Abstract
Context:
Vasomotor symptoms (VMS) are common. Whether VMS are associated with fracture incidence or bone mineral density (BMD) levels is unknown.
Objective:
This study aimed to examine associations of baseline VMS with fracture incidence and BMD.
Design:
This was a prospective observational study with mean (SD) followup of 8.2 (1.7) years (1993–2005).
Setting:
Forty United States clinical centers.
Participants:
We examined data from Women's Health Initiative Clinical Trial participants (n = 23 573) age 50–79 years not using menopausal hormone therapy, and 4,867 participants of the BMD sub-study.
Interventions:
None.
Main Outcome Measures:
We measured baseline VMS, incident adjudicated fractures, and BMD (baseline, annual visits 1, 3, 6, and 9).
Results:
After adjustment for baseline age, body mass index, race/ethnicity, smoking, and education, the hazard ratio for hip fracture among women with baseline moderate/severe VMS (vs no VMS) was 1.78 (95% confidence interval [CI], 1.20–2.64; P = .01). There was no association between VMS and vertebral fracture. VMS severity was inversely associated with BMD during followup (P = .004 for femoral neck, P = .045 for lumbar spine). In repeated measures models, compared with women who reported no VMS, women with moderate/severe VMS had 0.015 g/cm2 lower femoral neck BMD (95% CI, −0.025–−0.005) and 0.016 g/cm2 lower lumbar spine BMD (95% CI, −0.032–−0.004).
Conclusions:
Women with moderate/severe VMS have lower BMD and increased hip fracture rates. Elucidation of the biological mechanisms underlying these associations may inform the design of preventive strategies for at-risk women prior to occurrence of fracture.
Vasomotor symptoms (VMS), ie, hot flashes and/or night sweats, are common in women during the menopause transition. VMS peak in late perimenopause, when women have had no menses for at least 3 months but not yet experienced 12 months of amenorrhea (1). During the late perimenopause, approximately 60% of women experience VMS (2). VMS may persist well past the final menstrual period. Four years after the final menstrual period, nearly 50% of women are still symptomatic, and 10% of women continue having VMS 12 years or more after the final menstrual period (2). The peak prevalence of VMS coincides with the time period of accelerated bone loss at the hip and spine (3). Given the high prevalence and potentially long exposure to VMS, it is clinically important to examine whether these symptoms are indicative of adverse bone health.
A few studies examined associations between VMS and bone mineral density (BMD); they were cross sectional (4–8), brief (9, 10), or focused on premenopausal infertile women (11). Cross-sectional studies (4, 5, 7, 12), and a 30-month study (10) of peri- and postmenopausal women suggested that spine BMD is lower among women with VMS than among women without VMS. A longitudinal study of younger women progressing through the menopausal transition using repeated measures analysis found that the presence of VMS was inversely associated with spine and femoral neck BMD (13). Likewise, little is known about associations between VMS and fracture risk. A longitudinal study of women who had osteoporosis at baseline found no association of “bothersome” VMS with vertebral or nonvertebral fracture risk (14). To our knowledge, no prospective studies have examined these associations in a large cohort of postmenopausal women not selected for having osteoporosis at baseline.
Our goal was to examine associations of baseline VMS with subsequent BMD and fracture incidence using data from the Women's Health Initiative, a large cohort of United States postmenopausal women (15). We hypothesized that VMS would be associated with lower BMD and higher fracture rates. We also hypothesized that selective estrogen receptor modulator use, physical functioning, incident cancer, and sleep disruption (potentially leading to falls) may be potential mechanisms involved in associations between VMS and fracture risk.
Materials and Methods
Participants
The Women's Health Initiative (WHI) was conducted at 40 Unites States clinical centers (16, 17). The clinical trials (WHI-CT) and the observational study (WHI-OS) enrolled postmenopausal women age 50–79 years at baseline who were free from serious medical conditions (15, 18). The three WHI-CTs evaluated menopausal hormone therapy (HT), calcium and vitamin D supplementation, and a low-fat eating pattern (15).
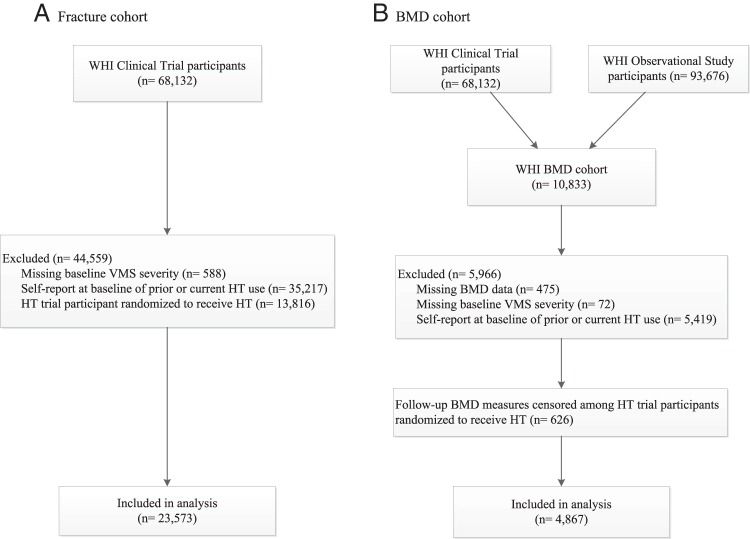
For the current study, analyses of the associations between VMS and fracture were performed using data from participants of the WHI-CT (Figure 1). Of the 68 132 women enrolled onto the WHI-CT, we excluded data from 588 participants for whom we lacked data regarding VMS severity. Because HT use can influence fracture risk, data from 35 217 participants who reported prior or current HT use, and 13 816 who were randomly assigned to receive HT (conjugated equine estrogens alone or conjugated equine estrogens + medroxyprogesterone acetate), were also excluded. Thus, our analytic sample size for incident fractures was 23 573 participants.
Figure 1.
Flow diagram of analytic cohorts.
Using data from the WHI BMD cohort, we analyzed associations between VMS and BMD. At the time of enrollment, participants at three of the 40 clinical centers (Tucson/Phoenix, Arizona; Pittsburgh, Pennsylvania; and Birmingham, Alabama; n = 10 833) underwent hip and anteroposterior lumbar dual-energy x-ray absorptiometry on a Hologic QDR2000 or 4500W machine (Hologic) using standard protocols for positioning and analysis (19–21). Quality assurance methods included cross-clinic calibration phantoms, further evaluation of scans with specific problems, and review of a random sample of scans (22).
Of 10 833 CT and OS participants from the WHI BMD cohort, 10 358 had at least one BMD measurement at baseline and/or followup. Among these, 5419 participants were excluded because of current or prior use of HT. (Women who reported never having used HT at baseline tended not to initiate HT during followup). A further 72 were missing baseline VMS severity data. Thus, the sample size for our BMD analyses was 4867 participants (Figure 1). Postrandomization BMD measures were censored for participants who were assigned to either HT preparation (n = 626).
Each institution obtained human subjects committee approval. All participants provided written informed consent.
Outcomes: Fracture incidence and BMD
Information regarding incident fractures was self reported semiannually. Self-reported fractures were then confirmed based on medical record adjudication. The questionnaire item asked participants whether they had experienced fracture events since the previous visit: “Has a doctor told you for the first time that you have a new broken, crushed, or fractured bone?” Which bone did you break?” Response choices included: hip, upper leg (not hip), pelvis, knee (patella), lower leg or ankle, foot (not toe), spine or back (vertebra), lower arm or wrist, hand (not finger), elbow, and upper arm or shoulder. For this analysis, we classified fractures into the following categories: hip, spine, and nonvertebral (including hip).
The BMD outcome was absolute BMD (g/cm2), measured at baseline, and annual followup visits 1, 3, 6, and 9 for the WHI-CT, and at baseline and annual followup visits 3, 6, and 9 for the WHI-OS. If information was missing regarding one or more BMD measurements for a participant, the participant still contributed to the model the BMD measurements that were available.
Predictor: Vasomotor symptom assessment
The primary exposure variable was VMS severity. Information regarding VMS at baseline was obtained on the baseline questionnaire. The questionnaire item stated, “Below is a list of symptoms people sometimes have. For each item, mark the one oval that best describes how bothersome the symptom was during the past 4 weeks for you.” Response choices were: symptom did not occur, mild (symptom did not interfere with usual activities), moderate (symptom interfered somewhat with usual activities), and severe (symptom was so bothersome that usual activities could not be performed). The symptom list included separate items for hot flashes and night sweats. As expected, the overlap between hot flashes and night sweats was high. Therefore, in this analysis, we made the a priori decision to define VMS as hot flashes and/or night sweats. Because of the low prevalence of severe VMS, we combined the moderate and severe categories of VMS into a single category for purposes of statistical analysis.
Other questionnaire-based information
We obtained information regarding age, race/ethnicity, education, family income, age at menopause, hysterectomy, oophorectomy, smoking, alcohol consumption, physical activity, previous fracture, sleep disturbance, incident falls, and medication use from baseline self-assessment questionnaires. Age at menopause was defined as previously described (23). Sleep disturbance was characterized using the validated WHI sleep disturbance construct (24). Participants were classified as never, past, or current users of HT based on questionnaire responses. Past HT use was defined as use of an estrogen or progestogen-containing pill or transdermal patch for 3 months or longer following menopause.
Weight and height were measured using standardized protocols. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared.
Statistical analysis
We examined baseline characteristics across categories of baseline VMS severity after adjustment for age, BMI, and smoking.
We analyzed associations between baseline VMS and incident fractures in the WHI-CT cohort. We analyzed associations between baseline VMS severity (none, mild, moderate/severe) and time to fracture using Cox proportional hazards regression, adjusting for covariates chosen a priori: baseline age, BMI, race/ethnicity, smoking, and education, and stratified by age and prior history of fracture. Hip, spine, and nonvertebral fractures each served as outcomes of separate models, where statistical significance was based on a 2-degree-of-freedom test for association.
In subgroup analyses, we tested for interactions between VMS and baseline characteristics chosen a priori: age, BMI, years since menopause, race/ethnicity, and physical activity. Statistical significance was based on a test of interaction. Twenty comparisons were made (5 outcomes × 4 subgroups); thus, one interaction was expected to be significant by chance alone. Additional post-hoc analyses investigated effect modification by baseline diabetes and history of cardiovascular disease.
Additional analyses were carried out to elucidate potential mechanisms of associations between VMS and fracture risk. Time-dependent covariates (ie, tamoxifen/selective estrogen receptor modulator use, sleep disturbance, physical functioning, incident cancer, number of incident falls) were added one at a time to the Cox regression model in which hip fracture was the outcome.
We analyzed associations between baseline VMS and BMD in the WHI BMD cohort. To do this, we examined associations between baseline VMS severity and longitudinal measures of BMD using repeated measures regression with an unstructured variance-covariance matrix, adjusting for baseline age, BMI, race/ethnicity, BMD scanner, smoking, and education. We compared mean BMD at baseline and during followup (averaged over followup visit) by baseline VMS severity categories using two 1-degree-of-freedom tests for trend. Femoral neck BMD and spine BMD each served as outcomes of separate regression models. Based on a priori hypotheses, we examined the associations of baseline VMS with BMD by subgroups of baseline age (50–59, 60–69, 70–79 y) and BMI (< 25, 25–<30, and ≥ 30 kg/m2) (2, 25). Statistical significance was based on a test for trend of the interaction of VMS severity with BMD at baseline and followup; one interaction was expected to be statistically significant by chance alone.
Because of the known influence of breast cancer therapy on both VMS and BMD, we performed a sensitivity analysis in which we repeated the regression models after excluding women with a history of breast cancer (n = 133).
All analyses were conducted by using SAS software version 9.3 (SAS Institute) and R software version 2.15 (R Foundation for Statistical Computing). All P values were two-sided and P ≤ .05 were regarded as statistically significant.
Results
Characteristics of the participants
Compared with excluded participants, participants in the analytic sample tended to be younger (mean age, 62.3 vs 63.5 y), thinner (mean BMI, 28.6 vs 29.6 kg/m2), and white (82 vs 78%) (data not shown).
At baseline, 15 418 participants (65%) reported having no VMS, 5766 (24%) reported mild VMS, and 2378 (10%) reported moderate/severe VMS (Table 1). Mean (SD) age of the participants of the fracture sample at baseline was 63.5 (7.0) years. Women in the fracture cohort who had moderate/severe VMS at baseline tended to be younger, were more likely to be non-White, and were more likely to have undergone bilateral oophorectomy compared with women with no VMS or mild VMS (all P < 0.05).
Table 1.
Baseline Characteristics of the Fracture Cohort (n = 23 573) by Severity of Baseline Vasomotor Symptoms
| Characteristic | Severity of Baseline Vasomotor Symptoms |
P Valuea | |||||
|---|---|---|---|---|---|---|---|
| None (n = 15 418) |
Mild (n = 5766) |
Moderate/Severe (n = 2389) |
|||||
| N | % | N | % | N | % | ||
| Age at screening, y | <.001 | ||||||
| 50–54 | 1093 | 7.1 | 1036 | 18.0 | 612 | 25.6 | |
| 55–59 | 2341 | 15.2 | 1497 | 26.0 | 662 | 27.7 | |
| 60–69 | 7911 | 51.3 | 2481 | 43.0 | 844 | 35.3 | |
| 70–79 | 4073 | 26.4 | 752 | 13.0 | 271 | 11.3 | |
| Race/ethnicity | <.001 | ||||||
| White | 12 746 | 82.7 | 4202 | 72.9 | 1363 | 57.1 | |
| Black | 1490 | 9.7 | 1102 | 19.1 | 735 | 30.8 | |
| Hispanic | 570 | 3.7 | 247 | 4.3 | 208 | 8.7 | |
| American Indian | 63 | 0.4 | 32 | 0.6 | 13 | 0.5 | |
| Asian/Pacific Islander | 355 | 2.3 | 106 | 1.8 | 28 | 1.2 | |
| Unknown | 194 | 1.3 | 77 | 1.3 | 42 | 1.8 | |
| Education | <.001 | ||||||
| ≤High school/GED | 3716 | 24.2 | 1503 | 26.3 | 815 | 34.4 | |
| School after high school | 5805 | 37.9 | 2219 | 38.8 | 946 | 40.0 | |
| ≥College degree | 5806 | 37.9 | 1998 | 34.9 | 606 | 25.6 | |
| Family income | <.001 | ||||||
| <20K | 2905 | 20.2 | 1025 | 19.0 | 654 | 29.5 | |
| 20–<35K | 4016 | 27.9 | 1396 | 25.9 | 513 | 23.1 | |
| 35–<50K | 3113 | 21.6 | 1147 | 21.3 | 404 | 18.2 | |
| 50–<75K | 2528 | 17.5 | 1016 | 18.9 | 356 | 16.1 | |
| ≥75K | 1850 | 12.8 | 798 | 14.8 | 289 | 13.0 | |
| US region | .008 | ||||||
| Northeast | 4825 | 31.3 | 1728 | 30.0 | 676 | 28.3 | |
| South | 3415 | 22.1 | 1381 | 24.0 | 720 | 30.1 | |
| Midwest | 3560 | 23.1 | 1423 | 24.7 | 562 | 23.5 | |
| West | 3618 | 23.5 | 1234 | 21.4 | 431 | 18.0 | |
| Time since menopause, y | <.001 | ||||||
| <5 | 1102 | 8.7 | 1254 | 25.2 | 622 | 29.8 | |
| 5–<10 | 1988 | 15.7 | 1030 | 20.7 | 414 | 19.9 | |
| 10–<15 | 2750 | 21.7 | 963 | 19.4 | 338 | 16.2 | |
| ≥15 | 6830 | 53.9 | 1728 | 34.7 | 711 | 34.1 | |
| Hysterectomy | 4129 | 26.8 | 1518 | 26.3 | 824 | 34.5 | .53 |
| Bilateral oophorectomy | 1362 | 9.1 | 474 | 8.4 | 262 | 11.4 | .01 |
| Smoking status | <.001 | ||||||
| Never | 8097 | 53.2 | 2922 | 51.2 | 1118 | 47.5 | |
| Past | 5970 | 39.2 | 2246 | 39.4 | 942 | 40.0 | |
| Current | 1164 | 7.6 | 535 | 9.4 | 294 | 12.5 | |
| Treated diabetes (pills or shots) | 851 | 5.5 | 326 | 5.7 | 191 | 8.0 | .11 |
| Alcohol consumption | .002 | ||||||
| Non/past drinker | 4687 | 30.6 | 1755 | 30.7 | 898 | 38.0 | |
| <1 drink/wk | 5275 | 34.5 | 2037 | 35.7 | 829 | 35.1 | |
| 1–14 drinks/wk | 4869 | 31.8 | 1750 | 30.6 | 581 | 24.6 | |
| >14 drinks/wk | 470 | 3.1 | 171 | 3.0 | 56 | 2.4 | |
| Moderate-strenuous physical activity ≥20 min, episodes/wk | .05 | ||||||
| No activity | 2665 | 18.9 | 1099 | 21.1 | 517 | 23.4 | |
| Some activity | 6419 | 45.5 | 2361 | 45.4 | 1000 | 45.3 | |
| 2–<4 episodes/wk | 2229 | 15.8 | 797 | 15.3 | 297 | 13.5 | |
| ≥4 episodes/wk | 2802 | 19.9 | 941 | 18.1 | 393 | 17.8 | |
| Osteoporosis prescription medication | 302 | 2.0 | 75 | 1.3 | 26 | 1.1 | .43 |
| Severity of Baseline Vasomotor Symptoms |
P Value | ||||||
|---|---|---|---|---|---|---|---|
| None (n = 15 418) |
Mild (n = 5766) |
Moderate/Severe (n = 2389) |
|||||
| Mean | sd | Mean | sd | Mean | sd | ||
| Age at screening, y | 64.9 | 6.7 | 61.2 | 6.8 | 59.9 | 6.9 | <.001 |
| BMI, kg/m2 | 29.5 | 6.2 | 29.6 | 6.1 | 30.7 | 6.3 | <.001 |
| Total calcium intake, mg | 1104.1 | 674.4 | 1059.6 | 663.7 | 1029.2 | 745.0 | .37 |
| Total vitamin d intake, IU | 339.4 | 260.7 | 326.4 | 266.4 | 305.4 | 251.5 | .14 |
| Physical functioning scale (RAND36) | 80.9 | 19.5 | 80.9 | 20.2 | 75.2 | 23.6 | <.001 |
| Total energy expenditure/wk from phys act, MET-h | 10.4 | 12.5 | 9.8 | 12.4 | 9.4 | 12.8 | .38 |
Abbreviations: GED, General Educational Diploma; MET-hrs, metabolic equivalent of task (hours/week); RAND36, 36-item Short-Form Health Survey.
P values adjusted for age, BMI, race/ethnicity, and smoking.
Likewise, women in the BMD cohort with moderate/severe VMS tended to be younger and non-White (all P < 0.05; Table 2). Average (SD) followup was 8.2 (1.7) years.
Table 2.
Baseline Characteristics of the BMD Cohort (n = 4867) by Severity of Baseline Vasomotor Symptoms
| Characterisic | Severity of Baseline Vasomotor Symptoms |
P Valuea | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None (n = 3161) |
Mild (n = 1157) |
Moderate/Severe (n = 549) |
|||||||||||
| N | % | Mean | sd | N | % | Mean | sd | N | % | Mean | sd | ||
| Age at screening, y | <.001 | ||||||||||||
| 50–54 | 251 | 7.9 | 238 | 20.6 | 142 | 25.9 | |||||||
| 55–59 | 437 | 13.8 | 264 | 22.8 | 127 | 23.1 | |||||||
| 60–69 | 1507 | 47.7 | 451 | 39 | 199 | 36.2 | |||||||
| 70–79 | 966 | 30.6 | 204 | 17.6 | 81 | 14.8 | |||||||
| Race/ethnicity | <.001 | ||||||||||||
| White | 2517 | 79.6 | 759 | 65.6 | 252 | 45.9 | |||||||
| Black | 381 | 12.1 | 276 | 23.9 | 213 | 38.8 | |||||||
| Hispanic | 180 | 5.7 | 91 | 7.9 | 66 | 12 | |||||||
| American Indian | 54 | 1.7 | 17 | 1.5 | 10 | 1.8 | |||||||
| Asian/Pacific Islander | 7 | 0.2 | 3 | 0.3 | 3 | 0.5 | |||||||
| Unknown | 22 | 0.7 | 11 | 1 | 5 | 0.9 | |||||||
| Education | <.001 | ||||||||||||
| ≤High school/GED | 1083 | 34.4 | 395 | 34.4 | 251 | 46.2 | |||||||
| School after high school | 1134 | 36 | 414 | 36.1 | 181 | 33.3 | |||||||
| ≥College Degree | 929 | 29.5 | 338 | 29.5 | 111 | 20.4 | |||||||
| Family income | .002 | ||||||||||||
| <20K | 946 | 32.3 | 343 | 32.7 | 234 | 46.7 | |||||||
| 20–<35K | 880 | 30 | 305 | 29 | 125 | 25 | |||||||
| 35–<50K | 503 | 17.2 | 177 | 16.9 | 65 | 13 | |||||||
| 50–<75K | 360 | 12.3 | 138 | 13.1 | 50 | 10 | |||||||
| ≥75K | 243 | 8.3 | 87 | 8.3 | 27 | 5.4 | |||||||
| US region | .08b | ||||||||||||
| Northeast | 1277 | 40.4 | 406 | 35.1 | 143 | 26 | |||||||
| South | 801 | 25.3 | 403 | 34.8 | 246 | 44.8 | |||||||
| West | 1083 | 34.3 | 348 | 30.1 | 160 | 29.1 | |||||||
| Time since menopause, y | <.001 | ||||||||||||
| <5 | 183 | 7.6 | 215 | 22.7 | 115 | 25.2 | |||||||
| 5–<10 | 308 | 12.8 | 183 | 19.3 | 71 | 15.6 | |||||||
| 10–<15 | 487 | 20.2 | 167 | 17.6 | 75 | 16.4 | |||||||
| ≥15 | 1427 | 59.3 | 383 | 40.4 | 195 | 42.8 | |||||||
| Hysterectomy | 1029 | 32.6 | 405 | 35 | 245 | 44.7 | .18 | ||||||
| Bilateral oophorectomy | 315 | 10.3 | 117 | 10.6 | 67 | 12.8 | .07 | ||||||
| Smoking status | .01 | ||||||||||||
| Never | 1797 | 57.6 | 638 | 55.8 | 272 | 50.5 | |||||||
| Past | 1072 | 34.4 | 408 | 35.7 | 201 | 37.3 | |||||||
| Current | 251 | 8 | 98 | 8.6 | 66 | 12.2 | |||||||
| Treated diabetes (pills or shots) | 215 | 6.8 | 75 | 6.5 | 75 | 13.7 | .22 | ||||||
| Alcohol consumption | .13 | ||||||||||||
| Non/past drinker | 1276 | 40.7 | 495 | 43.2 | 290 | 53.8 | |||||||
| <1 drink/wk | 1027 | 32.7 | 372 | 32.5 | 146 | 27.1 | |||||||
| 1–14 drinks/wk | 749 | 23.9 | 249 | 21.7 | 84 | 15.6 | |||||||
| >14 drinks/wk | 85 | 2.7 | 30 | 2.6 | 19 | 3.5 | |||||||
| Moderate-strenuous physical activity ≥20 min, episodes/wk | .26 | ||||||||||||
| No activity | 523 | 18.5 | 226 | 22.3 | 125 | 25.7 | |||||||
| Some activity | 1234 | 43.8 | 446 | 43.9 | 219 | 45 | |||||||
| 2–<4 episodes/wk | 420 | 14.9 | 128 | 12.6 | 65 | 13.3 | |||||||
| ≥4 episodes/wk | 643 | 22.8 | 215 | 21.2 | 78 | 16 | |||||||
| Osteoporosis prescription medication | 55 | 1.7 | 10 | 0.9 | 5 | 0.9 | .51 | ||||||
| Age at screening, y | 65.3 | 7 | 61.8 | 7.5 | 60.6 | 7.6 | <.001 | ||||||
| BMI, kg/m2 | 28.5 | 6.1 | 29.1 | 6 | 30.7 | 6.7 | .02 | ||||||
| Total calcium intake, mg | 1085.5 | 672.4 | 1055.6 | 691.4 | 942.2 | 592.8 | .49 | ||||||
| Total vitamin d intake, IU | 313.4 | 252.9 | 298.2 | 288.5 | 272.4 | 259 | .96 | ||||||
| Physical functioning scale (RAND36) | 80 | 20 | 78 | 20.9 | 69.9 | 25.7 | <.001 | ||||||
| Total energy expenditure/wk from phys act, MET-h | 11.5 | 14.5 | 10.4 | 13.2 | 9.1 | 13 | .34 | ||||||
Abbreviations: GED, General Educational Diploma; MET-h, metabolic equivalent of task (hours/week); RAND36, 36-item Short-Form Health Survey.
P adjusted for age, BMI, race/ethnicity, BMD scanner, and smoking.
Region and BMD scanner are confounded so P was not adjusted for scanner.
Associations between VMS and fracture incidence
After adjusting for baseline age, BMI, race/ethnicity, smoking, and education, baseline VMS severity was associated with increased hazard ratio (HR) for fracture (Table 3). Among women reporting baseline moderate/severe VMS (compared with no VMS), the HR for hip fracture was 1.78 (95% confidence interval [CI], 1.20–2.64, P = .01). Higher VMS severity was associated with increased HR for nonvertebral fractures (P = .04). However, VMS severity was not associated with the incidence of clinical spine fractures. VMS were not associated with lower arm/wrist fractures (data not shown).
Table 3.
Multivariablea-Adjusted Risk of Fracture Associated with Severity of Baseline Vasomotor Symptoms in the Fracture Study Population (n = 23 573), Stratified by Age and BMI
| Fracture Site | Subgroup | Severity of Baseline Vasomotor Symptoms |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None |
Mild |
Moderate/Severe |
P Valueb | |||||||||
| N | % | N | % | HR | (95% CI) | N | % | HR | (95% CI) | |||
| Hip | Overall | 265 | 0.21 | 53 | 0.11 | 0.93 | (0.69–1.27) | 29 | 0.15 | 1.78 | (1.20–2.64) | .01 |
| Age, y | .90 | |||||||||||
| 50–59 | 13 | 0.04 | 8 | 0.04 | 1.08 | (0.43–2.70) | 5 | 0.05 | 1.76 | (0.61–5.11) | ||
| 60–69 | 85 | 0.13 | 21 | 0.11 | 0.92 | (0.57–1.49) | 10 | 0.15 | 1.63 | (0.84–3.16) | ||
| 70–79 | 167 | 0.53 | 24 | 0.41 | 0.90 | (0.57–1.41) | 14 | 0.69 | 1.93 | (1.11–3.34) | ||
| BMI | .33 | |||||||||||
| Normal (<25) | 88 | 0.28 | 21 | 0.19 | 1.26 | (0.76–2.09) | 7 | 0.19 | 1.53 | (0.70–3.35) | ||
| Overweight (25–30) | 120 | 0.28 | 21 | 0.13 | 0.79 | (0.50–1.27) | 12 | 0.19 | 1.90 | (1.03–3.50) | ||
| Obese (≥30) | 57 | 0.11 | 11 | 0.06 | 0.75 | (0.38–1.48) | 10 | 0.11 | 1.95 | (0.98–3.87) | ||
| Spine | Overall | 226 | 0.18 | 66 | 0.14 | 1.16 | (0.87–1.54) | 21 | 0.11 | 1.09 | (0.68–1.75) | .59 |
| Age, y | .07 | |||||||||||
| 50–59 | 29 | 0.10 | 13 | 0.06 | 0.71 | (0.37–1.37) | 3 | 0.03 | 0.39 | (0.12–1.28) | ||
| 60–69 | 84 | 0.13 | 31 | 0.16 | 1.34 | (0.88–2.02) | 7 | 0.11 | 1.12 | (0.52–2.42) | ||
| 70–79 | 113 | 0.36 | 22 | 0.38 | 1.20 | (0.75–1.91) | 11 | 0.54 | 1.74 | (0.91–3.34) | ||
| BMI | .17 | |||||||||||
| Normal (<25) | 76 | 0.24 | 15 | 0.13 | 0.82 | (0.46–1.48) | 7 | 0.19 | 1.40 | (0.63–3.10) | ||
| Overweight (25–30) | 75 | 0.17 | 24 | 0.15 | 1.27 | (0.79–2.02) | 7 | 0.11 | 1.45 | (0.65–3.19) | ||
| Obese (≥30) | 74 | 0.15 | 27 | 0.14 | 1.38 | (0.88–2.18) | 7 | 0.07 | 0.75 | (0.32–1.74) | ||
| Otherc | Overall | 2240 | 1.94 | 673 | 1.51 | 0.93 | (0.85–1.02) | 289 | 1.57 | 1.12 | (0.98–1.27) | .04 |
| Age, y | .77 | |||||||||||
| 50–59 | 387 | 1.41 | 253 | 1.23 | 0.91 | (0.78–1.07) | 133 | 1.30 | 1.07 | (0.87–1.31) | ||
| 60–69 | 1115 | 1.88 | 295 | 1.57 | 0.92 | (0.80–1.04) | 109 | 1.75 | 1.15 | (0.95–1.41) | ||
| 70–79 | 738 | 2.55 | 125 | 2.32 | 1.00 | (0.82–1.22) | 47 | 2.52 | 1.14 | (0.84–1.55) | ||
| BMI | .49 | |||||||||||
| Normal (<25) | 647 | 2.26 | 169 | 1.61 | 0.89 | (0.74–1.06) | 54 | 1.58 | 0.92 | (0.69–1.23) | ||
| Overweight (25–30) | 798 | 2.00 | 266 | 1.73 | 1.03 | (0.89–1.19) | 101 | 1.72 | 1.21 | (0.97–1.50) | ||
| Obese (≥30) | 785 | 1.68 | 236 | 1.27 | 0.88 | (0.76–1.02) | 130 | 1.46 | 1.15 | (0.95–1.40) | ||
Adjusted for baseline age, BMI, race/ethnicity, smoking, education, and stratified by age group and prior history of fracture.
P corresponds to a 2-degree-of-freedom test of association for the 3-level nominal categorical predictor.
Includes hip fracture.
Associations between VMS severity and fracture incidence did not vary across subgroups of baseline age or BMI (Table 3). In addition, there was no interaction of VMS with years since menopause, race/ethnicity, baseline cardiovascular disease, or physical activity (data not shown).
The HR for hip fracture associated with moderate/severe VMS was attenuated by the inclusion of physical functioning in the Cox regression models, although VMS remained statistically significantly associated with hip fracture incidence; the HR (95% CI) changed from 1.78 (1.20–2.64) to 1.64 (1.09–2.46). None of the other potential confounders (tamoxifen/selective estrogen receptor modulator use, sleep disturbance, incident cancer, and incident number of falls) appreciably altered the HR for VMS (attenuation ranging between 0 and 6%), and VMS remained significantly associated with hip fracture incidence.
There were insufficient hip fracture events to allow stratification of results according to presence of diabetes mellitus or prevalent cardiovascular disease. Of the 29 hip fracture cases with moderate/severe symptoms at baseline, only one occurred in a participant who was treated for diabetes at baseline. In the subset of women without diabetes, the HR (95% CI) for hip fracture among women with moderate/severe VMS, compared with women without VMS, was 1.88 (1.25–2.81). VMS were not associated with diabetes, and adding diabetes as a covariate did not appreciably change the results: hip fracture HR (95% CI) was 0.93 (0.69–1.26) for mild VMS (vs none); and 1.77 (1.19–2.63) for moderate/severe VMS (vs none; P = .01).
In a sensitivity analysis, we excluded data from participants who reported use of selective estrogen receptor modulators (n = 1038) or prescription osteoporosis medications (n = 3621) at baseline or any time during the followup period. Results were similar to the results of the primary analyses (data not shown).
Associations between VMS and BMD
The baseline severity of VMS was not associated with baseline femoral neck or spine BMD. Associations between baseline VMS severity and baseline BMD did not significantly differ across categories of BMI, age, or years since menopause (interaction P > 0.05), except that associations of VMS severity with baseline lumbar spine BMD differed according to BMI category (interaction P = 0.04). In the subgroup of women with BMI ≥ 30 kg/m2, compared with women who reported no VMS, women with moderate/severe VMS had lower baseline femoral neck BMD (−0.015 g/cm2; 95% CI, −0.029–−0.001 g/cm2) and baseline lumbar spine BMD (−0.024 g/cm2; 95% CI, −0.045–−0.002 g/cm2).
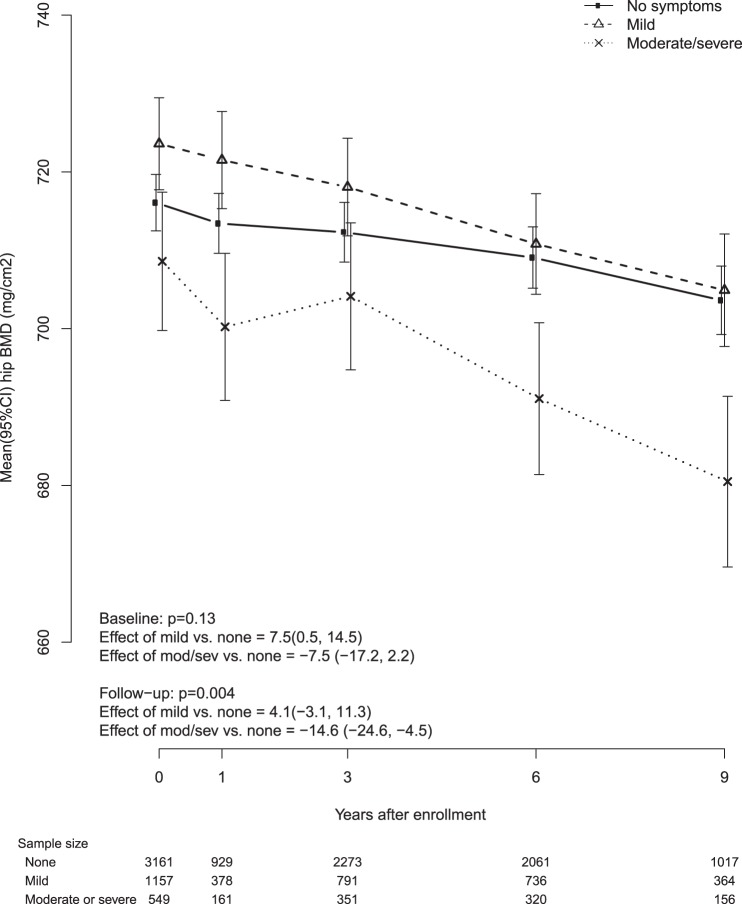
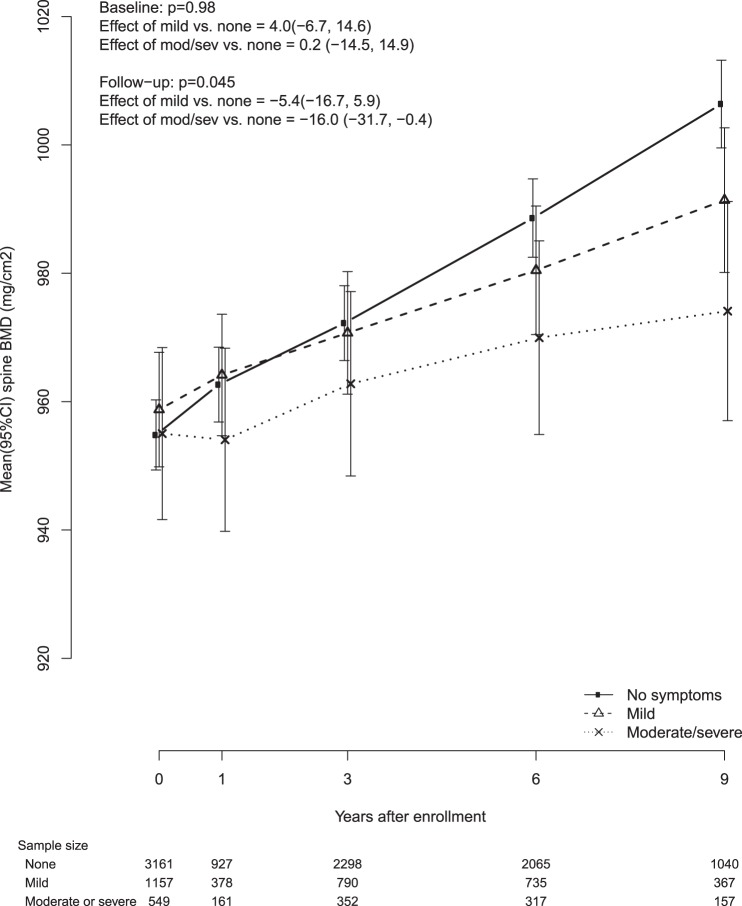
After adjusting for baseline age, BMI, race/ethnicity, smoking, and education, VMS severity was inversely associated with mean followup BMD both at the femoral neck and at the spine BMD (P for test of trend = .004 for femoral neck and .045 for spine). Compared with women who reported no VMS, women with moderate/severe VMS had 0.015 g/cm2 lower femoral neck BMD (95% CI, −0.025–−0.005, g/cm2) and 0.016 g/cm2 lower lumbar spine BMD (95% CI, −0.032–−0.004, g/cm2). Associations between VMS severity and BMD during followup did not differ by baseline age, years since menopause, or baseline BMI category (P > .05 for trend test). We show the trajectories of femoral neck BMD (Figure 2) and lumbar spine BMD (Figure 3) over time for each category of VMS severity (no VMS, mild VMS, moderate/severe VMS). Compared with participants without VMS, participants with moderate/severe VMS, had a steeper decline in femoral neck BMD and blunted increase in spine BMD.
Figure 2.
Femoral neck BMD by baseline vasomotor symptoms.
Figure 3.
Lumbar spine (L2–L4) BMD by baseline vasomotor symptoms.
In a sensitivity analysis that excluded women with a history of breast cancer at baseline (n = 133), associations between VMS and BMD were similar. Compared with women who reported no VMS, women with moderate/severe VMS had 0.013 g/cm2 lower femoral neck BMD (95% CI, −0.024–−0.003, g/cm2, P = .01) and 0.017 g/cm2 lower lumbar spine BMD (95% CI, −0.033–−0.007, g/cm2, P = .04).
Discussion
In this prospective study, compared with women who did not have VMS, women with moderate/severe VMS had lower BMD over time at the femoral neck and lumbar spine, and increased rates of hip fractures during an average (SD) of 8.2 years of followup. These associations did not differ by baseline age or BMI category. Despite being younger and heavier than asymptomatic women, characteristics associated with higher BMD, women with moderate/severe VMS had a higher risk of hip fractures that was also independent of other established risk factors for fractures.
To our knowledge, only one study has examined associations of VMS with subsequent fracture rates. That study included postmenopausal women with osteoporosis by BMD or prevalent fractures and found no association between hot flashes “that bothered them or interfered with their lives” in the previous 6 months with vertebral or nonvertebral fractures (14). However, followup duration was only 3 years and information regarding fracture locations was not analyzed. Two thirds of participants were randomly assigned to receive raloxifene, which is known to cause hot flashes in some women. The etiology of these hot flashes may be different than menopause-related VMS and may explain the discrepancy between results of the prior study and ours. Other longitudinal studies have found that postmenopausal women with VMS had lower BMD than those without VMS (13, 14). An additional longitudinal study found an association between “sweating” frequency and more rapid rates of loss in forearm BMD (9).
Lower estradiol levels in women with hot flashes may partly explain the associations we found between VMS and decreased bone density. We and others have previously found that higher estradiol levels are associated with lower rates of bone loss over the menopausal transition (26–28), and some longitudinal studies have found lower estradiol levels among women with VMS (29–31). Associations of estrogen level with VMS are likely complex, and may also depend on whether women have residual intermittent ovulatory activity (32). Information on estrogen levels is not available for the present study.
A possible explanation for our findings may be related to balance. However, moderate/severe VMS were not associated with increased risk for lower arm/wrist fracture. Moreover, including incident falls as a covariate did not attenuate the VMS HR for hip fracture. Our analysis does suggest that impaired physical functioning may partially explain the association between moderate/severe VMS and hip fracture. Falls are multifactorial and it is likely that we saw no attenuation because of our inability to directly control for balance.
In this study, increases in spine BMD over time were more pronounced among women without VMS than among women with moderate/severe VMS, yet spine BMD increased over time in all groups. A previous cross-sectional study found that age and BMD were strongly inversely related at the hip, but not at the lumbar spine (33). This may be because typical anteroposterior projection used in dual-energy x-ray absorptiometry to measure spine BMD is confounded by degenerative changes due to aging. For example, spinal degeneration and hyperostosis (osteophytes), vertebral fracture, and aortic calcification can spuriously increase bone density (34). It is also possible that increases in spine BMD are driven by the subgroup of WHI participants who received calcium and vitamin D in the WHI Calcium + Vitamin D trial (35).
Our study has limitations. Participants of WHI were healthier than similarly aged women in the general population, and the incidence of fractures in this cohort was low. Also, VMS were self reported and assessed at a single baseline visit. However, we suspect that this means of assessing VMS reflects what occurs in routine clinical practice. We cannot rule out residual confounding. The associations among age, VMS, BMD, fracture, and HT use in an observational setting are complex. However, in the current study, it is unlikely that HT use confounded our results because we included only women who reported that they had never used HT at baseline, and the overwhelming majority of these women did not initiate HT during followup. Because BMD was available only in a subset of participants (n = 4867), we were unable to adjust for BMD in the fracture models. Insufficient numbers of hip fracture events precluded a more detailed investigation into the joint effects of hot flash severity and night sweat severity and their corresponding interactions. Finally, information regarding serum levels of estrogen, vitamin D, and bone turnover markers was not available.
Strengths of our study include the large number of participants, the long followup duration, the collection of information regarding both hot flashes and night sweats, the direct measurement of BMI (which is associated with both VMS and BMD), the adjudication of fracture endpoints, and the detailed information regarding fracture risk factors and anatomical locations of fractures.
In conclusion, we found that women with moderate/severe VMS have lower BMD and increased hip fracture rates over followup. VMS are common, experienced by at least 60% of women (2). Information regarding VMS is easily assessable by clinicians. Women with VMS may benefit from greater attention to healthy lifestyle habits to maintain bone health. Elucidation of the biological mechanisms underlying these associations may inform the design of preventive strategies for at-risk women prior to occurrence of fracture.
Acknowledgments
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller. Clinical Coordinating Center: (Fred Hutchinson Cancer, Research Center, Seattle, Washington) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles, Kooperberg. Investigators and Academic Centers: (Brigham and Women's Hospital, Harvard Medical, School, Boston, Massachusetts) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, California), Marcia L. Stefanick; (The Ohio State University, Columbus, Ohio) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, Arizona) Cynthia A. Thomson; (University at Buffalo, Buffalo, New York), Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, Florida) Marian Limacher; (University of Iowa, Iowa/Davenport, Iowa) Robert Wallace; (University of Pittsburgh, Pittsburgh, Pennsylvania) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, North Carolina) Sally Shumaker, Women's Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services through Contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Disclosure Summary: C.J.C., E.L., M.J.O'S., A.L., J.C., J.M., M.V., J.W.-W., and A.A. have nothing to discolse. R.W. is a consultant to Merck and Novartis to perform safety monitoring of antiosteoporotic clinical trials. N.W. is cofounder, stockholder, and director of Osteodynamics. He has received honoraria for lectures from Amgen and Merck in the past year. He has received consulting fees from the following companies in the past year: AbbVie, Amarin, Amgen, Bristol-Myers Squibb, Corcept, Endo, Imagepace, Janssen, Lilly, Merck, Novartis, Noven, Pfizer/Wyeth, Radius, and Sanfo-Aventis. Through his health system, he has received research support from Merck and NPS.
Footnotes
- BMD
- bone mineral density
- BMI
- body mass index
- CI
- confidence interval
- HR
- hazard ratio
- HT
- hormone therapy
- VMS
- Vasomotor symptom
- WHI
- Women's Health Initiative
- WHI-CT
- Women's Health Initiative Clinical Trial
- WHI-OS
- Women's Health Initiative Observational Study.
References
- 1. Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: Study of women's health across the nation. Am J Public Health. 2006;96:1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Politi MC, Schleinitz MD, Col NF. Revisiting the duration of vasomotor symptoms of menopause: A meta-analysis. J Gen Intern Med. 2008;23:1507–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sowers MR, Jannausch M, McConnell D, et al. Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab. 2006;91:1261–1267. [DOI] [PubMed] [Google Scholar]
- 4. Gast GC, Grobbee DE, Pop VJ, et al. Vasomotor symptoms are associated with a lower bone mineral density. Menopause. 2008. [DOI] [PubMed] [Google Scholar]
- 5. Grainge MJ, Coupland CA, Cliffe SJ, Chilvers CE, Hosking DJ. Reproductive, menstrual and menopausal factors: Which are associated with bone mineral density in early postmenopausal women? Osteoporos Int. 2001;12:777–787. [DOI] [PubMed] [Google Scholar]
- 6. Scoutellas V, O'Neill TW, Lunt M, Reeve J, Silman AJ. Does the presence of postmenopausal symptoms influence susceptibility to vertebral deformity? European Vertebral Osteoporosis Study (EVOS) Group. Maturitas. 1999;32:179–187. [DOI] [PubMed] [Google Scholar]
- 7. Tural A, Yoldemir T, Erenus M. Assessment of bone mineral density should be considered earlier in perimenopausal women with vasomotor symptoms. Int J Gynaecol Obstet. 2009;107:114–116. [DOI] [PubMed] [Google Scholar]
- 8. von Mühlen DG, Soroko S, Kritz-Silverstein D, Barrett-Connor E. Vasomotor symptoms are not associated with reduced bone mass in postmenopausal women: The Rancho Bernardo Study. J Womens Health Gend Based Med. 2000;9:505–511. [DOI] [PubMed] [Google Scholar]
- 9. Naessen T, Persson I, Ljunghall S, Bergström R. Women with climacteric symptoms: A target group for prevention of rapid bone loss and osteoporosis. Osteoporos Int. 1992;2:225–231. [DOI] [PubMed] [Google Scholar]
- 10. Salamone LM, Gregg E, Wolf RL, et al. Are menopausal symptoms associated with bone mineral density and changes in bone mineral density in premenopausal women? Maturitas. 1998;29:179–187. [DOI] [PubMed] [Google Scholar]
- 11. Pal L, Norian J, Zeitlian G, Bevilacqua K, Freeman R, Santoro N. Vasomotor symptoms in infertile premenopausal women: A hitherto unappreciated risk for low bone mineral density. Fertil Steril. 2008;90:1626–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee SJ, Kanis JA. An association between osteoporosis and premenstrual symptoms and postmenopausal symptoms. Bone Miner. 1994;24:127–134. [DOI] [PubMed] [Google Scholar]
- 13. Crandall CJ, Zheng Y, Crawford SL, et al. Presence of vasomotor symptoms is associated with lower bone mineral density: A longitudinal analysis. Menopause. 2009;16:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang A, Grady D, Blackwell T, Bauer D. Hot flushes, bone mineral density, and fractures in older postmenopausal women. Obstet Gynecol. 2007;109:841–847. [DOI] [PubMed] [Google Scholar]
- 15. Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials.. 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 16. Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative Observational Study: Baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–S121. [DOI] [PubMed] [Google Scholar]
- 17. Anderson GL, Manson J, Wallace R, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003;13:S5–S17. [DOI] [PubMed] [Google Scholar]
- 18. Cauley JA, Wampler NS, Barnhart JM, et al. Incidence of fractures compared to cardiovascular disease and breast cancer: The Women's Health Initiative Observational Study. Osteoporos Int. 2008;19:1717–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beck TJ, Petit MA, Wu G, LeBoff MS, Cauley JA, Chen Z. Does obesity really make the femur stronger? BMD, geometry, and fracture incidence in the women's health initiative-observational study. J Bone Miner Res. 2009;24:1369–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. LaCroix AZ, Beck TJ, Cauley JA, et al. Hip structural geometry and incidence of hip fracture in postmenopausal women: What does it add to conventional bone mineral density? Osteoporos Int. 2010;21:919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Orchard TS, Larson JC, Alghothani N, et al. Magnesium intake, bone mineral density, and fractures: Results from the Women's Health Initiative Observational Study. Am J Clin Nutr. 2014;99:926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen Z, Arendell L, Aickin M, Cauley J, Lewis CE, Chlebowski R. Hip bone density predicts breast cancer risk independently of Gail score: Results from the Women's Health Initiative. Cancer. 2008;113:907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. [DOI] [PubMed] [Google Scholar]
- 24. Levine DW, Kaplan RM, Kripke DF, Bowen DJ, Naughton MJ, Shumaker SA. Factor structure and measurement invariance of the Women's Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15:123–136. [DOI] [PubMed] [Google Scholar]
- 25. Gold EB, Sternfeld B, Kelsey JL, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40–55 years of age. Am J Epidemiol. 2000;152:463–473. [DOI] [PubMed] [Google Scholar]
- 26. Crandall CJ, Tseng CH, Karlamangla AS, et al. Serum sex steroid levels and longitudinal changes in bone density in relation to the final menstrual period. J Clin Endocrinol Metab. 2013;98:E654–E663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dennerstein L, Lehert P, Guthrie JR, Burger HG. Modeling women's health during the menopausal transition: A longitudinal analysis. Menopause. 2007;14:53–62. [DOI] [PubMed] [Google Scholar]
- 28. Slemenda C, Longcope C, Peacock M, Hui S, Johnston CC. Sex steroids, bone mass, and bone loss. A prospective study of pre-, peri-, and postmenopausal women. J Clin Invest. 1996;97:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guthrie JR, Dennerstein L, Taffe JR, Lehert P, Burger HG. Hot flushes during the menopause transition: A longitudinal study in Australian-born women. Menopause. 2005;12:460–467. [DOI] [PubMed] [Google Scholar]
- 30. Ford K, Sowers M, Crutchfield M, Wilson A, Jannausch M. A longitudinal study of the predictors of prevalence and severity of symptoms commonly associated with menopause. Menopause. 2005;12:308–317. [DOI] [PubMed] [Google Scholar]
- 31. Ratka A, Miller V, Brown K, Jenschke M, Simpkins J. Association of various dimensions of hot flashes with systemic levels of gonadal steroids. Exp Biol Med (Maywood). 2009;234:395–402. [DOI] [PubMed] [Google Scholar]
- 32. Gold EB, Lasley B, Crawford SL, McConnell D, Joffe H, Greendale GA. Relation of daily urinary hormone patterns to vasomotor symptoms in a racially/ethnically diverse sample of midlife women: Study of women's health across the nation. Reprod Sci. 2007;14:786–797. [DOI] [PubMed] [Google Scholar]
- 33. Steiger P, Cummings SR, Black DM, Spencer NE, Genant HK. Age-related decrements in bone mineral density in women over 65. J Bone Miner Res. 1992;7:625–632. [DOI] [PubMed] [Google Scholar]
- 34. Rosen CJ., American Society for Bone and Mineral Research. 2013. Primer on the metabolic bone diseases and disorders of mineral metabolism. In: Bouillon R, Compston JE, Rosen V, eds. 8th ed Ames: Wiley-Blackwell; 349. [Google Scholar]
- 35. Shea B, Wells G, Cranney A, et al. Meta-analyses of therapies for postmenopausal osteoporosis. VII. Meta-analysis of calcium supplementation for the prevention of postmenopausal osteoporosis. Endocr Rev. 2002;23:552–559. [DOI] [PubMed] [Google Scholar]