Alzheimer's disease and schizophrenia (Yu et al., 2011; De Haan et al., 2012), and alterations in small-worldness have been described in multiple neurological disorders in relation to cognitive outcomes (He et al., 2012; Micheloyannis et al., 2006; Sanz-Arigita et al., 2010; Pandit et al., 2013; Batalle et al., 2012). Thus, graph analysis techniques afford the opportunity to elucidate the path from clinical risk factors to aberrant brain development to poor neurocognitive outcome.
The regionally-specific FA differences cited above in d-TGA patients (Rollins et al., 2014; Rivkin et al., 2013) lead us to hypothesize that differences in structural network topology (the “connectome”) may be related to the neurocognitive deficits seen in patients with d-TGA, since differences in global network organization can reflect altered FA values. We applied graph analysis to DTI data acquired from adolescents with d-TGA corrected surgically in early infancy and prospectively enrolled in the Boston Circulatory Arrest Study (BCAS). To determine the impact of complex congenital heart disease on large-scale brain networks, we compared network topological properties related to integration (efficiency), segregation (modularity, transitivity), and network optimization (small-worldness) among adolescents with d-TGA relative to healthy referent adolescents. Unlike regional FA values, these metrics incorporate a systems-level approach and inform about the topological characteristics of the brain as a network (or “connectome”). Next, we included these graph metrics in statistical mediation models including neurocognitive outcomes (as measured on a wide range of neuropsychological tests) as the independent variable and explored whether network topology mediates group differences in outcome. Finally, within the d-TGA cohort alone, we investigated whether network topology mediated differences in neurocognitive outcomes where patient or peri-operative variables were the independent variables. Mediation models (as opposed to simple correlative or multiple regression analyses) are necessary to investigate whether brain differences in fact underlie neurocognitive differences (e.g., Hayes, 2009).
2. Methods
2.1. Patients
We studied adolescents enrolled between April 1988 and February 1992 in the Boston Circulatory Arrest Study, a randomized trial comparing the neurological and developmental outcomes of children with d-TGA undergoing the arterial switch operation in early infancy with vital organ support using deep hypothermia with predominately total circulatory arrest (DHCA) or continuous low-flow cardiopulmonary bypass (LFBP). We have previously published trial methods and neurodevelopmental findings in the peri-operative period and at ages 1, 4, 8, and 16 years (Bellinger et al., 1995, 1999, 2009, 2011b). A referent group of healthy adolescents met the criteria adapted from those used in the National Institutes of Health MRI Study of Normal Brain Development (Evans and Brain, 2006). Our exclusion criteria included: children with known risk factors for brain disorders (e.g., intra-uterine exposure to toxicants, history of closed head injury with loss of consciousness, language disorder or Axis 1 psychiatric disorder, first degree relative with a lifetime history of an Axis 1 psychiatric disorder, or abnormality on neurological examination); subjects for whom MRI was contraindicated (e.g., pacemaker, metal implants); subjects with Trisomy 21; adolescents with other forms of CHD requiring surgical correction; and subjects whose primary language was not English. This study was approved by the Boston Children's Hospital Institutional Review Board and adhered to both institutional guidelines and the Declaration of Helsinki. Parents provided written informed consent, and adolescents provided assent.
2.2. Procedures
2.2.1. Image acquisition and analysis
All participants were scanned on a GE Twin 1.5 T system (General Electric, Milwaukee, WI) at Boston Children's Hospital using a quadrature head coil. Acquisition parameters were matrix = 64 × 64, TR/TE = 13,000 ms/108 ms, slice thickness = 4 mm, # of diffusion-weighted directions = 6, b = 750 s/mm2, and FOV = 240 mm. Axial slices were acquired. Repeated acquisitions were obtained for each subject. Due to unacceptable artifact, some subjects contributed fewer acquisitions than others; an analysis of variance showed that there was no significant effect of series number (data not shown).
2.2.2. Pre-processing
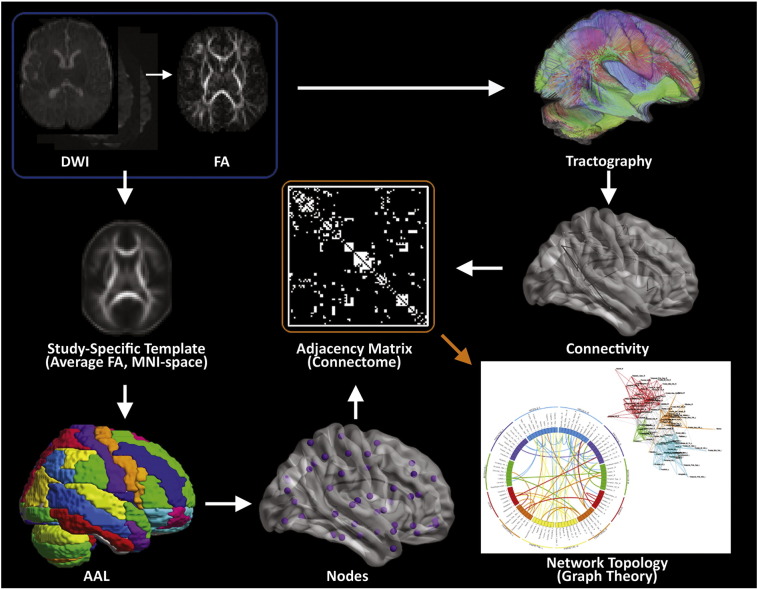
DTI data were pre-processed by first concatenating all runs into a single file and then corrected for motion, eddy current, and slice dropout artifacts using standard routines in FSL (FMRIB, Oxford UK). DTI metrics, including fractional anisotropy (FA), signal intensity without diffusion weighting (S0), and direction of the principal eigenvector were computed for each voxel and then deterministic tractography was performed using in-house software written in IDL (http://www.ittvis.com, Boulder, CO). Streamlines were computed from each white matter voxel (determined as all voxels with FA > 0.25) in both directions. Stopping thresholds for the tractography were: turning angle > 45 degrees or FA < 0.25. For graph analysis, the DTI data were segmented into 76 anatomical regions by applying the automated anatomic labeling (AAL) template (Tzourio-Mazoyer et al., 2002) from SPM8 (Wellcome Dept. of Cognitive Neurology, London, UK) (Fig. 1) (see next section for more details). Each of these regions served as a node in the subsequent topological analyses.
Fig. 1.
Methodology for structural network topology analysis. A flow diagram for the construction of WM structural networks from DTI including registration, segmentation, generation of WM fiber tracts using deterministic tractography, generation of adjacency matrix and nodes, and visualization of connectivity with spring-board and circle diagrams (see Methods section and Supplemental information for more details).
2.2.3. Image segmentation and registration details
Our segmentation and registration procedure obtains the greater between-participant accuracy obtainable via a study-specific template, while also ensuring the template corresponds to MNI space:
-
1
The T1-weighted images were segmented into GM, WM, and CSF (in native space).
-
2
The WM probability maps were then co-registered to the FA images.
-
3
The S0 images were normalized to the EPI template supplied by SPM8 (in MNI space).
-
4
Using the transformation from step 3, the FA images were transformed into MNI space.
-
5
A study-specific FA template was then computed by averaging the transformed FA images.
-
6
The FA images were then normalized to the study-specific FA template.
-
7
A second study-specific FA template was then computed by averaging the transformed FA images.
-
8
To ensure optimal correspondence with MNI space, the WM probability maps were also transformed into MNI space using the transformation found in step 7 and averaged across participants. The averaged WM probability map was normalized to the white matter template provided by SPM8, and the same transformation was applied to the second study-specific FA template.
-
9
The FA images were normalized to the second study-specific template.
-
10
The AAL parcellation atlas (Tzourio-Mazoyer et al., 2002) was normalized into native space by applying the reverse of the transformation found in step 9.
The anatomical scans were without gross anatomical abnormalities which could conceivably impact the spatial transformations (Rivkin et al., 2013) and all transformations were verified via visual inspection.
2.2.4. Graph analysis
Graph metrics (global efficiency, modularity, transitivity and small-worldness) were computed via the C++ modules available from the Brain Connectivity Toolbox (BCT; Indiana University). A brief description for each metric is given below (For further details, see the BCT documentation at https://sites.google.com/site/bctnet/ (Rubinov, and Sporns, 2010)).
Global efficiency is a measure of network integration (Achard and Bullmore, 2007; Bullmore and Sporns, 2012). The path length between two nodes is defined as the shortest distance between them. Global efficiency is defined as the mean of the reciprocal path length over all pairs of nodes (e.g., if every node was directly connected to every other node, the path lengths would all be one, and global efficiency ((mean(1/path length)) would be 1)). In a highly integrated network, the typical number of steps it takes to get from one node to another is low.
Modularity is a measure of network segregation (Meunier et al., 2010; Uehara et al., 2012). Modularity is defined as the fraction of the edges that fall within given modules minus the expected such fraction if the edges were distributed at random. In a more modular – or segregated – network, nodes within a given module are more highly interconnected, and less connected to nodes outside the module. Modularity was calculated using the Louvain algorithm (Blondel et al., 2008).
Transitivity, another measure of segregation at the local or nodal level, is calculated as the proportion of triangles (i.e., where A−B, A−C and B−C are all directly connected) relative to incomplete triangles (i.e., where A−B and A−C are directly connected, but B−C are not) and quantifies the frequency of localized clusters within the overall network.
Small-worldness represents the balance of integration and segregation (Bassett and Bullmore, 2006; Telesford et al., 2011). Small-worldness is calculated as the ratio of transitivity to characteristic path length, divided by the ratio of transitivity to characteristic path length for a random graph with the same degree distribution; and quantifies the extent to which the network balances overall efficiency and localized clustering (Humphries and Gurney, 2008). In a small-world network, there is only a slight increase in characteristic path length as compared to a random network (and hence only slightly less integration), but a large increase in transitivity (and hence much greater segregation).
2.2.5. Graph visualization details (spring-load and circle diagrams)
For visualization purposes, the spring-loading technique (Fruchterman and Reingold, 1991) was used: graphs were modeled with each node as a positively-charged particle (repelling each other) but connected via springs, with spring constants equal to the connection strength. This procedure results in more strongly-connected nodes appearing closer together in the graph. The visualizations were color-coded according to the specific modules to which each node belonged, with intra-modular connections drawn as a specific color (red, orange, cyan, or green) and inter-modular connections drawn as black. Circle connectivity plots were generated using Circos (Krzywinski et al., 2009). Anatomical regions were segmented and grouped using existing AAL template labels, with groups arranged by hemisphere and physical anatomical location.
2.3. Cognitive outcome measures
Neuropsychological test scores of adolescents with d-TGA and healthy referent adolescents were reported previously in detail (Bellinger et al., 2011b). To assess overall cognitive functioning, we chose the Intelligence Quotient (IQ), measured in the d-TGA subjects at age 8 years using the Wechsler Intelligence Scale for Children-Third Edition with the expectation that Wechsler IQ scores measured at 8 and 16 years would demonstrate relative stability across this age range (Mortensen et al., 2003). While IQ was not obtained at 16 years of age, detailed cognitive testing was conducted on adolescents concurrently with acquisition of MRI scan including academic achievement, memory, executive function, and visual-spatial function (refer to SI for details) (Bellinger et al., 2011b).
To assess academic achievement, we used the Wechsler Individual Achievement Test-Second Edition (WIAT) mathematics and reading composite scores, in addition to subtest scores (Tables S1–3) (Wechsler, 1991). For learning and memory, we used the General Memory Index of the Children's Memory Scale (CMS) (Cohen, 2011), in addition to subtest scores (Tables S1–3), as well as the Delayed Rey–Osterrieth Complex Figure Test (ROCF) (Tables S1–3). An executive function summary score was derived from the Delis–Kaplan Executive Function System (D-KEFS) by averaging standard scores on the letter fluency and category fluency trials of verbal fluency, primary combined measure on design fluency, combined conditions score on sorting, total consecutively correct score on word context, and total achievement score on tower (Delis et al., 2001). In addition, individual subset scores of the D-KEFS were also used (Supplemental Tables 1–3). Visual-spatial function was measured using the Test of Visual-Perceptual Skills (TVPS) and the ROCF (Gardner, 1996).
2.4. Demographic and medical variables
We collected demographic and medical variables including gestational age, age at MRI, sex, social class at 16 years of age (defined as Hollingshead Four Factor Index of Social Status score), total cooling duration, presence of clinical or electroencephalographic seizures, length of hospital stay, duration of stay in ICU, duration of intubation, any open-heart surgery after the ASO, and high catheterization exposure (defined as ≥3 diagnostic catheterizations or ≥2 interventional catheterizations), as previously described. (Bellinger et al., 2011b)
2.5. Statistical approach
Comparisons between d-TGA patients and referents were performed using a General Linear Model with d-TGA status as the variable of interest and age, sex, and square root of the number of DTI acquisitions as nuisance covariates.
2.5.1. Analysis of indirect effects
To more precisely determine the effect of d-TGA on network topology, (and, ultimately, on neurocognitive outcome), we performed a mediation analysis (Hayes, 2009) (see Supplemental methods for more detail) and investigated indirect effects of d-TGA status on neurocognitive outcome, mediated by graph theory metrics. In these analyses, d-TGA status was the independent variable, neurocognitive outcome was the dependent variable, and the graph metrics were the mediating variables (the same covariates as above were included). Bootstrapping (25,000 iterations, resampling with replacement) was used to test for statistical significance, as the parameter of interest (the product of two regression parameters) has a well-known non-normal distribution. Bias-corrected and accelerated confidence intervals, (Diciccio and Efron, 1996) shown to provide accurate control with optimal power for mediation analyses (Hayes and Scharkow, 2013) were computed. Because many mediating variables were tested, the false discovery rate (FDR) method (Benjamini and Hochberg, 2000) was used to control for false positives at q < 0.05. Additionally, to assess the possible effects of peri-operative variables, further analyses were conducted on the cohort of d-TGA patients, with peri-operative variables the independent variable, neurocognitive outcome the dependent variable, and graph metrics the mediating variables. These analyses were performed on a global basis (refer to SI for statistical analysis details).
3. Results
3.1. Clinical trial participants
The scans of 33 adolescents with d-TGA and 11 referent subjects were excluded from analysis due to unacceptable signal artifact. Following exclusion of these 44 adolescents, data from 49 adolescents with d-TGA and 29 referent subjects were included in the final analysis; clinical and neurocognitive characteristics of this sub-cohort are described in Rivkin et al. (2013). Demographic characteristics (gestational age, sex, and age at MRI) in the excluded subjects did not differ from those of subjects included in the final analysis. Adolescents with d-TGA, compared to referent subjects, were older at MRI, more likely to be male, and lower in social class, but had similar gestational age.
3.2. Network topological difference between d-TGA and referent subjects
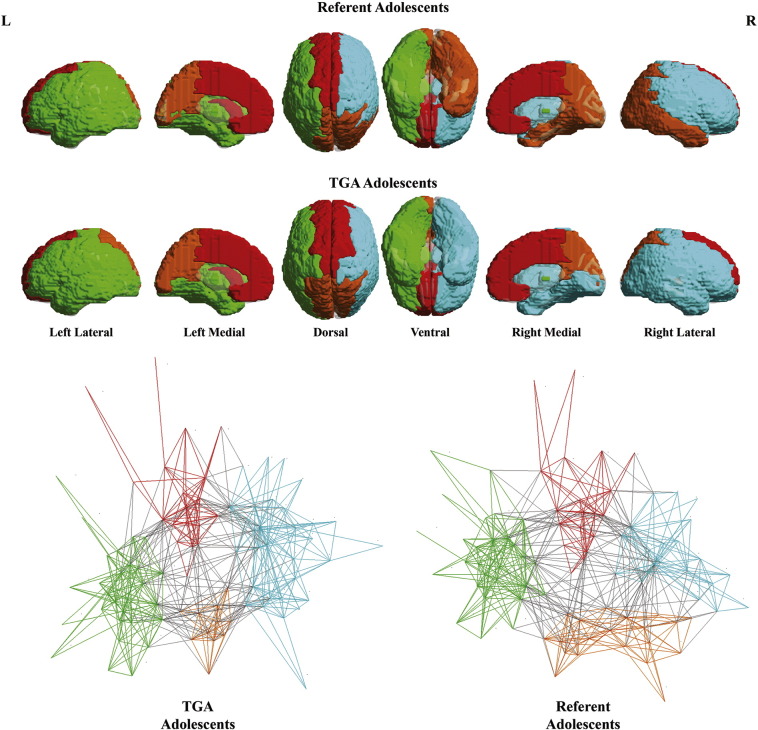
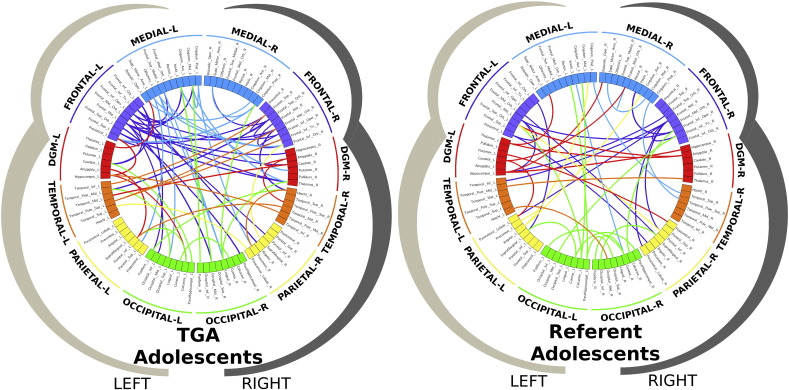
Comparisons between the d-TGA adolescents and referent subjects demonstrated that structural white matter network topology in the d-TGA adolescents was altered globally. Specifically, in the d-TGA cohort, global efficiency was reduced (albeit at only a trend level), modularity was increased, and small-worldness was increased compared to the referent group (Table 1). To better understand the basis of these topological differences, we visualized the network topology of the d-TGA adolescents and the referents with regard to community structure (Fig. 2) and then to small-world architecture (Fig. 3). Utilizing the spring-loaded technique, which maps the nodes of a network in accordance with their connection strength rather than anatomical proximity, Fig. 2 demonstrated that, in the d-TGA cohort, the global network topology was more tightly clustered into separate modules or subnetworks, with fewer connections between subnetworks. Similarly, in Fig. 3, visualization of the small-world architecture using a circle diagram demonstrated a tendency toward more short-range connections (i.e., smaller “arcs”, suggesting localized processing) as well as differences in the spatial localization of long-range connections (i.e., with long-range connections appearing more clustered in the d-TGA cohort rather than uniformly distributed as in the referent cohort).
Table 1.
Global network topology parameters between d-TGA and referent subjects.
| Graph metric | TGA cohort Mean ± SEM |
Healthy referent cohort Mean ± SEM |
Percentdifference | p-value |
|---|---|---|---|---|
| Global efficiency | 0.31 ± 0.008 | 0.33 ± 0.012 | −7.0 | −0.061 |
| Modularity | 0.53 ± 0.004 | 0.52 ± 0.005 | 2.0 | 0.012 |
| Transitivity | 0.31 ± 0.010 | 0.33 ± 0.014 | −4.0 | −0.22 |
| Small-worldness | 2.81 ± 0.048 | 2.69 ± 0.060 | 5.0 | 0.026 |
Fig. 2.
Community structure (modularity). Graph analyses indicated that global network topology was optimally described as comprising four modules (top), or subnetworks, including: an anteromedial interhemispheric subnetwork (red), posterior interhemispheric subnetwork (yellow-orange), right intrahemispheric subnetwork (green) and left intrahemispheric subnetwork (cyan). Spring-loaded visualization (bottom) demonstrated that the global network topology was more tightly clustered into separate modules in the d-TGA cohort, with fewer connections between modules. The gray lines represent intermodular connections. The colored lines present intramodular connections. See Fig. S1 for anatomic parcellation labels.
Fig. 3.
Small-world architecture. Visualization of the small-world architecture with a circle diagram representing the short-range (arcs) and long-range connection (lines) between different regions of the brain including frontal lobes (blue and purple), subcortical nuclei (red), temporal lobes (orange), parietal lobes (yellow), and occipital lobes (green). The d-TGA circle diagram (left) demonstrated a tendency toward more short-range connections (in the frontal regions compared to occipital regions), compared to the referent group (right), which demonstrate more short-range connections posteriorly. In addition, there is a difference in the spatial localization of long-range connections: long-range connections appear more clustered in the d-TGA cohort as compared to the more uniform distribution found in the referent cohort.
3.3. Cognitive mediation analysis: d-TGA compared to referent subjects
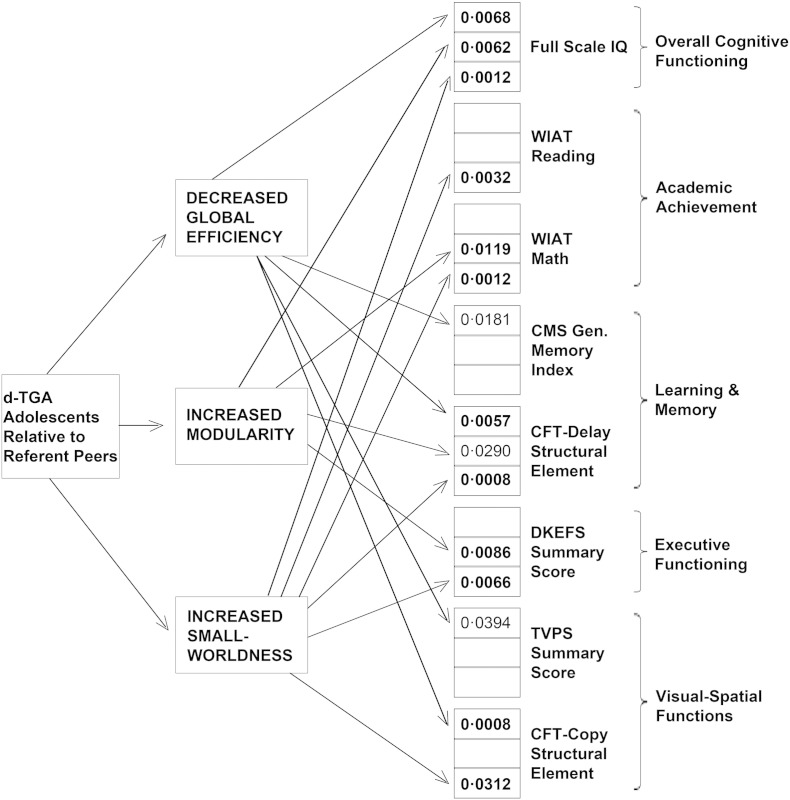
Our primary aim was to determine whether the differences in network topology mediated differences in cognitive functioning among the d-TGA adolescents relative to referent subjects. As summarized in Fig. 4 and Table S1, poorer neurocognitive functioning in the d-TGA group was mediated by differences in network topology (i.e., decreased global efficiency, increased modularity, and increased small-worldness) across multiple domains (i.e., overall cognitive functioning, academic achievement, learning and memory, executive functions, and visual-spatial functions, although a significant result at FDR-corrected q < 0.05 was not obtained for every single subtest), indicating that the differences in cognitive functioning arose, at least in part, from differences in the white matter topological organization among the d-TGA adolescents relative to referent subjects.
Fig. 4.
Structural network topology mediates neurocognitive differences between d-TGA adolescents and healthy referent peers. Results from the mediation analyses demonstrate different paths by which lower efficiency (less network integration) and increased modularity (more network segregation) mediate neurocognitive differences between d-TGA adolescents and healthy referent peers. Values represent significant p-values for the mediation (i.e., a × b—refer to supplement for more details). Note that p-values in bold are significant with correction for multiple comparisons (FDR < 0.05), whereas p-values in regular type are only nominally significant (p < 0.05, uncorrectEd.) See Table S1 for results pertaining to the complete list of neuropsychological tests and subtests.
3.4. Cognitive mediation analysis—medical and demographic variables
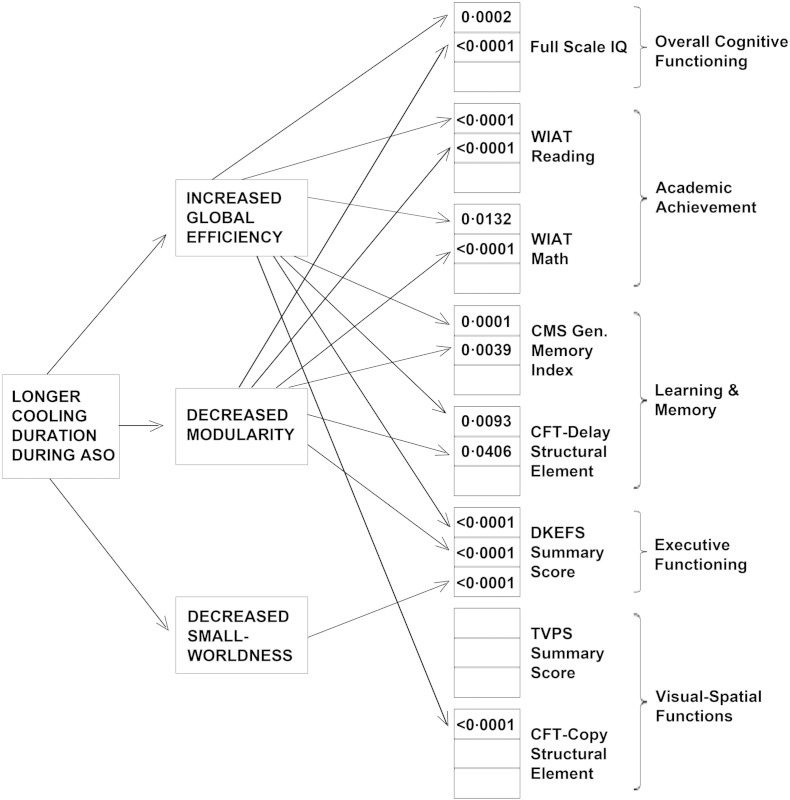
Within the d-TGA cohort, we explored whether the effects of medical, surgical, and demographic variables were mediated by differences in network topology. These analyses demonstrated that duration of intraoperative cooling during the ASO exerted a strong neuroprotective effect, enhancing neurocognitive functioning across all domains assessed, mediated by network topology (Fig. 5) (Table S2). In contrast, longer hospital stay exerted deleterious effects, mediated by network topology (Table S3). Of note, we found no effect on network topology or long-term neurocognitive outcome of vital organ support technique (DHCA versus LFBP), duration of total circulatory arrest time, or the presence or absence of a ventricular septal defect.
Fig. 5.
Better neurocognitive outcomes, associated with longer cooling duration during hypothermic cardiopulmonary bypass, are mediated by global network topology. Results from the mediation analyses demonstrate different paths by which longer cooling duration during the ASO mediated better neurodevelopmental outcomes. In contrast to Fig. 4, here, within the d-TGA group, longer cooling was associated with increased global efficiency (more network integration) and decreased modularity (less network segregation). These effects, in turn, resulted in better neurocognitive performance across all neurocognitive domains. Values represent significant p-values for the mediation (i.e., a × b—refer to supplement). Note that p-values in bold are significant with correction for multiple comparisons (FDR < 0.05), whereas p-values in regular type are only nominally significant (p < 0.05, uncorrectEd.) See Tables S2 and S3 for peri-operative mediation results pertaining to the complete list of neuropsychological tests and subtests.
4. Discussion
Patients with complex CHD are at increased risk for abnormalities of brain growth, cerebral metabolism, and tissue microstructure, as well as white matter injury (Donofrio et al., 2011; Mussatto et al., 2014). Moreover, regional brain abnormalities have been shown to correlate with neurocognitive outcomes (Rollins et al., 2014; Von Rhein et al., 2014). However, the mechanisms linking patient and perioperative risk factors, abnormal brain development, evidence of structural abnormality on MRI, and poor neurocognitive outcome remain poorly understood. Here, we demonstrate for the first time that worse neurocognitive function in adolescents with d-TGA is mediated by global differences in white matter structural network topology, suggesting that disruption of this organization of large-scale networks within the human brain gives rise to neurocognitive dysfunction in children with complex CHD. Finally, we demonstrate that structural network topology mediates the neuroprotective effect of longer duration of core cooling during reparative neonatal cardiac surgery, as well as the detrimental effect of prolonged hospitalization on neurodevelopmental outcomes.
Our network topological analyses demonstrated that segregation (modularity/transitivity) and optimized balance of segregation and integration (small-worldness) differed significantly in the d-TGA group from those of referent adolescents, while integration (efficiency) differed at a trend level. In addition, these differences were related to cognitive differences observed between the two groups. Both small-worldness and modularity are dependent on long-range connections that allow for synchronous processing of information. Disruption of these connections is likely to impair cognitive functioning. Similarly, connections among modules are critical to maintenance of community organization. Taken together, these findings suggest that cognitive dysfunction in d-TGA is likely related at least in part to global alteration in distributed neural networks, an inference that is concordant with the constellation of diffuse white and gray matter abnormalities detected by neuropathological and neuroimaging techniques in fetuses and neonates with complex CHD (Volpe, 2014; Kinney et al., 2005).
The structural network topological differences found in this study are also likely related to the white matter microstructure or fractional anisotropy (FA) that is often regarded as an indicator of white matter fiber tract organization. We previously reported, using voxel based morphometric techniques, that d-TGA adolescents had diffusely reduced FA in deep frontal, parietal, and temporal white matter regions, a subset of which correlated with poor cognition (Rollins et al., 2014; Rivkin et al., 2013). These regions are traversed by multiple long-range fiber tracts involved in several domains of cognition. The findings in the current study build upon these earlier findings by demonstrating a more global view of cognitive function adversely affected by widespread disruption of white matter connections within and across distributed networks in the brain of d-TGA adolescents.
Our findings that adolescents with d-TGA not only displayed lower network integration and higher segregation, but also that these topological differences mediated neurocognitive outcomes, raise the possibility that network topology may be a potent biomarker for neurocognitive outcomes in children with complex CHD. Further research, particularly of a multi-institutional nature, is needed to determine whether biomarkers derived from brain network topological analysis improve the ability of known patient and medical risk factors to predict later neurodevelopmental disabilities.
Additionally, within the d-TGA group, we found that duration of intraoperative core cooling of infants with dTGA was directly related to long-term neurodevelopmental outcomes and was mediated by global structural network topology. Used during cardiothoracic surgery for more than 50 years, therapeutic hypothermia (TH) has garnered considerable attention in the last decade as a mechanism for neuroprotection, with documented efficacy in animal models (Van Der Worp et al., 2007; Tooley et al., 2003; Johnston et al., 2011) and large-scale human neonatal clinical trials after hypoxic–ischemic encephalopathy (Johnston et al., 2011).
What might be the clinical value of the topological differences that we have documented in this study? Similar to other neurological disorders, network topological measurements have the capability to inform us about the neurological underpinnings of poor cognitive dysfunction in long term survivors with complex congenital heart disease. There have been a number of neuropsychiatric and neurocognitive disorders that have been designated as “disconnection syndromes” because of the disruption of white matter tracts that allow for communication between specialized sensorimotor and association cortices (Geschwind, and Levitt, 2007; Catani, 2005; Geschwind, 1965; Sheline et al., 2010). Indeed, our study of network topology in children with complex congenital heart disease does suggest the possibility that a type of “disconnection” syndrome may exist in the patients we have studied — placing them at risk for neurobehavioral and neuropsychiatric impairment. As we start to understand more about the etiologies (including genetics) that may underlie the pathogenesis of cognitive dysfunction in children with complex congenital heart disease, a similar use of network topology measurement may have clinical value with regard to early detection of neurological dysfunction in patients with complex congenital heart disease. As an example, network topology measurements have been used to demonstrate brain network dysfunction before the development of molecular abnormalities in the setting of neurodegeneration (i.e., APOE 4 genetic risk for Alzheimer's disease) (Sheline et al., 2010).
Network topological measurements also have the capacity to inform us about what independent risk factors (modifiable or non-modifiable) may incite neurocognitive or neurobehavioral abnormalities in children with complex congenital heart disease. This potential clinical value of network topology is paramount because of the multiplicity of clinical variables not only in the pre-, peri-, and postoperative periods, but also during the vulnerable and extended period of postnatal development in children with complex CHD. This underscores the need to include network topology measurements in pediatric cardiology clinical trials that incorporate neurodevelopment as an outcome measure. As such, the need for these “connectome” neuroimaging biomarkers as potential treatment response biomarkers is also heralded by advances in cardiac neuroprotection that are on the horizon (Andropoulos et al., 2010; Albers, Bichell, and Mclaughlin, 2010; Stam, 2014). Lastly, brain network measurements, especially applied longitudinally in this at-risk population, have the clinical potential to help us understand how the nervous system – in the setting of complex congenital heart disease – has the capacity to repair and reorganize in response to multiple stressors during the prenatal and postnatal periods. In this context, it has recently been proposed that alterations in relation to “hub architecture” or “rich club” organization are directly related to brain network reorganization (Stam, 2014), which also may be applicable to children with complex CHD and underlie some of the global changes that we have detected in our cohort. The view of organizational difference found in adolescents treated for d-TGA early in life, as compared to healthy children, also raises attractive therapeutic possibilities. More recently developed tools such as transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (TMS) have demonstrated the ability to enhance both cortical network activity and functional performance, and may prove therapeutically beneficial in clinical groups with altered cerebral organization particularly if found early in life (Polanía et al., 2011; Khedr et al., 2013; Grefkes and Fink, 2011; Fox et al., 2012; Simmonds et al., 2014).
Our study has certain limitations. We used a DTI sequence with six diffusion-encoding gradient directions, the minimum necessary to compute the diffusion tensor. Six direction encoded DTI studies, with different degrees of signal average, have been used to study brain development in the adolescent period (Simmonds et al., 2014; Schmithorst et al., 2002; Barnea-Goraly et al., 2005; Sidaros et al., 2008) and certain disease processes like neurotrauma (Sidaros et al., 2008). In addition, six direction DTI has recently been used in other pathologies (e.g., multiple sclerosis) to show differences in graph metrics, which have also been correlated with lesion load and neurobehavioral outcomes (Shu et al., 2011). However, six direction DTI may be considered suboptimal, as greater SNR is present when more directions are used (Jones, 2004; Lebel et al., 2012); this effect is particularly relevant for reconstruction of certain longitudinal fiber pathways (cortical association fibers) (Lebel et al., 2012; Vaessen et al., 2010). The fact that significant results were obtained even with our sub-optimal acquisition method points to the large effect sizes present in our results. We hope in future work to use more advanced diffusion sequences (such as Q-ball, HARDI, etc.) to study this group and disease process in even more detail and to find additional effects which were missed in this study. Future work using diffusion spectrum imaging or Q ball imaging involving a larger number of directions will also likely enhance the ability to visualize cortical association fibers, and improve the neuroanatomic specificity of our generalized global findings which have been derived using a smaller number of encoded directions (Griffa et al., 2015). Additionally, the FSIQ score measured at age 8 may vary from the true FSIQ at age 16 by up to 1 standard deviation (Ramsden et al., 2011), so the true effect on FSIQ may be greater than what we have found in this study. Brain network topology may also be calculated using resting state functional MRI, which may result in a more comprehensive understanding of how disruptions in brain networks are associated with functional cognitive deficits in patients with complex congenital heart disease.
In conclusion, we found that adolescents with d-TGA who underwent open heart surgery in early infancy, compared with a healthy referent group, demonstrated alterations in integration and segregation of global brain topology that mediate differences across multiple neurocognitive domains. We also show that the beneficial and adverse neurodevelopmental effects, respectively, of longer core cooling during bypass for the neonatal ASO and prolonged hospitalization were mediated by network topology. Future research using graph theory analyses in patients with other CHD lesions and management strategies may provide new insights regarding the interplay of peri-operative risk factors, aberrant brain development, and outcomes.
The following are the supplementary data related to this article.
Supplemental Material: Additional Statistical Methods and Anatomic Labels for Figure 2 (Supplemental Figure S1).
Mediation: Cognitive difference between d-TGA and referent.
Mediation: total cooling time at ASO in d-TGA group.
Mediation: length of hospital stay (days) in d-TGA group.
Conflicts of interest
There have been no identified potential conflicts of interest.
Funding
This work was supported by funding from the National Heart, Lung, and Blood Institute (RO1 HL77681), NINDS K23063371, The Children's Heart Foundation (Chicago), the Farb Family Fund, the Kostin Family Fund, the Ian Harrison Family Neonatal Brain Injury Fund and the Pittsburgh Children's Hospital Foundation.
Acknowledgements
We thank Vince Lee and Rafael Ceschin with excellent technical assistance with the figures. We thank Carolyn Lenhart with editorial assistance with the supplemental tables.
References
- Achard S., Bullmore E. Efficiency and cost of economical brain functional networks. PLOS Comput. Biol. 2007;3(2):e17. doi: 10.1371/journal.pcbi.0030017. 17274684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers E.L., Bichell D.P., Mclaughlin B. New approaches to neuroprotection in infant heart surgery. Pediatr. Res. 2010;68(1):1–9. doi: 10.1203/PDR.0b013e3181df5402. 20351657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andropoulos D.B., Brady K.M., Easley R.B., Fraser C.D., Jr Neuroprotection in pediatric cardiac surgery: what is on the horizon? Prog. Pediatr. Cardiol. 2010;29(2):113–122. doi: 10.1016/j.ppedcard.2010.06.005. 20802846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N., Menon V., Eckert M., Tamm L., Bammer R., Karchemskiy A., Dant C.C., Reiss A.L. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb. Cortex. 2005;15(12):1848–1854. doi: 10.1093/cercor/bhi062. 15758200 [DOI] [PubMed] [Google Scholar]
- Bassett D.S., Bullmore E. Small-world brain networks. Neuroscientist. 2006;12(6):512–523. doi: 10.1177/1073858406293182. 17079517 [DOI] [PubMed] [Google Scholar]
- Batalle D., Eixarch E., Figueras F., Muñoz-Moreno E., Bargallo N., Illa M., Acosta-Rojas R., Amat-Roldan I., Gratacos E. Altered small-world topology of structural brain networks in infants with intrauterine growth restriction and its association with later neurodevelopmental outcome. Neuroimage. 2012;60(2):1352–1366. doi: 10.1016/j.neuroimage.2012.01.059. 22281673 [DOI] [PubMed] [Google Scholar]
- Beca J., Gunn J.K., Coleman L., Hope A., Reed P.W., Hunt R.W., Finucane K., Brizard C., Dance B., Shekerdemian L.S. New white matter brain injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest. Circulation. 2013;127(9):971–979. doi: 10.1161/CIRCULATIONAHA.112.001089. 23371931 [DOI] [PubMed] [Google Scholar]
- Bellinger D.C., Jonas R.A., Rappaport L.A., Wypij D., Wernovsky G., Kuban K.C., Barnes P.D., Holmes G.L., Hickey P.R., Strand R.D. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N. Engl. J. Med. 1995;332(9):549–555. doi: 10.1056/NEJM199503023320901. 7838188 [DOI] [PubMed] [Google Scholar]
- Bellinger D.C., Wypij D., Rivkin M.J., DeMaso D.R., Robertson R.L., Dunbar-Masterson C., Rappaport L.A., Wernovsky G., Jonas R.A., Newburger J.W. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation. 2011;124(12):1361–1369. doi: 10.1161/CIRCULATIONAHA.111.026963. 21875911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger D.C., Newburger J.W., Wypij D., Kuban K.C., duPlesssis A.J., Rappaport L.A. Behaviour at eight years in children with surgically corrected transposition: the Boston Circulatory Arrest Trial. Cardioil. Young. 2009;19(1):86–97. doi: 10.1017/S1047951108003454. 19079812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger D.C., Wypij D., Kuban K.C., Rappaport L.A., Hickey P.R., Wernovsky G., Jonas R.A., Newburger J.W. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation. 1999;100(5):526–532. doi: 10.1161/01.cir.100.5.526. 10430767 [DOI] [PubMed] [Google Scholar]
- Bellinger D.C., Wypij D., Rivkin M.J., Demaso D.R., Robertson R.L., Jr., Dunbar-Masterson C., Rappaport L.A., Wernovsky G., Jonas R.A., Newburger J.W. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation. 2011;124(12):1361–1369. doi: 10.1161/CIRCULATIONAHA.111.026963. 21875911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. On the adaptive control of the false discovery rate in multiple testing with independent statistics. J. Educ. Behav. Statist. 2000;25(1):60–83. [Google Scholar]
- Bertholdt S., Latal B., Liamlahi R., Prêtre R., Scheer I., Goetti R., Dave H., Bernet V., Schmitz A., Von Rhein M., Knirsch W., Research Group Heart and Brain Cerebral lesions on magnetic resonance imaging correlate with preoperative neurological status in neonates undergoing cardiopulmonary bypass surgery. Eur. J. Cardiothorac. Surg. 2014;45(4):625–632. doi: 10.1093/ejcts/ezt422. 24031043 [DOI] [PubMed] [Google Scholar]
- Blondel V.D., Guillaume J., Lambiotte R., Lefebvre E. Fast unfolding of communities in large networks. J. Stat. Mech. 2008;2008(10):10008. [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10(3):186–198. doi: 10.1038/nrn2575. 19190637 [DOI] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. The economy of brain network organization. Nat. Rev. Neurosci. 2012;13(5):336–349. doi: 10.1038/nrn3214. 22498897 [DOI] [PubMed] [Google Scholar]
- Catani M., ffytche D.H. The rises and falls of disconnection syndromes. Brain. 2005;128(10):2224–2239. doi: 10.1093/brain/awh622. 16141282 [DOI] [PubMed] [Google Scholar]
- Clouchoux C., Du Plessis A.J., Bouyssi-Kobar M., Tworetzky W., Mcelhinney D.B., Brown D.W., Gholipour A., Kudelski D., Warfield S.K., Mccarter R.J., Robertson R.L., Evans A.C., Newburger J.W., Limperopoulos C. Delayed cortical development in fetuses with complex congenital heart disease. Cereb. Cortex. 2013;23(12):2932–2943. doi: 10.1093/cercor/bhs281. 22977063 [DOI] [PubMed] [Google Scholar]
- Cohen M.J. Children's Memory Scale, Encyclopedia of Clinical Neuropsychology. Springer; 2011. pp. 556–559. [Google Scholar]
- De Haan W., Van Der Flier W.M., Koene T., Smits L.L., Scheltens P., Stam C.J. Disrupted modular brain dynamics reflect cognitive dysfunction in Alzheimer's disease. Neuroimage. 2012;59(4):3085–3093. doi: 10.1016/j.neuroimage.2011.11.055. 22154957 [DOI] [PubMed] [Google Scholar]
- Delis D., Kaplan E., Kramer J. The Delis–Kaplan Executive Function System (D-KEFS) The Psychological Corporation; San Antonio, TX.: 2001. [Google Scholar]
- Diciccio T.J., Efron B., Hall P., Martin M.A., Canty A.J., Davison A.C., Hinkley D.V., Gleser L.J., Lee S.M.S., Young G.A., DiCiccio T.J., Efron B. Bootstrap confidence intervals. Stat. Sci. 1996;11(3):189–228. [Google Scholar]
- Donofrio M.T., Duplessis A.J., Limperopoulos C. Impact of congenital heart disease on fetal brain development and injury. Curr. Opin. Pediatr. 2011;23(5):502–511. doi: 10.1097/MOP.0b013e32834aa583. 21881507 [DOI] [PubMed] [Google Scholar]
- Evans A.C., Brain Development Cooperative Group. The NIH MRI study of normal brain development. Neuroimage. 2006;30(1):184–202. doi: 10.1016/j.neuroimage.2005.09.068. 16376577 [DOI] [PubMed] [Google Scholar]
- Fair D.A., Dosenbach N.U., Church J.A., Cohen A.L., Brahmbhatt S., Miezin F.M., Barch D.M., Raichle M.E., Petersen S.E., Schlaggar B.L. Development of distinct control networks through segregation and integration. Proc. Natl. Acad. Sci. U. S. A. 2007;104(33):13507–13512. doi: 10.1073/pnas.0705843104. 17679691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M., Van Den Heuvel M.P., Fornito A., He Y., Hulshoff Pol H.E., Agosta F., Comi G., Rocca M.A. Assessment of system dysfunction in the brain through MRI-based connectomics. Lancet Neurol. 2013;12(12):1189–1199. doi: 10.1016/S1474-4422(13)70144-3. 24120645 [DOI] [PubMed] [Google Scholar]
- Fornito A., Zalesky A., Breakspear M. Graph analysis of the human connectome: promise, progress, and pitfalls. Neuroimage. 2013;80:426–444. doi: 10.1016/j.neuroimage.2013.04.087. 23643999 [DOI] [PubMed] [Google Scholar]
- Fox M.D., Halko M.A., Eldaief M.C., Pascual-Leone A. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS) Neuroimage. 2012;62(4):2232–2243. doi: 10.1016/j.neuroimage.2012.03.035. 22465297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruchterman T.M., Reingold E.M. Graph drawing by force-directed placement. Softw. Practice Experience. 1991;21:1129–1164. [Google Scholar]
- Gardner M. Test of Visual-Perceptual Skills (nm) Revised. Psychological and Educational Publications. Inc; 1996. [Google Scholar]
- Geschwind D.H., Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr. Opin. Neurobiol. 2007;17(1):103–111. doi: 10.1016/j.conb.2007.01.009. 17275283 [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. II. Brain. 1965;88(3):585–644. doi: 10.1093/brain/88.3.585. 5318824 [DOI] [PubMed] [Google Scholar]
- Goch C.J., Oztan B., Stieltjes B., Henze R., Hering J., Poustka L., Meinzer H.-P., Yener B., Maier-Hein K.H. Global Changes in the Connectome in Autism Spectrum Disorders, Computational Diffusion MRI and Brain Connectivity. Springer; 2014. pp. 239–247. [Google Scholar]
- Goch C.J., Stieltjes B., Henze R., Hering J., Poustka L., Meinzer H.-P., Maier-Hein K.H. Quantification of changes in language-related brain areas in autism spectrum disorders using large-scale network analysis. Int. J. Comput. Assist. Radiol. Surg. 2014;9(3):357–365. doi: 10.1007/s11548-014-0977-0. 24459035 [DOI] [PubMed] [Google Scholar]
- Grefkes C., Fink G.R. Reorganization of cerebral networks after stroke: new insights from neuroimaging with connectivity approaches. Brain. 2011;134(5):1264–1276. doi: 10.1093/brain/awr033. 21414995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffa A., Baumann P.S., Ferrari C., Do K.Q., Conus P., Thiran J.P., Hagmann P. Characterizing the connectome in schizophrenia with diffusion spectrum imaging. Hum. Brain Mapp. 2015;36:354–366. doi: 10.1002/hbm.22633. 25213204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffa A., Baumann P.S., Thiran J.P., Hagmann P. Structural connectomics in brain diseases. Neuroimage. 2013;80:515–526. doi: 10.1016/j.neuroimage.2013.04.056. 23623973 [DOI] [PubMed] [Google Scholar]
- Hayes A.F. Beyond Baron and Kenny: statistical mediation analysis in the new millennium. Commun. Monogr. 2009;76:408–420. [Google Scholar]
- Hayes A.F., Scharkow M. The relative trustworthiness of inferential tests of the indirect effect in statistical mediation analysis: does method really matter? Psychol. Sci. 2013;24(10):1918–1927. doi: 10.1177/0956797613480187. 23955356 [DOI] [PubMed] [Google Scholar]
- He H., Sui J., Yu Q., Turner J.A., Ho B.-C., Sponheim S.R., Manoach D.S., Clark V.P., Calhoun V.D. Altered small-world brain networks in schizophrenia patients during working memory performance. PLOS One. 2012;7(6):e38195. doi: 10.1371/journal.pone.0038195. 22701611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries M.D., Gurney K. Network ‘small-world-ness': a quantitative method for determining canonical network equivalence. PLOS One. 2008;3(4) doi: 10.1371/journal.pone.0002051. 18446219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M.V., Fatemi A., Wilson M.A., Northington F. Treatment advances in neonatal neuroprotection and neurointensive care. Lancet Neurol. 2011;10(4):372–382. doi: 10.1016/S1474-4422(11)70016-3. 21435600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.K. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magn. Reson. Med. 2004;51(4):807–815. doi: 10.1002/mrm.20033. 15065255 [DOI] [PubMed] [Google Scholar]
- Kaiser M. The potential of the human connectome as a biomarker of brain disease. Front. Hum. Neurosci. 2013;7:484. doi: 10.3389/fnhum.2013.00484. 23966935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr E.M., Shawky O.A., El-Hammady D.H., Rothwell J.C., Darwish E.S., Mostafa O.M., Tohamy A.M. Effect of anodal versus cathodal transcranial direct current stimulation on stroke rehabilitation: a pilot randomized controlled trial. Neurorehabil. Neural Repair. 2013;27 doi: 10.1177/1545968313484808. 23609526 [DOI] [PubMed] [Google Scholar]
- Kinney H.C., Panigrahy A., Newburger J.W., Jonas R.A., Sleeper L.A. Hypoxic–ischemic brain injury in infants with congenital heart disease dying after cardiac surgery. Acta Neuropathol. 2005;110(6):563–578. doi: 10.1007/s00401-005-1077-6. 16244891 [DOI] [PubMed] [Google Scholar]
- Krzywinski M., Schein J., Birol I., Connors J., Gascoyne R., Horsman D., Jones S.J., Marra M.A. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19(9):1639–1645. doi: 10.1101/gr.092759.109. 19541911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Benner T., Beaulieu C. Six is enough? Comparison of diffusion parameters measured using six or more diffusion-encoding gradient directions with deterministic tractography. Magn. Reson. Med. 2012;68(2):474–483. doi: 10.1002/mrm.23254. 22162075 [DOI] [PubMed] [Google Scholar]
- Li H., Xue Z., Ellmore T.M., Frye R.E., Wong S.T. Network-based analysis reveals stronger local diffusion-based connectivity and different correlations with oral language skills in brains of children with high functioning autism spectrum disorders. Hum. Brain Mapp. 2014;35(2):396–413. doi: 10.1002/hbm.22185. 23008187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limperopoulos C., Tworetzky W., Mcelhinney D.B., Newburger J.W., Brown D.W., Robertson R.L., Jr., Guizard N., Mcgrath E., Geva J., Annese D., Dunbar-Masterson C., Trainor B., Laussen P.C., Du Plessis A.J. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121(1):26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. 20026783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J.M., Buckley E.M., Schwab P.J., Mccarthy A.L., Winters M.E., Busch D.R., Xiao R., Goff D.A., Nicolson S.C., Montenegro L.M., Fuller S., Gaynor J.W., Spray T.L., Yodh A.G., Naim M.Y., Licht D.J. Time to surgery and preoperative cerebral hemodynamics predict postoperative white matter injury in neonates with hypoplastic left heart syndrome. J. Thorac. Cardiovasc. Surg. 2014;148:2181–2188. doi: 10.1016/j.jtcvs.2014.05.081. 25109755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier D., Lambiotte R., Bullmore E.T. Modular and hierarchically modular organization of brain networks. Front. Neurosc. 2010;4:200. doi: 10.3389/fnins.2010.00200. 21151783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheloyannis S., Pachou E., Stam C.J., Breakspear M., Bitsios P., Vourkas M., Erimaki S., Zervakis M. Small-world networks and disturbed functional connectivity in schizophrenia. Schizophr. Res. 2006;87(1–3):60–66. doi: 10.1016/j.schres.2006.06.028. 16875801 [DOI] [PubMed] [Google Scholar]
- Miller S.P., Mcquillen P.S., Hamrick S., Xu D., Glidden D.V., Charlton N., Karl T., Azakie A., Ferriero D.M., Barkovich A.J., Vigneron D.B. Abnormal brain development in newborns with congenital heart disease. N. Engl. J. Med. 2007;357(19):1928–1938. doi: 10.1056/NEJMoa067393. 17989385 [DOI] [PubMed] [Google Scholar]
- Mortensen E.L., Andresen J., Kruuse E., Sanders S.A., Reinisch J.M. IQ stability: the relation between child and young adult intelligence test scores in low-birthweight samples. Scand. J. Psychoil. 2003;44(4):395–398. doi: 10.1111/1467-9450.00359. 12887561 [DOI] [PubMed] [Google Scholar]
- Mussatto K.A., Hoffmann R.G., Hoffman G.M., Tweddell J.S., Bear L., Cao Y., Brosig C. Risk and prevalence of developmental delay in young children with congenital heart disease. Pediatrics. 2014;133(3):e570–e577. doi: 10.1542/peds.2013-2309. 24488746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen M., Sørensen H.T., Hjortdal V.E., Christensen T.D., Pedersen L. Congenital heart defects and developmental and other psychiatric disorders: a Danish nationwide cohort study. Circulation. 2011;124(16):1706–1712. doi: 10.1161/CIRCULATIONAHA.110.002832. 21947292 [DOI] [PubMed] [Google Scholar]
- Pandit A.S., Expert P., Lambiotte R., Bonnelle V., Leech R., Turkheimer F.E., Sharp D.J. Traumatic brain injury impairs small-world topology. Neurology. 2013;80(20):1826–1833. doi: 10.1212/WNL.0b013e3182929f38. 23596068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.-J., Friston K. Structural and functional brain networks: from connections to cognition. Science. 2013;342(6158) doi: 10.1126/science.1238411. 24179229 [DOI] [PubMed] [Google Scholar]
- Polanía R., Nitsche M.A., Paulus W. Modulating functional connectivity patterns and topological functional organization of the human brain with transcranial direct current stimulation. Hum. Brain Mapp. 2011;32(8):1236–1249. doi: 10.1002/hbm.21104. 20607750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M., Wig G.S., Barnes K.A., Church J.A., Vogel A.C., Laumann T.O., Miezin F.M., Schlaggar B.L., Petersen S.E. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. 22099467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden S., Richardson F.M., Josse G., Thomas M.S., Ellis C., Shakeshaft C., Seghier M.L., Price C.J. Verbal and non-verbal intelligence changes in the teenage brain. Nature. 2011;479(7371):113–116. doi: 10.1038/nature10514. 22012265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivkin M.J., Watson C.G., Scoppettuolo L.A., Wypij D., Vajapeyam S., Bellinger D.C., Demaso D.R., Robertson R.L., Jr, Newburger J.W. Adolescents with d-transposition of the great arteries repaired in early infancy demonstrate reduced white matter microstructure associated with clinical risk factors. J. Thorac. Cardiovasc. Surg. 2013;146(3):543–549. doi: 10.1016/j.jtcvs.2012.12.006. 23375991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins C.K., Watson C.G., Asaro L.A., Wypij D., Vajapeyam S., Bellinger D.C., Demaso D.R., Robertson R.L., Newburger J.W., Rivkin M.J. White matter microstructure and cognition in adolescents with congenital heart disease. J. Pediatr. 2014;165:936–944. doi: 10.1016/j.jpeds.2014.07.028. 25217200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. 19819337 [DOI] [PubMed] [Google Scholar]
- Sanz-Arigita E.J., Schoonheim M.M., Damoiseaux J.S., Rombouts S.A., Maris E., Barkhof F., Scheltens P., Stam C.J. Loss of ‘small-world’networks in Alzheimer's disease: graph analysis of FMRI resting-state functional connectivity. PLOS One. 2010;5(11):e13788. doi: 10.1371/journal.pone.0013788. 21072180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer C., Von Rhein M., Knirsch W., Huber R., Natalucci G., Caflisch J., Landolt M.A., Latal B. Neurodevelopmental outcome, psychological adjustment, and quality of life in adolescents with congenital heart disease. Dev. Med. Child Neurol. 2013;55(12):1143–1149. doi: 10.1111/dmcn.12242. 23937239 [DOI] [PubMed] [Google Scholar]
- Schmithorst V.J., Wilke M., Dardzinski B.J., Holland S.K. Correlation of white matter diffusivity and anisotropy with Age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222(1):212–218. doi: 10.1148/radiol.2221010626. 11756728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y.I., Morris J.C., Snyder A.Z., Price J.L., Yan Z., D'Angelo G., Liu C., Dixit S., Benzinger T., Fagan A., Goate A., Mintun M.A. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Aβ42. J. Neurosci. 2010;30(50):17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. 21159973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu N., Liu Y., Li K., Duan Y., Wang J., Yu C., Dong H., Ye J., He Y. Diffusion tensor tractography reveals disrupted topological efficiency in white matter structural networks in multiple sclerosis. Cereb. Cortex. 2011;21(11):2565–2577. doi: 10.1093/cercor/bhr039. 21467209 [DOI] [PubMed] [Google Scholar]
- Sidaros A., Engberg A.W., Sidaros K., Liptrot M.G., Herning M., Petersen P., Paulson O.B., Jernigan T.L., Rostrup E. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain. 2008;131(2):559–572. doi: 10.1093/brain/awm294. 18083753 [DOI] [PubMed] [Google Scholar]
- Simmonds D.J., Hallquist M.N., Asato M., Luna B. Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. Neuroimage. 2014;92:356–368. doi: 10.1016/j.neuroimage.2013.12.044. 24384150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. Network attributes for segregation and integration in the human brain. Curr. Opin. Neurobiol. 2013;23(2):162–171. doi: 10.1016/j.conb.2012.11.015. 23294553 [DOI] [PubMed] [Google Scholar]
- Stam C.J. Modern network science of neurological disorders. Nat. Rev. Neurosci. 2014;15(10):683–695. doi: 10.1038/nrn3801. 25186238 [DOI] [PubMed] [Google Scholar]
- Tabbutt S., Gaynor J.W., Newburger J.W. Neurodevelopmental outcomes after congenital heart surgery and strategies for improvement. Curr. Opin. Cardiol. 2012;27(2):82–91. doi: 10.1097/HCO.0b013e328350197b. 22274574 [DOI] [PubMed] [Google Scholar]
- Telesford Q.K., Joyce K.E., Hayasaka S., Burdette J.H., Laurienti P.J. The ubiquity of small-world networks. Brain Connectivity. 2011;1(5):367–375. doi: 10.1089/brain.2011.0038. 22432451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooley J.R., Satas S., Porter H., Silver I.A., Thoresen M. Head cooling with mild systemic hypothermia in anesthetized piglets is neuroprotective. Ann. Neurol. 2003;53(1):65–72. doi: 10.1002/ana.10402. 12509849 [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. 11771995 [DOI] [PubMed] [Google Scholar]
- Uehara, T., Tobimatsu, S., Kan, S., Miyauchi, S. (Eds.), Modular organization of intrinsic brain networks: A graph theoretical analysis of resting-state fMRI. complex medical engineering (CME). ICME International Conference (2012) IEEE
- Vaessen M.J., Hofman P.A., Tijssen H.N., Aldenkamp A.P., Jansen J.F., Backes W.H. The effect and reproducibility of different clinical DTI gradient sets on small world brain connectivity measures. Neuroimage. 2010;51(3):1106–1116. doi: 10.1016/j.neuroimage.2010.03.011. 20226864 [DOI] [PubMed] [Google Scholar]
- Van Der Linde D., Konings E.E., Slager M.A., Witsenburg M., Helbing W.A., Takkenberg J.J., Roos-Hesselink J.W. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2011;58(21):2241–2247. doi: 10.1016/j.jacc.2011.08.025. 22078432 [DOI] [PubMed] [Google Scholar]
- Van Der Worp H.B., Sena E.S., Donnan G.A., Howells D.W., Macleod M.R. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain. 2007;130(12):3063–3074. doi: 10.1093/brain/awm083. 17478443 [DOI] [PubMed] [Google Scholar]
- Vlooswijk M.C., Vaessen M.J., Jansen J.F., De Krom M.C., Majoie H.J., Hofman P.A., Aldenkamp A.P., Backes W.H. Loss of network efficiency associated with cognitive decline in chronic epilepsy. Neuroil. 2011;77(10):938–944. doi: 10.1212/WNL.0b013e31822cfc2f. 21832213 [DOI] [PubMed] [Google Scholar]
- Volpe J.J. Encephalopathy of congenital heart disease — destructive and developmental effects intertwined. J. Pediatr. 2014;164(5):962–965. doi: 10.1016/j.jpeds.2014.01.002. 24529617 [DOI] [PubMed] [Google Scholar]
- Von Rhein M., Buchmann A., Hagmann C., Huber R., Klaver P., Knirsch W., Latal B. Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain. 2014;137(1):268–276. doi: 10.1093/brain/awt322. 24277720 [DOI] [PubMed] [Google Scholar]
- Wang Z., Dai Z., Gong G., Zhou C., He Y. Understanding structural-functional relationships in the human brain: a large-scale network perspective. Neuroscientist. 2014 doi: 10.1177/1073858414537560. 24962094 [DOI] [PubMed] [Google Scholar]
- Wechsler D. WISC-III: Wechsler Intelligence Scale for Children. Psychological Corporation; San Antonio, TX.: 1991. [Google Scholar]
- Wen W., He Y., Sachdev P. Structural brain networks and neuropsychiatric disorders. Curr. Opin. Psychiatry. 2011;24(3):219–225. doi: 10.1097/YCO.0b013e32834591f8. 21430538 [DOI] [PubMed] [Google Scholar]
- Wen W., Zhu W., He Y., Kochan N.A., Reppermund S., Slavin M.J., Brodaty H., Crawford J., Xia A., Sachdev P. Discrete neuroanatomical networks are associated with specific cognitive abilities in old age. J. Neurosci. 2011;31(4):1204–1212. doi: 10.1523/JNEUROSCI.4085-10.2011. 21273405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Plis S.M., Erhardt E.B., Allen E.A., Sui J., Kiehl K.A., Pearlson G., Calhoun V.D. Modular organization of functional network connectivity in healthy controls and patients with schizophrenia during the resting state. Front. Syst. Neurosci. 2011;5:103. doi: 10.3389/fnsys.2011.00103. 22275887 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material: Additional Statistical Methods and Anatomic Labels for Figure 2 (Supplemental Figure S1).
Mediation: Cognitive difference between d-TGA and referent.
Mediation: total cooling time at ASO in d-TGA group.
Mediation: length of hospital stay (days) in d-TGA group.