Abstract
Context:
Anaplastic thyroid carcinoma (ATC) is one of the most deadly human malignancies. It is 99% lethal, and patients have a median survival of only 6 months after diagnosis. Despite these grim statistics, the mechanism underlying the tumorigenic capability of ATC cells is unclear.
Objective:
S100A8 and S100A9 proteins have emerged as critical mediators in cancer. The aim was to investigate the expression and function of S100A8 and S100A9 in ATC and the mechanisms involved.
Design:
We determined the expression of S100A8 and S100A9 in human ATC by gene array analysis and immunohistochemistry. Using RNAi-mediated stable gene knockdown in human ATC cell lines and bioluminescent imaging of orthotopic and lung metastasis mouse models of human ATC, we investigated the effects of S100A8 and S100A9 on tumorigenesis and metastasis.
Results:
We demonstrated that S100A8 and S100A9 were overexpressed in ATC but not in other types of thyroid carcinomas. In vivo analysis in mice using ATC cells that had S100A8 knocked down revealed reduced tumor growth and lung metastasis, as well as significantly prolonged animal survival. Mechanistic investigations showed that S100A8 promotes ATC cell proliferation through an interaction with RAGE, which activates the p38, ERK1/2 and JNK signaling pathways in the tumor cells.
Conclusions:
These findings establish a novel role for S100A8 in the promoting and enhancing of ATC progression. They further suggest that the inhibition of S100A8 could represent a relevant therapeutic target, with the potential of enabling a more effective treatment path for this deadly disease.
Anaplastic thyroid carcinoma (ATC) is one of the most aggressive human cancers. It arises from the follicular cells of the thyroid gland and is characterized by loss of differentiation. Although it accounts for less than 2% of all thyroid cancer cases, mortality due to ATC exceeds that of all other endocrine cancers combined. The major challenges of ATC are due to a lack of diagnostic markers for early detection and a poor response to all currently available therapies. As a result, more than 90% of patients die within six months of diagnosis. This poor prognosis has improved only modestly in recent years with the advent of aggressive multimodal therapies (1).
S100 proteins, a superfamily of EF-hand calcium-binding proteins with more than 20 members, have emerged as critical proinflammatory mediators in chronic and acute inflammatory diseases. Among them, S100A8 and S100A9 were originally identified as immunogenic proteins that formed a stable heterodimeric complex, also known as calprotectin. They are constitutively expressed and secreted by neutrophils and myeloid cells, and they, like their other family members, function in inflammation (2). Many tumors including ATC also exhibit significant up-regulation of the S100A8 and S100A9 genes (3–7). However, the roles of S100A8 and S100A9 in ATC are unknown.
Here, we investigate the possible tumorigenic functions and signaling pathways of S100A8 and S100A9 in ATC in order to identify potential targets for therapeutic intervention. Our findings provide convincing evidence that ATC cells isolated from patients, as well as ATC cell lines examined both in vitro and in vivo express elevated levels of S100A8, and that S100A8 is an important regulator of tumor development. These results suggest that inhibition of S100A8 activity may be therapeutically beneficial for the treatment of ATC.
Materials and Methods
S100A8 and S100A9 mRNA expression in clinical tissues
DNA microarray experiments were performed using the Affymetrix HG-U133 Plus 2.0 at the Mayo Clinic Genomic Core Facility in accordance with standard protocols. Use of human tissues was approved by the Mayo Institutional Review Board Committee. Microarray Suite version 5.0 (MAS 5.0 - Affymetrix) was used to process and analyze the data in terms of the background correction, probe summarization, and data normalization. Default settings and global scaling were used as a normalization method. GeneSpring GX 7.3. (Agilent Technology) was used to detect differentially expressed genes, defined as those with a greater than twofold difference in expression (P < .05).
Cell culture and transfection
Human ATC cell lines (THJ-11T and THJ-16T) were cultured in an RPMI-1640 medium as previously described (8). In some experiments, cells were transfected with a vector expressing the firefly luciferase gene (pSIN-luc; a gift of Dr. Yasuhiro Ikeda of Mayo Clinic) to generate stable clones. A detailed description of the construction and transfection protocol has been published (9).
shRNA knockdown.
To establish cell lines in which S100A8 or S100A9 expression is stably knocked down, cells were infected with lentiviral particles containing either five human S100A8-specific or S100A9-specific shRNA gene silencer sequences according to the manufacturer's instructions (Santa Cruz Biotechnology). Following transduction, cells were selected with puromycin (10 μg/mL) in media. Control cell lines were infected with scrambled shRNA lentiviral particles that did not target any known mammalian mRNA (Santa Cruz Biotechnology).
RNA isolation, reverse transcription, and qRT-PCR analysis.
Total RNA was isolated and reverse transcribed into cDNA using the Thermoscript First Strand Synthesis System (Invitrogen). The mRNA levels were quantified in triplicate by qRT-PCR on a ViiA7 PCR System (Applied Biosystems). Oligonucleotide primers for S100A8 and S100A9 were purchased from Santa Cruz Biotechnology. Human GAPDH was used as the housekeeping gene during the amplifications.
Cell viability assay
Cells were seeded in triplicate into 96-well plates at a concentration of 5 × 103 cells/well in RPMI/10% FBS and incubated at 37°C. For cell proliferation assays, Alamar Blue dye (Invitrogen) was added directly to the culture media to a final concentration of 10%. As a negative control, Alamar blue was added to cell-free medium. After 1 h of incubation, the plates were read in a fluorescent plate reader.
Apoptosis assay
Cells were seeded in triplicate into 96-well plates at a concentration of 5 × 103 cells/well in RPMI/10% FBS and incubated at 37°C. Apoptosis was determined using a luminescence-based assay method that quantitates caspase-3 and caspase-7 activities. Caspase 3/7 activities were measured using the Caspase-Glo 3/7 assay kit (Promega) and the plates were read in a luminometer according to the manufacturer's protocol.
Flow cytometry analysis
Cells were dissociated with trypsin/EDTA and then labeled with antibodies to S100A8 or RAGE (Abcam). Side and forward scatter profiles were used to eliminate cell doublets. Flow cytometric analysis was performed on a fluorescence-activated cell sorting (FACS) Calibur flow cytometer (BD Biosciences). FACS data were generated using FlowJo software (FlowJo LLC).
Phosphoprotein array profiling
Cells were treated with 20 μg/mL of S100A8 for 3 h and then lysed in NP-40 lysis buffer with protease inhibitors. Lysates were probed with human phosphokinase array blots (R & D Systems, Catalog No. ARY003B).
Western blotting
Protein sample was resolved by the SDS/PAGE followed by immunoblotting with anti-S100A8 (1:300, Sigma), anti-S100A9 (1:1000, Abcam), anti-p-ERK1/2 (T202/Y204) (1:2000, Cell Signaling), anti-ERK1/2 (1:4000, Cell Signaling), anti-p-SAPK/JNK (T183/Y185) (1:500, Cell Signaling), anti-SAPK/JNK (1:500, Cell Signaling), anti-p38 (1:4000; Santa Cruz), anti-p-p38 (T180/Y182) (1:1000; Santa Cruz), anti-p-AKT (Ser473) (1:500, Santa Cruz), anti-AKT (1:500, Santa Cruz), β-actin (1:2000; Sigma), and GAPDH (1:4000, Cell Signaling) antibodies. Anti-IgG conjugated with horseradish peroxidase was used as a secondary antibody. The membranes were developed using a chemiluminescence system (ECL detection reagent; Amersham Pharmacia).
Immunohistochemistry and indirect immunofluorescence analysis
Immunohistochemistry was performed on paraformaldehyde-fixed, paraffin-embedded tissue specimens. The slides were subsequently incubated with antihuman S100A8 (1:50; Sigma) and RAGE (1:100; Abcam) antibodies and visualized with Elite vector Stain ABC systems (Vector Laboratories) and DAB substrate (DakoCytomation) and counterstained with hematoxylin. For indirect immunofluorescence, the following primary antibodies were used: rabbit antihuman S100A8 (1:100) and rabbit antihuman RAGE (1:100). Images were captured using a Leica DMI3000B inverted fluorescent microscope with Leica AF6000E software (North Central Instruments).
Mouse tumor models
Eight-week-old female NOD/SCIDIl2rg−/− mice were obtained from the Jackson laboratory and maintained under pathogen-free conditions with the approval of the Institutional Animal Care and Use Committee of Saint Louis University. For orthotopic transplantation, 5 × 105 cells were suspended in 10 μL of Matrigel/RPMI at a 1:1 dilution and injected into the right thyroid gland. Mice were sacrificed three to five weeks after injection. Tumors and adjacent tissues were collected and analyzed by immunohistochemistry. In separate experiments, lung metastases were experimentally induced by injection of 3 × 105 cells into the lateral tail vein. After four weeks, mice were sacrificed and lung metastases were examined by histology and immunohistochemistry.
Bioluminescent imaging
Anesthetized mice were injected with D-luciferin (150 μg/kg) and imaged with an IVIS Spectrum. The light emitted from the bioluminescent tumors was detected, digitized, and displayed, and regions of interest from displayed images were quantified as total photon counts (photons/s) using Living Image software (Caliper Life Sciences).
Statistical analysis
For the survival studies, statistical analyses were performed with Kaplan-Meier survival curves and p values calculated using GraphPad Prism 5.0 software. Numerical data were expressed as mean ± S.E.M. Statistical differences are considered significant at P < .05.
Results
S100A8 and S100A9 mRNA and protein levels are highly elevated in ATC tissues
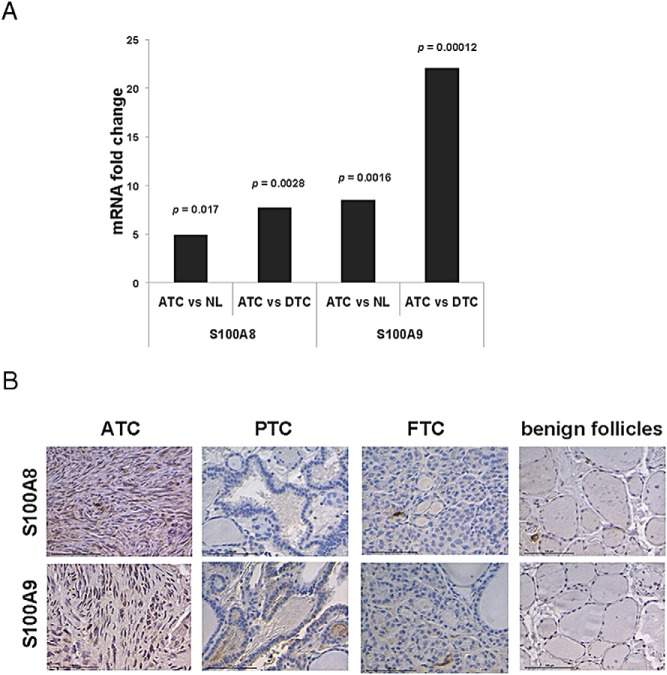
We performed gene array analysis to investigate whether the mRNA levels of S100A8 and S100A9 are elevated in the tissues of ATC patients. S100A8 and S100A9 mRNA levels were fivefold (P < .05) and 8.5-fold (P < .005) higher, respectively, in ATC tissues than in normal thyroid tissue. This increase in mRNA levels was even more pronounced [7.7-fold for S100A8 mRNA and 22-fold for S100A9 (P < .005 and P < .0005, respectively)] when ATC tissues were compared to those of differentiated thyroid carcinomas (DTC) (Figure 1A). This increase in the mRNA levels for S100A8 and S100A9 in ATC tissues as compared to other thyroid carcinomas was mirrored in the levels of the proteins themselves. We found that S100A8 and S100A9 levels were highly elevated in ATC, but not in papillary or follicular thyroid carcinomas or benign follicles (Figure 1B). These results agree with previous findings (7) and indicate that S100A8 and S100A9 levels are preferentially elevated in ATC tissues.
Figure 1.
S100A8 and S100A9 are overexpressed in ATC. A, Gene array results of normal thyroid (NL), DTC, and ATC patient tissues. Mean values were calculated for each variable group and data was plotted as mRNA fold change between groups as shown with P values. ATC, n = 16; NL, n = 17; and DTC, n = 11. B, Immunohistochemical detection of S100A8 and S100A9 in ATC. Note that PTC, FTC and benign follicles were immunonegative for S100A8 and S100A9. Scale bar, 100 μM.
S100A8 stimulates the proliferation of ATC cells in vitro
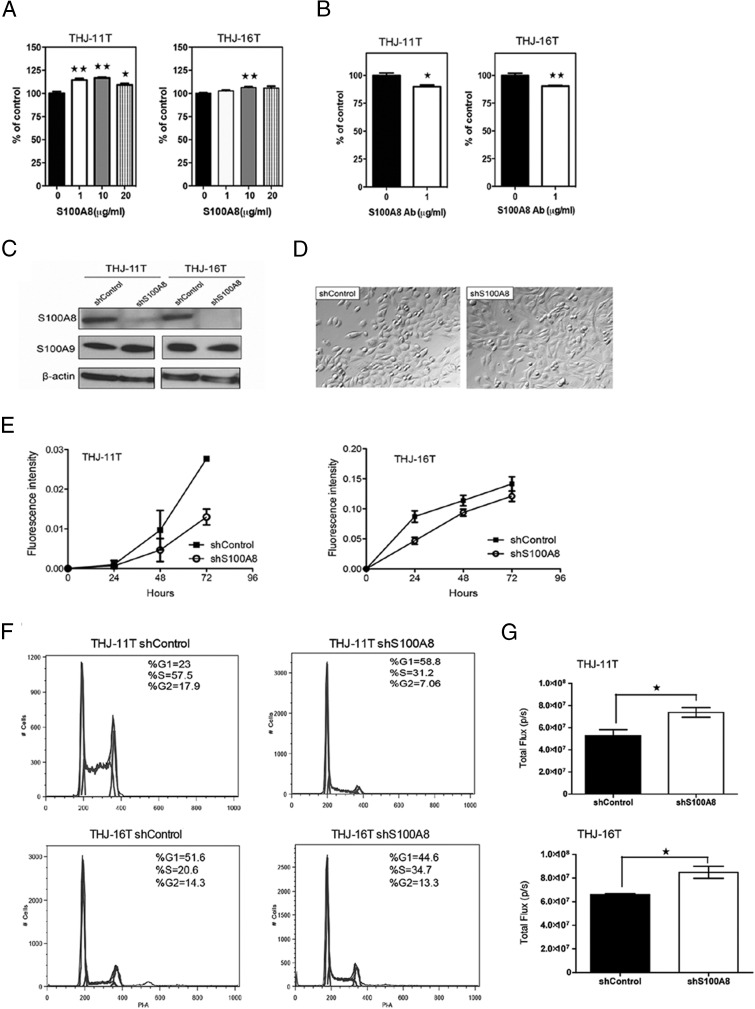
To investigate the effect on cell proliferation of S100A8, two human ATC cell lines, THJ-11T and THJ-16T (8), were starved overnight and then treated with increasing concentrations of S100A8 for 24 h. As little as 1 μg/mL of S100A8 stimulated the proliferation of THJ-11T. We also observed a modest, but significant, increase in the proliferation of THJ-16T cells in the presence of 10 μg/mL of S100A8 (Figure 2A). We next treated both THJ-11T and THJ-16T with anti-S100A8 antibody for 48 h, which decreased the rate of proliferation of both cell lines (Figure 2B). Our findings indicate that S100A8 mediates the proliferation of ATC cells.
Figure 2.
S100A8 stimulates the proliferation of ATC cells in vitro. A, THJ-11T and THJ-16T cells were starved overnight followed by treatment with increasing concentrations of S100A8 protein for 24 h. Cell proliferation was determined by Alamar blue assay and data was plotted as percent of control. Mean values were calculated from two independent experiments. *, P < .05 and **, P < .01. B, Cells were treated with anti-S100A8 antibody (1 μg/mL) for 48 h. Cell proliferation was determined by Alamar blue assay. Mean values were calculated from two independent experiments. C, S100A8 protein depletion by S100A8 shRNA was confirmed by Western blot analysis. S100A8 protein was nearly undetectable in both cell lines expressing S100A8 shRNA. The control shRNA had no effect on S100A8 expression. S100A8 shRNA also did not affect the expression of S100A9 as confirmed by Western blot analysis. β-actin was used as loading control. D, Representative bright field images showing that depletion of S100A8 by shRNA did not affect cell morphology. E, Growth curve of THJ-11T or THJ-16T cells expressing control shRNA or S100A8 shRNA as determined by Alamar blue assay. Each point represents the mean ± S.E.M. Similar results were obtained in three independent experiments. F, The cell-cycle distribution of THJ-11T and THJ-16T cells expressing control shRNA or S100A8 shRNA and the percentage of cells in G1, S, and G2 phases is indicated. G, A luminescence-based apoptosis assay showing that depletion of S100A8 by shRNA activated caspases-3 and caspase-7 activities in THJ-11T and THJ-16T cells.
Inhibition of cell proliferation by shRNA-mediated S100A8 knockdown
To explore whether endogenous S100A8 is responsible for the rapid proliferation of ATC cells that occurs in patients, we used commercial lentivirus shRNA to stably knockdown the S100A8 gene. Control cells were infected with scrambled shRNA sequences that do not target any known mammalian mRNA. S100A8 was nearly undetectable in both cell lines when S100A8 was silenced by shRNA and it did not affect the expression of S100A9 as confirmed by Western blot analysis (Figure 2C). Although S100A8 knockdown did not affect cell morphology (Figure 2D), cells infected with S100A8 shRNA proliferated more slowly than the controls after 72 h in culture (Figure 2E), indicating that S100A8-shRNA inhibits tumor cell growth in vitro. A cell cycle analysis revealed that depletion of S100A8 increased by more than twofold the number of cells in the G1-phase of the cell cycle in THJ-11T cells. In contrast, depletion of S100A8 did not affect the cell cycle profile in THJ-16T cells (Figure 2F). Additional apoptosis assay that measures caspase-3 and caspase-7 activities showed that S100A8 depletion induced apoptosis in both THJ-11T and THJ-16T cells (Figure 2G). Together, these results indicate that depletion of S100A8 more-robustly inhibited tumor cell growth by THJ-11T than by THJ-16T cells.
S100A8 is required for tumorigenesis and lung metastasis
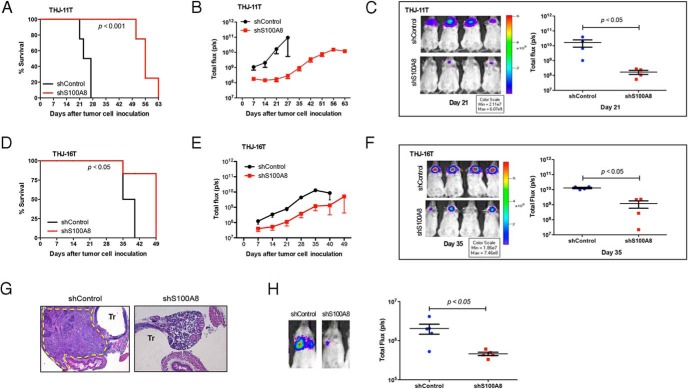
To explore whether ATC cells in which S100A8 translation was silenced could initiate tumors in vivo, we engineered THJ-11T and THJ-16T cells expressing pSIN-luciferase and either control shRNA or S1008 shRNA. Stable clones were orthotopically injected into the thyroids of NOD/SCID Il2rg−/− mice (10), and tumor initiation and growth were monitored via bioluminescent imaging. Tumor volume, assessed as total photon counts per second, revealed that all mice injected with control-shRNA-expressing THJ-11T cells developed tumors within seven days of injection (Figure 3). In contrast, mice injected with S100A8-shRNA-expressing THJ-11T cells (n = 4 per group) experienced a delayed onset of ATC and slower disease progression, resulting in overall prolonged survival (Figure 3A). In particular, it took about 21 days for mice injected with S100A8-shRNA-expressing THJ-11T cells to begin to develop tumors (P < .05) (Figure 3, B and C) and these mice survived a total of about 63 days (P < .001) (Figure 3A). Similar, but less marked, results were observed in mice injected with S100A8-shRNA-expressing THJ-16T cells (P < .05) (Figure 3, D-F). If one were to extrapolate this survival advantage into human years, these results are astounding considering that the median survival of ATC patients is only about 6 months from diagnosis (11, 12).
Figure 3.
shRNA knockdown of S100A8 slows tumor onset and progression and prolongs survival. A, Kaplan-Meier survival curve of mice injected with THJ-11T expressing control shRNA or S100A8 shRNA. P < .001. B, Bioluminescent tumor signals were quantified as total flux (p/s) using Living image software and plotted against the days since implantation. All mice (n = 4 per group) were reimaged using the same setting of the IVIS Imaging System. C, Bioluminescent images of mice injected with THJ-11T expressing control shRNA or S100A8 shRNA were taken at day 21 using the IVIS (left panel). Right panel, bioluminescent signals were quantified as total flux (p/s). P < .05. D, Survival curve of mice (n = 4 per group) injected with THJ-16T expressing control shRNA or S100A8 shRNA. P < .05. E, Bioluminescent tumor signals were quantified as total flux (p/s) from mice injected with THJ-16T expressing control shRNA or S100A8 shRNA and plotted against the days since implantation. F, Bioluminescent images of mice injected with THJ-16T expressing control shRNA or S100A8 shRNA were taken at day 35 using the IVIS (left panel). Right panel, bioluminescent signals were quantified as total flux (p/s). P < .05. G, Representative histology images of thyroid tumors of mice injected with THJ-11T expressing control shRNA at day 27 (the endpoint of control-shRNA-expressing THJ-11T group). Note that mice injected with THJ-11T expressing control shRNA displayed aggressive tumor tissues (broken yellow line) with local invasion to adjacent trachea, esophagus and smooth muscles. Tr, trachea. Mice injected with THJ-11T expressing S100A8 shRNA displayed normal thyroid follicles. H, Representative bioluminescent images of mice with lung metastasis (left panel). Right panel, bioluminescent signals were quantified as total flux (p/s) and plotted at 4 weeks after tail vein injection of THJ-11T expressing control shRNA or S100A8 shRNA. P < .05.
Further histopathological analysis at day 27 (the endpoint of control-shRNA-expressing THJ-11T group) determined that the tumors arising in the control mice displayed clinical features of ATC, and confirmed the absence of tumors in mice injected with S100A8-shRNA-expressing THJ-11T cells (Figure 3G). The results were similar when we performed a tail vein assay for lung metastasis. Mice injected in the tail vein with S100A8 silenced cells remained free of tumors, but control mice exhibited significant lung metastases four weeks after injection (P < .05) (Figure 3H). Collectively, these results suggest that S100A8 contributes to tumor growth and lung metastasis.
Knockdown of S100A9 expression in ATC cells does not affect tumorigenesis and metastasis
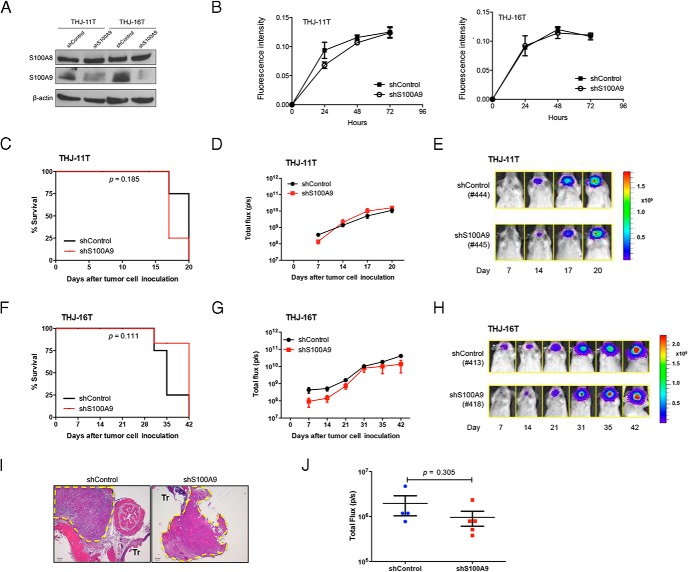
It has been suggested that S100A8 interacts with S100A9 to form a heterodimeric complex in many tumor cells. To explore whether S100A9 also affects proliferation and tumor growth in ATC, we used S100A9 shRNA to specifically block S100A9 expression in THJ-11T and THJ-16T cells. As shown in Figure 4A, S100A9 expression was inhibited in both cell lines by S100A9 shRNA. Unlike when S100A8 was silenced, however, the knockdown of S100A9 had no effect on the proliferation (Figure 4B). The effect of S100A9 was further evaluated in xenograft tumor models. We injected NOD/SCID Il2rg−/− mice orthotopically with THJ-11T or THJ-16T cells expressing either control or S1009 shRNA and monitored tumor formation weekly with IVIS imaging. The time to tumor onset and progression in all of the mice (n = 4 per group) was similar (P > .05) (Figure 4, C and F), and the animals displayed similar tumor volumes (Figure 4, D, E, G, and H). Histopathological analysis confirmed the presence of tumors in both control and shS100A9 mice (Figure 4I). Furthermore, mice injected in the tail vein with S100A9 silenced cells showed lung metastases similar to those seen in control mice four weeks after injection (P > .05) (Figure 4J). These results suggest that S100A9 does not affect tumor growth and lung metastasis.
Figure 4.
RNAi-mediated S100A9 knockdown does not prolong survival. A, Western blot analysis of S100A8 and S100A9 protein levels in THJ-11T or THJ-16T cells expressing control shRNA or S100A9 shRNA. β-actin was used as loading control. B, Growth curve of THJ-11T and THJ-16T cells expressing control shRNA or S100A9 shRNA as determined by Alamar blue assay. Each point represents the mean ± S.E.M. Similar results were obtained in two independent experiments. C, Survival curve of mice injected with THJ-11T expressing control shRNA or S100A9 shRNA. n = 4 mice per group. D, Bioluminescent signals were quantified as total flux (p/s) and plotted against the days since implantation. Mice injected with THJ-11T cells in which S100A9 expression was silenced displayed a similar time to tumor onset and progression as control mice. E, Representative bioluminescent images of mice injected with THJ-11T expressing control shRNA or S100A9 shRNA. All mice were reimaged using the same setting of the IVIS. F, Survival curve of mice injected with THJ-16T expressing control shRNA or S100A9 shRNA. n = 4 mice per group. G, Bioluminescent signals were quantified as total flux (p/s) and plotted against the days since implantation. Note that mice injected with THJ-16T cells in which S100A9 expression was silenced displayed a similar time to tumor onset and progression as control mice. H, Representative bioluminescent images of mice injected with THJ-16T expressing control shRNA or S100A9 shRNA. I, Representative histology images of thyroid tumors at day 20 of mice injected with THJ-11T expressing control shRNA or S100A9 shRNA. Broken yellow lines indicate tumors. Tr, trachea. Scale bar, 100 μm. J, Bioluminescent signals of lung metastasis were quantified as total flux (p/s) and plotted at 4 weeks after tail vein injection of THJ-11T expressing control shRNA or S100A9 shRNA.
S100A8 interaction with RAGE promotes the proliferation of ATC cells by activating p38, ERK1/2, and JNK signaling pathways
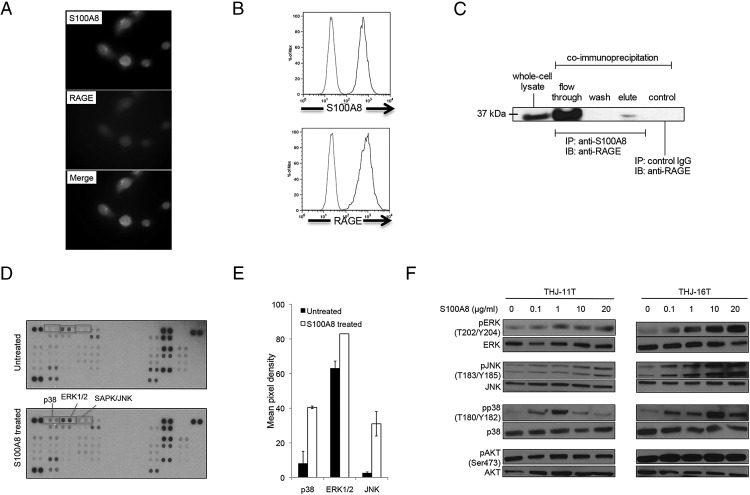
Several observations in colon, prostate, and breast tumors indicate that S100A8 interacts with RAGE to activate multiple signaling pathways (13–15). Therefore, we investigated whether S100A8 stimulates cellular proliferation through RAGE. As shown in Figure 5A, S100A8 and RAGE expression in ATC cells was detected by immunofluorescence and confirmed by FACS analysis (Figure 5B). To determine whether S100A8 binds RAGE on ATC cells, we performed immunoprecipitation assays. THJ-11T cell lysates were immunoprecipitated with anti-S100A8 or an irrelevant control rabbit IgG. Whole-cell lysates and immunoprecipitated proteins were then separated on SDS-PAGE and immunoblotted with anti-RAGE antibodies. RAGE was clearly detectable when the anti-S100A8 antibody was used for immunoprecipitation, but not when the control antibody was used (Figure 5C), indicating that S100A8 physically interacts with RAGE on ATC cells.
Figure 5.
Screening of signaling pathways activated by S100A8. A, Immunofluorescent staining of S100A8 and RAGE in THJ-11T cells. B, FACS analysis of S100A8 and RAGE in THJ-11T cells. Red lines represent isotype controls, blue lines show positive staining for S100A8 or RAGE. C, Immunoprecipitation experiments demonstrated that S100A8 was physically associated with RAGE. THJ-11T cell lysates were immunoprecipitated with anti-S100A8 or an irrelevant control rabbit IgG. Whole-cell lysates and immunoprecipitated proteins were separated on SDS-PAGE and immunoblotted with anti-RAGE antibodies. Results depicted are representative of three experiments. D, Cells were either left untreated or treated with 20 μg/mL of S100A8 for 3 h. Whole-cell lysates were probed with a human phosphokinase array. Proteins showing increased phosphorylation upon S100A8 treatment are highlighted. E, An analysis of the mean pixel density of highlighted spots (p38, ERK1/2, JNK) of the array. F, Cells were starved overnight followed by treatment with increasing S100A8 protein concentrations (0.1 to 20 μg/mL) for 1 h. Whole-cell lysates were examined by Western blot analysis using phosphospecific antibodies, pERK (T202/Y204), ERK, pJNK (T183/Y185), JNK, pp38 (T180/Y182), p38, pAKT (S473), and AKT. Data are representative of two independent experiments.
To determine whether the effect of S100A8 on cell proliferation is mediated through common signaling pathways, we performed a phosphokinase array assay. Treatment of THJ-11T cells with exogenous S100A8 for three h activated p38 MAPK, ERK1/2 (p44/42 MAPK), and SAPK/JNK signaling pathways (Figure 5, D and E). Our results indicate that S100A8 induces ERK1/2 and JNK activation in both cell lines in a concentration- and time-dependent manner, with a significant effect observed at 1 μg/mL and maximal effect achieved at 20 μg/mL after an incubation of 60 min. S100A8 also induced phosphorylation of p38, with maximal phosphorylation observed at 1 μg/mL in THJ-11T and 10 μg/mL in THJ-16T cells (Figure 5F). In contrast, no significant differences in the phosphorylation of AKT were observed in either cell line upon S100A8 stimulation (Figure 5F). This suggests that S100A8 preferentially activates three MAPK signaling pathways, p38, ERK1/2, and JNK.
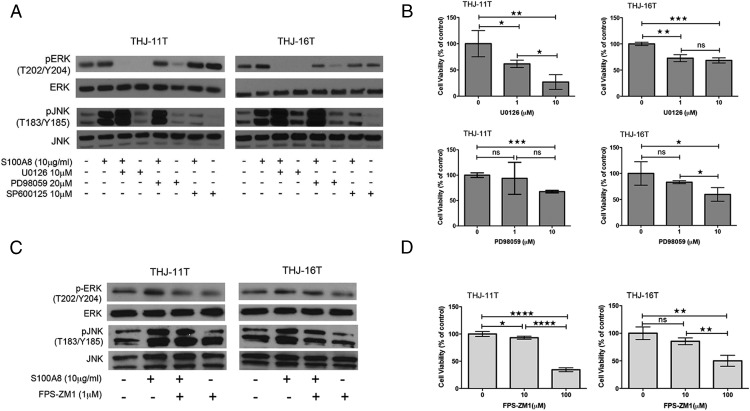
To verify the involvement of specific signaling pathways, cells were pretreated with inhibitors of ERK1/2 (U0126 and PD98059) or an inhibitor of JNK (SP600125) for 60 min before S100A8 treatment. S100A8 was then added, and the cells were incubated for an additional 60 min before phosphorylation levels were measured. S100A8-induced phosphorylation of ERK1/2 and JNK was reversed by pretreatment with their respective inhibitors (Figure 6A), suggesting that S100A8 works through the ERK1/2 and JNK pathways. In addition, we found differential effects of U0126 and PD98059 on ERK1/2 activation. U0126 completely abolished ERK1/2 phosphorylation. In contrast, PD98059 only slightly decreased ERK1/2 phosphorylation in both cell lines. This suggests that U0126 inhibited ERK1/2 more potently than PD98059. We also found that 1 μM of U0126 reduced the proliferation of THJ-11T (P < .05) and THJ-16T (P < .01) cells after 48 h as compared to control cells, while 10 μM PD98059 had a more significant inhibitory effect on cell growth (THJ-11T, P < .001; THJ-16T, P < .05, respectively) (Figure 6B).
Figure 6.
S100A8 activates ERK1/2 and JNK signaling pathways through RAGE. A, Cells were pretreated with ERK1/2 inhibitors U0126 and PD98059 and the JNK inhibitor SP600125 for 60 min before the application of S100A8 (10 μg/mL). The phosphorylation was measured in a period of 60 min by immunoblotting with antibodies to pERK, ERK, pJNK, and JNK. B, Alamar blue assay at 48 h showing cells treated with U0126 or PD98059 displayed decreased cell proliferation compared with their control cells. ns, P > .05; * P < .05; ** P < .01; ***, P < .001. C, Cells were pretreated with an inhibitor of RAGE, FPS-ZM1 (1 μM), for 2 h before stimulation with S100A8 (10 μg/mL) for 1 h. Phosphorylation of ERK and JNK was measured in cell lysates by immunoblotting. There was a decrease in pERK/ERK and pJNK/JNK in FPS-ZM1-treated THJ-11T and THJ-16T cells as compared to control cells. D, Alamar blue assay at 72 h showing cells treated with FPS-ZM1 displayed decreased cell proliferation as compared to control cells. ns, P > .05; * P < .05; ** P < .01; ****, P < .0001.
To verify the involvement of RAGE in S100A8-induced signaling pathways, cells were pretreated for 2 h with the RAGE inhibitor FPS-ZM1 (1 μM), followed by stimulation with 10 μg/mL S100A8 for 1 h. ERK and JNK phosphorylation was analyzed in cell lysates by immunoblotting. The results show that RAGE inhibition reduced the S100A8-induced phosphorylation of ERK1/2 and JNK (Figure 6C), suggesting that S100A8 regulates MAPK signaling pathways through RAGE. We also found that, although 10 μM FPS-ZM1 reduced the proliferation of THJ-11T cells as compared to control (P < .05) after 72 h, 100 μM FPS-ZM1 had a more significant effect on both cell lines (THJ-11T, P < .0001; THJ-16T, P < .01, respectively) (Figure 6D). Together, these data indicate that S100A8 promotes cell growth through RAGE signaling and MAPK activation.
S100A8 activation of the MAPK signaling pathway in vivo
We next sought to confirm our in vitro findings about the effect of S100A8 treatment in animal models. Immunohistochemical analysis of THJ-11T and THJ-16T orthrotopic xenograft tumors indicated S100A8, S100A9, and RAGE were highly expressed in the resulting ATC tumors. Cellular inflammatory infiltrates in the tumors also expressed S100A8. In contrast, normal mouse thyroid follicles did not express any of these proteins (Supplemental Figure 1A). Consistent with the in vitro data showing that S100A8 and RAGE regulates MAPK signaling pathways, thyroid xenograft tumors exhibited activation of p38, ERK1/2, and JNK (Supplemental Figure 1B). These results suggest that S100A8 signaling is active and is consistent with tumorigenicity.
Discussion
This study has three major novel findings. First, S100A8 is activated in ATC. Second, S100A8 interacts with RAGE and promotes the proliferation of ATC cells by activating p38, ERK1/2, and JNK signaling pathways. Third, S100A8 inhibition can reduce the oncogenic and metastatic potential of ATC cells. We conclude that S100A8 is a relevant intervention target for ATC treatment.
We found that overexpression of the S100A8 and S100A9 mRNA and protein levels occurred only in ATC, and not in papillary or follicular thyroid carcinomas or benign follicles, consistent with previous studies (7). The correlation of elevated S100A8 and S100A9 levels with ATC is of particular clinical relevance since one of the major challenges in treating ATC patients is the lack of early diagnostic markers. This finding suggests that S100A8 and S100A9 could potentially serve as biomarkers for ATC.
This study used two authenticated ATC cell lines, THJ-11T and THJ-16T, established by our laboratory in 2010 (8). As detailed previously, these cell lines were derived from tumors removed from ATC patients. They have been extensively characterized, and the presence of unique genetic mutations and short tandem DNA repeat sequences confirms that each cell line matches its presumed tumor of origin (8). The advantages of these human cancer cell lines over primary tissue include the ability to culture them over long periods of time and grow them in large quantities for drug-screening applications. Despite these advantages, it is necessary to confirm our findings in primary tumors because cell lines do not always recapitulate all aspects of primary tumors. However, the rarity and rapidly fatal nature of this malignancy has made this difficult to achieve in laboratory settings.
S100A8 and S100A9 are highly expressed in breast cancer, hepatocellular carcinomas, and pulmonary adenocarcinoma, and their expression is correlated with poor differentiation (16, 17). In contrast, S100A8 and S100A9 are often downregulated in poorly differentiated esophageal squamous cell carcinoma (18). In this study, we observed a modest but significant increase in the proliferation of both THJ-11T and THJ-16T cells upon S100A8 stimulation. In contrast, anti-S100A8 antibody decreased the rate of proliferation of both cell lines. We further applied gene-silencing methods to individually downregulate S100A8 or S100A9 expression in ATC cells without affecting the expression of their presumed partner. As expected, cells with S100A8 knockdown proliferated more slowly than the controls. We also found that the difference in growth rate obtained with either S100A8 stimulation or inhibitory antibodies are not as significant as those with the S100A8 shRNA alone. This could be partially explained by the fact that tumor cells also produce S100A8, therefore, exogenous S100A8 stimulation or inhibitory antibodies cannot reach the maximum effects as the complete deletion of S100A8 by shRNA. In addition, we observed striking and significantly prolonged animal survival when S100A8 was silenced in either THJ-11T or THJ-16T cells prior to orthotopic injection or tail vein lung metastasis assays. In contrast, we did not see an effect on tumorigenesis and metastasis when S100A9 expression was inhibited in ATC cells. This may indicate S100A9 does not directly affect tumorigenesis. It is unclear at this time why S100A9 expression is elevated in ATC patients. Further studies are required to elucidate the possible contribution of S100A9 to the ATC disease process.
Depletion of S100A8 more-robustly inhibited xenograft tumorigenesis by THJ-11T than by THJ-16T cells. The differences we observed in the time to tumor onset and progression between THJ-11T and THJ-16T cells may be due to inherited genetic differences between these two cell lines. THJ-11T cell line has a KRAS mutation at codon 12 while THJ-16T cell line has phosphatidylinositol 3-kinase (PIK3CA) (1622T, S541F) mutations (8). The frequency of KRAS mutations in thyroid cancer is around 3%. Mutation of the PIK3CA gene in ATC is more than 20% and often associated with poor prognosis. How exactly the nature of these different driver mutations affects the response to S100A8 down-regulation remains unknown and warrants further investigation. Collectively, however, our in vivo tumorigenesis experiments demonstrate for the first time that S100A8 plays a role in tumor promotion and progression in ATC.
It should be pointed out that inflammatory cells that infiltrate ATC tumors also express high levels of S100A8, and numerous studies of human carcinoma cells have identified a clear correlation between the levels of S100A8/S100A9 in tumors and enhanced cancer cell survival. Notably, breast cancer cells express the chemokines CXCL1 and 2, which attract S100A8/S100A9-expressing myeloid cells (6). It is possible that ATC cells not only express S100A8 themselves, but that they also attract inflammatory cells that secrete a storm of S100A8 and chemokines in the tumor microenvironment. Many of these chemokines and cytokines in the tumor microenvironment have been linked to chemoresistance (6). The important role of S100A8 in ATC suggests that its potential relevance as a therapeutic target may extend beyond simply suppressing tumor growth. Inhibiting its expression or activity may also increase the chemosensitivity of the cancer cells.
RAGE has been shown to serve as the primary receptor for S100A8 (13, 15, 19, 20). We confirmed an interaction between RAGE and S100A8 in ATC cells by co-immunoprecipitation. We further demonstrated that S100A8 binding to ATC cells stimulates RAGE-dependent activation of the p38, ERK1/2, and JNK signaling pathways. This finding was confirmed with specific ERK and JNK inhibitors. However, we found differential effects of U0126 and PD98059 on S100A8-induced ERK phosphorylation. Western blot analysis indicates that U0126 inhibited ERK more potently than PD98059 in both THJ-11T and THJ-16T cell lines. U0126 and PD98059 have long been used as specific inhibitors of the MEK pathway. The differential effects of U0126 and PD98059 in ATC cells suggest their actions may not be exclusively due to an inhibition of the MEK pathway. Alternatively, U0126 and PD98059 may have different action mechanisms as MEK inhibitors. More studies will need to be done to understand the specific effects of these inhibitors on different signaling pathways in order to utilize them in cancer therapy. Furthermore, we showed that treatment of ATC cells with the RAGE inhibitor FPS-ZM1 reduced phosphorylation of ERK and JNK and blocked S100A8-induced cell proliferation. FPS-ZM1 is a small, nontoxic molecule that aggressively inhibits the RAGE-mediated influx of circulating amyloid-β peptide into the brain and suppresses the inflammatory response in a mouse model of Alzheimer disease (AD) (21). FPS-ZM1 may be an attractive candidate for preclinical testing for the treatment of ATC because of its ability to inhibit ATC cell proliferation and its known anti-inflammatory activity in vivo.
In conclusion, our results provide mechanistic insights into the link between S100A8 levels and ATC. Our findings identify S100A8 as a novel progression and survival factor in ATC. They raise the possibility of therapeutically targeting this molecule to limit the proliferation and metastasis of cancer cells, which we believe could lead to a pronounced survival benefit for ATC patients.
Acknowledgments
We thank Y. Ikeda for the pSIN-luc vector, B. Nagel and M. Roth for tissue sectioning, and K. Toth for IVIS imaging.
This work was supported in part by the Saint Louis University President's Research Fund (Grant No. 8271) and the Washington University Institute of Clinical and Translational Sciences Grant (No. UL1 TR000448) to R.Y.L., National Cancer Institute Grant No. R01CA136665 to J.A.C. and R.C.S., the Department of Health, State of Florida, Bankhead-Coley Cancer Program (Grants No. 07BB-12297 and 9BW02) to J.A.C. and R.C.S., and a generous gift from Alfred D. and Audrey M. Petersen to R.C.S.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ATC
- anaplastic thyroid carcinoma
- DTC
- differentiated thyroid carcinomas
- FACS
- fluorescence-activated cell sorting.
References
- 1. Smallridge RC, Ain KB, Asa SL, et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22:1104–1139. [DOI] [PubMed] [Google Scholar]
- 2. Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol. 2003;170:3233–3242. [DOI] [PubMed] [Google Scholar]
- 3. Wang L, Chang EW, Wong SC, Ong SM, Chong DQ, Ling KL. Increased Myeloid-Derived Suppressor Cells in Gastric Cancer Correlate with Cancer Stage and Plasma S100A8/A9 Proinflammatory Proteins. J Immunol. 2013;190:794–804. [DOI] [PubMed] [Google Scholar]
- 4. Hermani A, Hess J, De Servi B, et al. Calcium-binding proteins S100A8 and S100A9 as novel diagnostic markers in human prostate cancer. Clin Cancer Res. 2005;11:5146–5152. [DOI] [PubMed] [Google Scholar]
- 5. Kim HJ, Kang HJ, Lee H, et al. Identification of S100A8 and S100A9 as serological markers for colorectal cancer. J Proteome Res. 2009;8:1368–1379. [DOI] [PubMed] [Google Scholar]
- 6. Acharyya S, Oskarsson T, Vanharanta S, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ito Y, Arai K, Nozawa R, et al. S100A8 and S100A9 expression is a crucial factor for dedifferentiation in thyroid carcinoma. Anticancer Res. 2009;29:4157–4161. [PubMed] [Google Scholar]
- 8. Marlow LA, D'Innocenzi J, Zhang Y, et al. Detailed molecular fingerprinting of four new anaplastic thyroid carcinoma cell lines and their use for verification of RhoB as a molecular therapeutic target. J Clin Endocrinol Metab. 2010;95:5338–5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hasegawa K, Nakamura T, Harvey M, et al. The use of a tropism-modified measles virus in folate receptor-targeted virotherapy of ovarian cancer. Clin Cancer Res. 2006;12:6170–6178. [DOI] [PubMed] [Google Scholar]
- 10. Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McIver B, Hay ID, Giuffrida DF, et al. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery. 2001;130:1028–1034. [DOI] [PubMed] [Google Scholar]
- 12. Venkatesh YS, Ordonez NG, Schultz PN, Hickey RC, Goepfert H, Samaan NA. Anaplastic carcinoma of the thyroid. A clinicopathologic study of 121 cases. Cancer. 1990;66:321–330. [DOI] [PubMed] [Google Scholar]
- 13. Hermani A, De Servi B, Medunjanin S, Tessier PA, Mayer D. S100A8 and S100A9 activate MAP kinase and NF-kappaB signaling pathways and trigger translocation of RAGE in human prostate cancer cells. Exp Cell Res. 2006;312:184–197. [DOI] [PubMed] [Google Scholar]
- 14. Ichikawa M, Williams R, Wang L, Vogl T, Srikrishna G. S100A8/A9 activate key genes and pathways in colon tumor progression. Mol Cancer Res. 2011;9:133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yin C, Li H, Zhang B, et al. RAGE-binding S100A8/A9 promotes the migration and invasion of human breast cancer cells through actin polymerization and epithelial-mesenchymal transition. Breast Cancer Res Treat. 2013;142:297–309. [DOI] [PubMed] [Google Scholar]
- 16. Arai K, Yamada T, Nozawa R. Immunohistochemical investigation of migration inhibitory factor-related protein (MRP)-14 expression in hepatocellular carcinoma. Med Oncol. 2000;17:183–188. [DOI] [PubMed] [Google Scholar]
- 17. Arai K, Teratani T, Kuruto-Niwa R, Yamada T, Nozawa R. S100A9 expression in invasive ductal carcinoma of the breast: S100A9 expression in adenocarcinoma is closely associated with poor tumour differentiation. Eur J Cancer. 2004;40:1179–1187. [DOI] [PubMed] [Google Scholar]
- 18. Wang J, Cai Y, Xu H, et al. Expression of MRP14 gene is frequently down-regulated in Chinese human esophageal cancer. Cell Res. 2004;14:46–53. [DOI] [PubMed] [Google Scholar]
- 19. Ghavami S, Rashedi I, Dattilo BM, et al. S100A8/A9 at low concentration promotes tumor cell growth via RAGE ligation and MAP kinase-dependent pathway. J Leukoc Biol. 2008;83:1484–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Turovskaya O, Foell D, Sinha P, et al. RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis. 2008;29:2035–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deane R, Du Yan S, Submamaryan RK, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nature medicine. 2003;9:907–913. [DOI] [PubMed] [Google Scholar]