Abstract
Context:
Papillary thyroid carcinoma (PTC) is the most common endocrine malignancy. It has been demonstrated that micro-RNAs (miRNAs) are involved in the development of PTC. The miRNA-chromatin immunoprecipitation microarray assay revealed down-regulation of miR-219–5p; however, the effect of miR-219–5p on PTC cell growth remains unknown. This result implied the critical role of miR-219–5p in the development of PTC.
Methods:
We investigated the association between miR-219–5p and PTC development. Expression of miR-219–5p was monitored in 30 PTC tissue specimens and compared with that in 30 normal thyroid tissue specimens. The effect of miR-219–5p on PTC development was studied by cell proliferation, migration, and apoptosis assays. The underlying mechanism was clarified by a reporter assay and rescue experiment.
Results:
The current study confirmed that miR-219–5p expression was inhibited in PTC tissue samples. There were statistically significant differences in the expression of miR-219–5p with regard to sex, tumor size, and lymph node metastasis in patients with PTC. Forced expression of miR-219–5p suppressed PTC cell proliferation and migration and promoted apoptosis. Further study showed that estrogen receptor (ER) α was the direct target of miR-219–5p and mediated the effect of miR-219–5p on PTC occurrence. Expression of miR-219–5p was inversely correlated with that of ERα. Importantly, ERα overexpression in PTC cells rescued the inhibitory effect of miR-219–5p on PTC cell proliferation and migration. Thus, our results indicated that miR-219–5p played a critical role in PTC growth by inhibiting ERα.
Conclusion:
Our investigation identified miR-219–5p as a negative regulator of PTC development through targeting of ERα.
Papillary thyroid carcinoma (PTC) originates from the thyroid epithelial follicular cells and is the most common endocrine malignancy (1, 2). The incidence of PTC worldwide has increased in recent years, accounting for 80% of all thyroid cancers (3). PTC can occur at any age but rarely has been diagnosed as a congenital tumor. Most PTC cases are diagnosed in patients aged 30 to 50 years. Women are affected more frequently than men at ratios of 2:1 to 4:1 (4, 5). It has been shown that several signaling pathways are involved in PTC development. Dysregulation of the RET/PTC-RAS-BRAF signaling pathway is evident in PTC, especially mutations in BRAF and RET/PTC genes (6, 7). The BRAF T1799A mutation is found in most patients with PTCs. Standard therapies, such as surgery and 131I therapy, have an excellent prognosis with survival rates of 95% at 25 years (8). However, 10% to 15% of patients with PTCs have a relapse and metastasis after therapy, depending on age at diagnosis, tumor stage, and initial treatment. Thus, there is an urgent need to develop new strategies to prevent PTC occurrence.
Micro-RNAs (miRNAs) constitute a class of small endogenous noncoding RNAs of 19 to 23 nucleotides that negatively regulate gene expression (9). miRNAs bind to the 3′-untranslated region (UTR) of the target mRNAs, causing a block of protein translation or mRNA degradation, depending on the level of complementarity (10, 11). It has been suggested that miRNAs regulate ∼30% of the human genome and control cellular processes such as cell proliferation, development, apoptosis, and the immune response. Recently, increasing evidence has emphasized the role of miRNA in PTC development. The miRNA-chromatin immunoprecipitation microarray assay revealed up-regulation of a set of miRNAs, including miR-221, miR-222, miR-146, miR-21, miR-155, miR-181a, and miR-181b, in PTCs compared with those in normal thyroid as well as a series of down-regulated miRNAs, such as miR-26a-1, miR-219–5p, and miR-345 (12, 13). The critical role of some of these miRNAs has been confirmed in experimental models. Previous investigations have shown that miR-26a suppresses cell growth and tumorigenesis of PTC by targeting CKS2 (14), miR-146a targets PRKCE to modulate PTC (15), and miR-221 and miR-222 are endogenous regulators of the PTC cell cycle by modulating p27Kip1 protein expression (16). Collectively, miRNAs are linked to the development of PTC.
According to recent investigations, miR-219–5p is significantly down-regulated in tumor samples, such as hepatocellular carcinoma (17) and lung cancer (18). Further studies have shown that miR-219–5p inhibits tumor size and cancer cell proliferation, suggesting that it is a negative regulator of tumor development (17). However, whether miR-219–5p is involved in the pathogenesis of PTC remains unknown. In the current study, we clarified the important role of miR-219–5p in suppressing PTC development by targeting estrogen receptor (ER) α.
Materials and Methods
Human tissue samples
This study was approved by the Medical Ethics Committee of The First Affiliated Hospital of Bengbu Medical College, China, and all works were conducted in accordance with the Declaration of Helsinki. All participants gave written informed consent before participating in this study. A total of 30 PTC and 30 normal thyroid tissue samples adjacent to a PTC were collected from patients undergoing curative-intent surgery at the Department of Surgery, The First Affiliated Hospital of Bengbu Medical College between 2010 and 2013. All tissues were immediately dissected, placed on ice, snap-frozen in liquid nitrogen, and stored at −80°C until processing. The histological sections were reviewed by 2 expert pathologists to verify the diagnosis and define the histological variants. None of the patients had received any preoperative treatment.
Cell culture and transfection
The human PTC cell line K1 was purchased from the American Type Culture Collection; cell line W3 was a kind gift from Dr Robert Gagel (MD Anderson Cancer Center, University of Texas). Thyroid cell line (human thyroid follicular epithelial) Nthy-ori 3–1 was purchased from the European Collection of Animal Cell Cultures (ECACC). K1 cells were cultured in DMEM-Ham's F12-MCDB 105 (2:1:1) (Invitrogen) with 10% fetal bovine serum (FBS) (Gibco), 100 μg/mL streptomycin, and 100 U/mL penicillin. W3 cells were cultured in DMEM with 10% FBS, 100 μg/mL streptomycin, and 100 U/mL penicillin. Nthy-ori 3–1 cells were cultured in RPMI-1640 (Invitrogen) containing 10% FBS), 100 U/mL penicillin, and 100 mg/mL streptomycin. HEK-293T cells were maintained in DMEM containing 10% FBS with 100 U/mL penicillin and 100 μg/mL streptomycin. All of the cells were incubated in a humidified atmosphere of 5% CO2 in air at 37°C. For the cell transfection assay, the synthetic miR-219–5p mimic and the antisense RNA oligonucleotides specific for miR-219–5p (anti-miR-219–5p) were transfected into cells using a Lipofectamine RNAiMAX kit (Invitrogen) at ∼50% confluence, following the manufacturer's instructions.
Plasmid construction
The human miR-219–5p precursor and ERα coding sequence were cloned into a lentivirus pCDH vector to generate stably transfected cell lines. Viral production was performed in 293T cells. 293T cells were cotransfected with lentiviral vector pCDH and a packaging plasmid. Cells were incubated overnight at 37°C and 5% CO2. The supernatant was collected at 24 and 48 hours posttransfection. A fragment of the ERα 3′-UTR containing the predicted binding site for miR-219–5p and the binding site mutated 3′-UTR were inserted into the psiCHECK-2 vector for the dual-luciferase reporter assay.
RNA extraction and quantitative PCR (qPCR)
Total RNA was isolated from tissue samples and cells using the RNeasy Plus Micro Kit (QIAGEN). For the detection of mature miRNA, miRNA was reverse transcribed using the TaqMan miRNA Reverse Transcription Kit (Applied Biosystems). qPCR involved the appropriate TaqMan miRNA assay (Applied Biosystems) and a Prism 7500 instrument (Applied Biosystems). Quantitation of U6 was performed as an endogenous control. For detection of the expression of ERα, mRNA was reverse transcribed using PrimeScript reverse transcriptase and random primers (TaKaRa), followed by qPCR with SYBR Green chemistry (Applied Biosystems) with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an endogenous control.
Cell proliferation assays
Cell viability was evaluated by a 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells were seeded in triplicate in 96-well plates at a density of 1 × 104 cells/well and maintained in a humidified atmosphere of 5% CO2 in air at 37°C. If transfection with anti-miR-219–5p was needed, cells were plated at day 1 after transfection. At 1, 2, 3, and 4 days after seeding, cells were treated with 0.125 mg/mL MTT (Amresco) for 3 hours before harvest. The medium was removed after incubation with MTT, and cells were solubilized in dimethylsulfoxide (100 μL/well) to dissolve the insoluble purple formazan product. The OD490 in each well, which reflected the number of viable cells, was determined by a microplate reader. Assays were performed in triplicate wells. Data are presented as means and SD from 3 independent experiments.
A cell counter was used to measure cell numbers directly. The cells were seeded in triplicate in 96-well plates at a density of 1 × 104 cells/well. At different time intervals, the cells were trypsinized and suspended in PBS, and viable cells were counted using a Coulter Z1 Cell Particle Counter (Beckman Coulter).
Cell migration assay
The cell migration assay was performed using a Transwell chamber. Cells were suspended in serum-free medium and added to the upper chamber. RPMI-1640 medium containing 10% FBS was added to the lower chamber. Cells were allowed to migrate for 12 hours at 37°C. Nonmigrating cells on the upper surface of the membrane were gently removed. The migrated cells were then fixed with methanol and stained with crystal violet. The stained cells on the lower surface of the membrane were photographed using an inverted light microscope and counted manually using 5 randomly selected areas. The migration assays were performed in triplicate.
Apoptosis assay
The apoptosis assay was performed by fluorescence-activated cell sorting on a flow cytometer according to annexin V–fluorescein isothiocyanate (FITC) and propidium iodide (PI) double staining. Cells were harvested and washed twice with cold PBS, followed by resuspension to the single cell suspension. The cells were fixed in cold 75% ethanol at 4°C overnight. The fixed cells were collected, washed twice with PBS, and resuspended in PBS containing annexin V–FITC and PI and then were incubated at 4°C for at least 30 minutes in the dark. The percentage of apoptotic cells was measured by flow cytometry (Beckman Coulter). The living cells were annexin V–FITC and PI double negative, whereas the late apoptotic or secondary necrotic cells were double positive. The early apoptotic cells were only annexin V–FITC positive, whereas the isolated nuclei or cellular debris were only PI positive.
Western blotting
The cells were lysed with buffer containing 1% SDS, 50 mM Tris-HCl (pH 6.8), 10 mM dithiothreitol, 10% glycerol, 0.002% bromphenol blue, and a protease inhibitor mixture (Roche). Equal amounts of protein were separated by SDS-PAGE, transferred to a polyvinylidene difluoride membrane (Millipore), immunoblotted with antibodies, and visualized with horseradish peroxidase–coupled secondary antibodies. The antibodies against ERα and ERβ were purchased from Abcam, the GAPDH antibody was from Sigma-Aldrich, and antibodies against phospho (p)-p38 (MAPK, p38 MAPK kinase, phospho-ERK1/2, and ERK1/2 were obtained from Cell Signaling Technology.
Selected targets mRNA for miR-219–5p
Target mRNAs for miR-219–5p were predicted using TargetScan (http://www.targetscan.org/) and PicTar (http://pictar.mdc-berlin.de/). Gene Ontology was used in a subsequent screening step. We determined that the 3′-UTR of ERα was the best candidate target mRNA.
Luciferase reporter assays
Luciferase reporter assays were performed using the dual-luciferase reporter assay system (psiCHECK-2 vector; Promega). A fragment of the ERα 3′-UTR containing the predicted binding site for miR-219–5p and the binding site mutated 3′-UTR were inserted into psiCHECK-2 vector. All constructs were verified by DNA sequencing. The psiCHECK-2 vector containing wild-type (WT) or mutated ERα was transfected into PTC cells with or without the synthetic miR-219–5p mimic. Thirty-six hours after transfection, luciferase activity was detected using a dual-luciferase reporter assay system and normalized to Renilla activity.
Hematoxylin and eosin staining
Standard hematoxylin and eosin staining was performed on 5-μm paraffin sections of PTC specimens. Tissues that were fixed, processed, embedded, and sectioned were stained in hematoxylin solution for 3 minutes. The slides were placed under running tap water at room temperature for at least 5 minutes. Then, the samples were stained in working eosin Y solution for 2 minutes and dehydrated. A drop of Permount was placed over the tissue on each slide and a coverslip was added. The slides were viewed with a microscope.
Immunohistochemistry
PTC specimens were fixed in 10% neutralized formalin and embedded in paraffin blocks. Sections were then prepared for immunohistochemical examination. After deparaffinization and rehydration, antigen retrieval was performed by boiling samples in 10 mM citrate buffer (pH 6.0) for 10 minutes. After inhibition of endogenous peroxidase activity for 30 minutes with methanol containing 0.3% H2O2, sections were blocked with 2% BSA in PBS for 30 minutes and incubated with ERα antibody (Abcam). The immune complex was visualized, and nuclei were counterstained with hematoxylin.
Statistical analysis
SPSS 19.0 software was used for statistical analysis. All values are expressed as means ± SD for at least 3 separate experiments, using an independent t test to perform comparisons of 2 independent groups.
Results
Expression of miR-219–5p is decreased in clinical PTC specimens and PTC cells
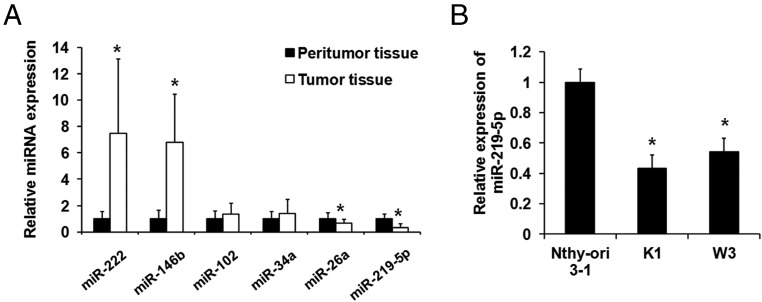
To study the role of miR-219–5p in PTC development, we detected expression of several miRNAs in 30 PTC samples and 30 normal thyroid tissues using qPCR. There was no difference between the 2 groups in age and sex. Consistent with the previous study, miR-146b and miR-222 were up-regulated in PTC tissues (Figure 1A and Table 1). The critical roles of these miRNAs, in addition to that of miR-219–5p, in PTC occurrence have been identified. The average level of miR-219–5p was significantly lower in PTC specimens than in normal thyroid tissues (Figure 1A and Table 1), which is consistent with previous miRNA-CHIP data (12, 13). As shown in Table 2, there were significant differences in the expression of miR-219–5p (relative quantification (RQ) value) with regard to sex. At the same time, tumor size tended to be larger and the incidence of lymph node metastasis tended to be higher in cases with low rather than high expression of miR-219–5p. In addition, miR-219–5p was down-regulated in PTC cell lines K1 and W3 compared with thyroid cell line Nthy-ori 3–1 (Figure 1B).
Figure 1.
miR-219–5p was down-regulated in PTC tissue and cells. A, Expression of miR-222, miR-146b, miR-102, miR-34a, miR-26a, and miR-219–5p was detected by qPCR in PTC peritumoral and tumor tissue. Data were normalized to the expression level in peritumoral tissue. B, Expression of miR-219–5p was detected by qPCR in PTC cell lines K1 and W3 and thyroid cell line Nthy-ori 3–1. Data were normalized to the expression level in the Nthy-ori 3–1 cells. *, P < .05.
Table 1.
Expression of miRNAs in PTC Tissue
| miRNA | Normal Thyroid Tissue | PTC Tissue | P Value |
|---|---|---|---|
| hsa-miR-222 | 1 ± 0.567 | 7.459 ± 5.637 | <.001 |
| hsa-miR-146b | 1 ± 0.656 | 6.769 ± 3.644 | <.001 |
| hsa-miR-155 | 1 ± 0.751 | 2.233 ± 1.873 | .00144 |
| hsa-miR-221 | 1 ± 0.790 | 2.181 ± 1.723 | .00115 |
| hsa-miR-220 | 1 ± 0.729 | 2.024 ± 1.580 | .00154 |
| hsa-miR-181b | 1 ± 0.650 | 1.959 ± 1.381 | .00107 |
| hsa-miR-34a | 1 ± 0.555 | 1.384 ± 1.066 | .08462 |
| hsa-miR-213 | 1 ± 0.824 | 1.366 ± 0.667 | .06346 |
| hsa-miR-102 | 1 ± 0.571 | 1.332 ± 0.846 | .08435 |
| hsa-miR-26a | 1 ± 0.448 | 0.689 ± 0.292 | .00229 |
| hsa-miR-138-1 | 1 ± 0.456 | 0.663 ± 0.357 | .00232 |
| hsa-miR-345 | 1 ± 0.651 | 0.645 ± 0.275 | <.001 |
| hsa-miR-219–5p | 1 ± 0.371 | 0.31 ± 0.291 | <.001 |
miRNA expression was detected by qPCR in 30 normal thyroid tissues and 30 PTC tissues. All RQ values are expressed as means ± SD. Data are normalized to the expression level in normal thyroid tissues.
Table 2.
Correlation of miR-219–5p Expression With Clinicopathologic Features in PTC
| Clinicopathologic Parameters | Case No. | miR-219–5p Expression | P Value |
|---|---|---|---|
| Total cases | 30 | ||
| Age | .4979 | ||
| ≤60 y | 16 | 1.139 ± 0.671 | |
| >60 y | 14 | 1.325 ± 0.815 | |
| Sex | .0102 | ||
| Male | 12 | 1.669 ± 0.531 | |
| Female | 18 | 1.057 ± 0.634 | |
| Tumor | .0041 | ||
| ≤5 cm | 14 | 1.637 ± 0.620 | |
| >5 cm | 16 | 0.980 ± 0.533 | |
| Lymph node metastasis | .0150 | ||
| Negative | 13 | 1.783 ± 0.917 | |
| Positive | 17 | 1.039 ± 0.658 | |
| Extrathyroid extension | |||
| None | 13 | 1.783 ± 0.917 | |
| Microscopic | 11 | 1.125 ± 0.790 | .0753 |
| Macroscopic | 6 | 0.881 ± 0.304 | .0479 |
| PTC subtype | |||
| Typical PTC | 12 | ||
| Papillary microcarcinoma | 4 | ||
| Tall cell variant | 3 | ||
| Intracystic papillary carcinoma | 2 | ||
| Diffuse sclerosing variant | 2 | ||
| Oncocytic variant | 2 | ||
| Follicular variant | 2 | ||
| Solid variant | 2 | ||
| Insular variants | 1 |
Clinicopathologic information of patients with PTC and miR-219–5p expression in different clinicopathologic features. All RQ values are expressed as means ± SD.
miR-219–5p inhibited proliferation and migration and promoted apoptosis in PTC cells
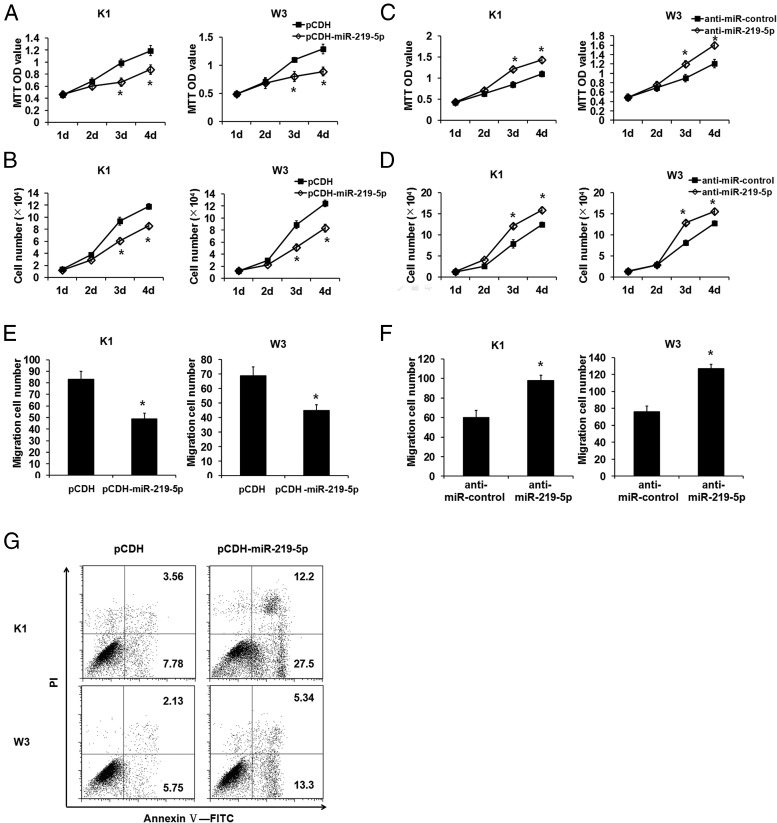
To characterize the effect of miR-219–5p on cell proliferation, migration, and apoptosis, which are required for tumorigenesis and metastasis, we performed overexpression studies through lentivirus infection and inhibition studies using miR-219–5p–specific antisense oligonucleotide inhibitor (anti-miR-219–5p), respectively, with PTC cells. As shown in the MTT assay (Figure 2A) and cell counter assay (Figure 2B), miR-219–5p overexpression significantly suppressed proliferation of K1 and W3 cells (Figure 2, A and B). In contrast, anti-miR-219–5p enhanced cell proliferation in the 2 PTC cells (Figure 2, C and D). The cell migration assay showed that forced expression of miR-219–5p inhibited cell migration (Figure 2E) and that inhibition of miR-219–5p promoted cell migration (Figure 2F). In addition, miR-219–5p overexpression dramatically increased the ratio of apoptotic cells in both PTC cell lines (Figure 2G). Thus, miR-219–5p was a negative regulator of PTC cell proliferation and migration.
Figure 2.
miR-219–5p inhibited proliferation and migration and promoted apoptosis of PTC cells. A and B, miR-219–5p expression was forced in PTC cells by lentivirus infection. Proliferation of PTC cells was inhibited by overexpression of miR-219–5p as indicated in the MTT (A) and cell count (B) assays. C and D, miR-219–5p was inhibited by antisense RNA oligonucleotides anti-miR-219–5p. Proliferation of PTC cells was augmented by inhibition of miR-219–5p as indicated in the MTT (C) and cell count (D) assays. E and F, Migration activity as indicated by the Transwell assay was suppressed by miR-219–5p overexpression (E) and augmented by miR-219–5p inhibition (F). G, The indicated PTC cells were incubated with the annexin-V–FITC antibody and stained with PI for apoptosis analysis by flow cytometry. Dot plots display PI fluorescence on the y-axis and FITC fluorescence on the x-axis. The percentages of apoptotic cells are shown in the upper and lower right quadrants. *, P < .05.
ERα was the target of miR-219–5p
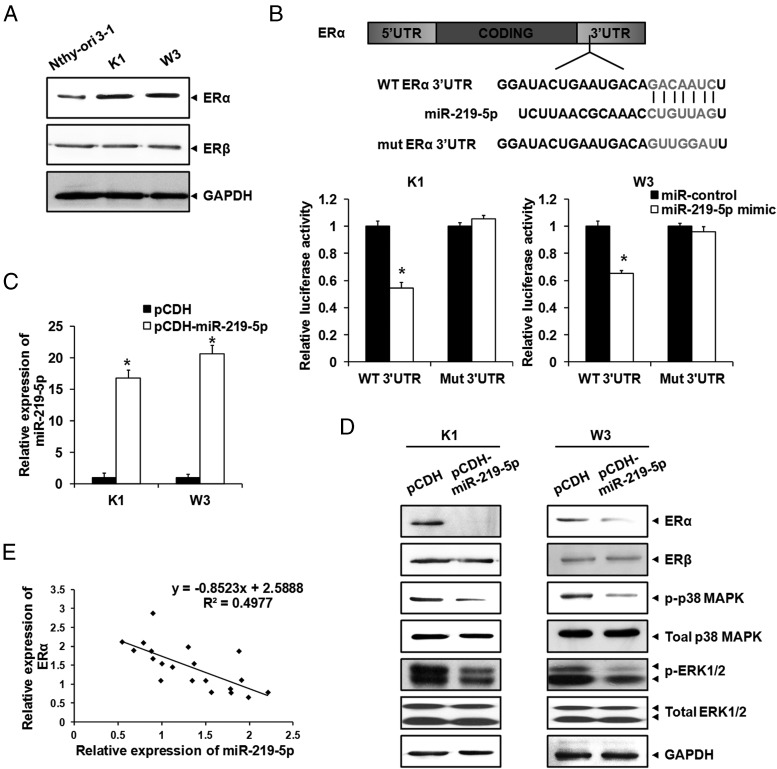
Previous studies have shown that women are affected by PTC more frequently than men at ratios of 2:1 to 4:1, indicating that an estrogen-related signaling pathway might play an important role in PTC development (4, 5). We searched 2 publicly available miRNA target prediction tools and established that ERα was a potential target of miR-219–5p. We found that expression of ERα, but not ERβ, was up-regulated in the PTC cells compared with the normal thyroid cell line, suggesting the potential critical role of ERα in PTC development (Figure 3A).
Figure 3.
ERα was the target of miR-219–5p. A, Protein levels of ERα and ERβ in PTC cells were detected by Western blotting, with GAPDH as an endogenous control. B, The WT or mutant (mut) 3′-UTR of ERα was transfected into PTC cells with the miR-219–5p mimic. Luciferase reporter assays were conducted after transfection for 36 hours. Data were normalized to the luciferase activity in PTC cells transfected with the miR control. C, miR-219–5p was overexpressed in PTC cells by lentivirus infection. Expression of miR-219–5p was measured by qPCR. D, Protein levels of ERα, ERβ, p-p38 MAPK, total MAPK, p-ERK1/2, and total ERK1/2 in PTC cells were monitored by Western blotting, with GAPDH as an endogenous control. E, Relationship of relative miR-219–5p and ERα expression. The images displayed are representative of 3 independent experiments *, P < .05.
To assess whether miR-219–5p could directly alter the expression of ERα, a fragment of the 3′-UTR of ERα mRNA (WT 3′-UTR) containing the putative miR-219–5p binding sequence (or the mutant sequence, mutant 3′-UTR), was cloned into the psiCHECK-2 vector (Figure 3B). The PTC cells were then transfected with the WT or mutant 3′-UTR of ERα and miR-219–5p mimic. Luciferase reporter assays were conducted after transfection for 36 hours, with the results that luciferase activity was decreased by ∼50% when the WT 3′-UTR and miR-219–5p mimic were cotransfected, whereas the mutant 3′-UTR had no effect on luciferase activity, even on miR-219–5p transfection (Figure 3B). We induced miR-219–5p overexpression in PTC cells to verify whether the expression of ERα was regulated by miR-219–5p. We first verified that expression levels of miR-219–5p were higher in pCDH-miR-219–5p cells than in control cells (Figure 3C). The Western blotting assay demonstrated that overexpression of miR-219–5p inhibited expression of ERα and the estrogen downstream signaling pathway, including p-p38 MAPK and p-ERK1/2, but had no effect on the expression of ERβ (Figure 3D). Furthermore, we found that expression of miR-219–5p in human PTC samples was negatively related to that of ERα (Figure 3E). A trendline was added, and the formula was calculated according to a scatter diagram (R2 = 0.4977). Collectively, these results indicated that ERα was a target of miR-219–5p and was regulated by miR-219–5p.
ERα promoted PTC cell proliferation and migration
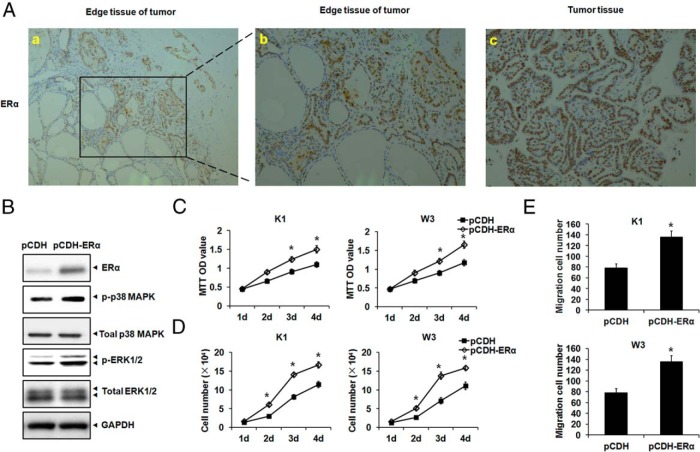
To further evaluate the effect of ERα on PTC development, we detected ERα expression in PTC by immunohistochemical staining, establishing that ERα was up-regulated in PTC samples (Figure 4A, c) compared with that in edge tissue of PTC (Figure 4A, a and b). The edge tissue of PTC is the border of tumor and normal tissue, where we also observed that ERα expression was relatively low in the normal section, whereas ERα expression was relative high in the tumor section (Figure 4A, a and b). ERα was ectopically expressed by lentivirus infection in PTC cells, with activation of the downstream signaling pathway, including p-p38 MAPK and p-ERK1/2 (Figure 4B), which are activators of tumor development. Proliferation and migration in both PTC cells were further augmented upon ERα overexpression (Figure 4, C–E).
Figure 4.
ERα overexpression promoted PTC cell growth. A, ERα was up-regulated in PTC specimens as shown by immunohistochemistry. In the edge tissue of PTC, ERα was up-regulated in the tumor section compared with the normal section (a, ×100 magnification; b, ×200 magnification). ERα showed high expression in PTC tissue (c, ×200 magnification). B, ERα was ectopically expressed in K1 cells by lentivirus infection. The protein levels of ERα, p-p38 MAPK, total MAPK, p-ERK1/2, and total ERK1/2 were monitored by Western blotting, with GAPDH as an endogenous control. C and D, ERα was ectopically expressed in PTC cells by lentivirus infection. The proliferation of PTC cells upon overexpression of ERα was measured by the MTT (C) and cell count (D) assays. E, Migration of PTC cells upon overexpression of ERα was measured by the Transwell assay. *, P < .05.
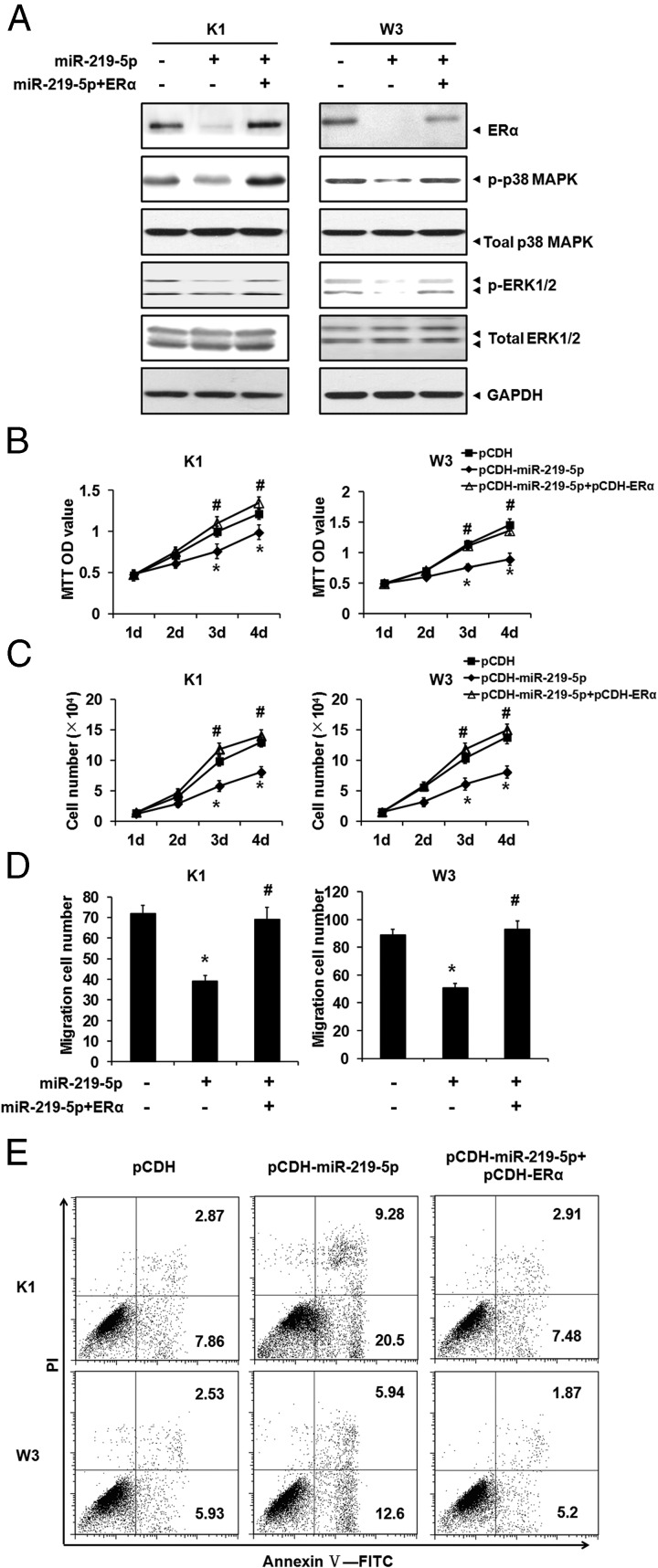
ERα overexpression rescued the growth-suppressive effect of miR-219–5p
To confirm that miR-219–5p suppressed PTC cell growth by targeting ERα, we performed a rescue experiment. ERα expression was forced in PTC cells overexpressing miR-219–5p, which was verified by Western blotting (Figure 5A). ERα totally abolished the impaired p-p38 MAPK and p-ERK1/2 by miR-219–5p overexpression (Figure 5A). The effect of ERα on PTC cell growth was investigated when miR-219–5p was overexpressed, as indicated by cell proliferation (Figure 5, B and C), migration (Figure 5D), and apoptosis (Figure 5E) assays, which showed that ERα significantly rescued the inhibitory effect of miR-219–5p on PTC cell growth.
Figure 5.
ERα rescued impaired PTC cells growth by miR-219–5p overexpression. A, ERα and miR-219–5p were ectopically expressed in PTC cells by lentivirus infection. The protein levels of ERα, p-p38 MAPK, total MAPK, p-ERK1/2, and total ERK1/2 were monitored by Western blotting, with GAPDH as an endogenous control. B and C, PTC cells were used in the MTT (B) and cell count (C) assays to monitor proliferation activity. D, Migration of PTC cells was measured by the Transwell assay. E, PTC cells were incubated with the annexin-V–FITC antibody and stained with PI for apoptosis analysis. Dot plots display PI fluorescence on the y-axis and FITC fluorescence on the x-axis. The percentages of apoptotic cells are shown in the upper and lower right quadrants. *, P < .05; #, P < .05.
Discussion
Recently, several miRNAs, such as miR-221, miR-222, and miR-26a, have been identified as playing roles in PTC development (12). In this study, we linked miR-219–5p to PTC with extensively evidence: first, miR-219–5p was down-regulated in PTC specimens and cells; second, the difference in miR-219–5p expression between men and women was found to be statistically significant; third, tumor size tended to be larger in cases with low rather than high expression of miR-219–5p; fourth, the incidence of lymph node metastasis tended to be higher in cases with low rather than high expression of miR-219–5p; fifth, overexpression of miR-219–5p inhibited proliferation and migration of PTC cells and promoted apoptosis of PTC cells; and finally, miR-219–5p directly inhibited ERα expression, thereby suppressing PTC cell growth. Collectively, our results identified miR-219–5p as a negative regulator of PTC occurrence. The anticancer role of miR-219–5p was also clarified in other cancer types, such as hepatocellular carcinoma (17) and glioblastoma (19). Rao et al (19) have shown that the down-regulation of miR-219–5p in patients with glioblastomas contributes to the increased activity of the RTK pathway by up-regulation of endothelial growth factor receptor (EGFR), thereby promoting glioblastoma growth. It is well known that EGFR signaling is involved in human thyroid carcinoma progression and is responsible for aggressive biological behavior (20); therefore, EGFR might be another target of miR-219–5p in PTC pathogenesis. It was of particular interest to investigate whether miR-219–5p regulated EGFR in PTC. However, we found that ERα overexpression rescued the growth-suppressive effect of miR-219–5p, indicating that ERα may mainly mediate the function of miR-219–5p.
Estrogens are involved in the growth and differentiation of the normal mammary gland (21). The biological effect of estrogen is mediated by several receptors, including ERα and ERβ. In this study, we found that miR-219–5p targeted to ERα, not ERβ, suggesting its high specificity. ERα, encoded by the gene ESR1 in humans, is a nuclear receptor that has a key role in cell proliferation and differentiation. The classic pathway of ERα involves direct binding to estrogen response elements in the control regions of genes, such as c-Myc and cyclin D1, which are essential to cancer development (22). Furthermore, ERα can act through nongenomic mechanisms to regulate signal transduction through pathways such as ERK, MAPK, and phosphatidylinositol 3-kinase. Previous investigations have shown that ERα contributes to the development of gynecological tumors, especially breast cancer. In thyroid carcinoma development, ERα induces cell growth, whereas ERβ plays the opposite role (23, 24). Here, we found that ERα was up-regulated in K1 and W3 cells, whereas no difference was found in another PTC cell line (TCP1) compared with that in Nthy-ori 3–1 cells (data not shown).
Increasing evidence has shown that women are more susceptible to PTC than men are (4, 5) and that estrogen is involved in proliferation of thyroid gland cells by coordinating the action of THS (25), which suggests that ER is involved in PTC occurrence. In addition, our previous studies have found that 53% of PTC tissues were ER positive and that expression of ER is related to metastasis of PTC (data not shown). Our current study demonstrated that ERα induced proliferation and migration of PTC cells, which mediated the effect of miR-219–5p on PTC development. However, the detailed mechanism about the promoted effect of ERα on PTC needs to be clarified. On the basis of previous studies, the classic downstream ERα signaling pathway mainly included the ERK and p38 MAPK signaling pathways, which was confirmed in our studies. Whether an alternative pathway existed in PTC development was worthy of study.
It is well acknowledged that PTC is a heterogeneous cancer, with a number of histologic variants, including papillary microcarcinoma, tall cell variants, columnar cell variants, diffuse sclerosing variants, insular variants, oncocytic variants, and so on (26). Compared with typical PTC, the tall cell, diffuse sclerosing, and insular variants of PTC have been thought to be more aggressive variants (26). The PTC histologic variants (World Health Organization Classification of Tumors, 2004) included in the current investigation are shown in Table 2 and Supplemental Figure 1. It is difficult to exactly define miRNA-219–5p expression in each PTC variant, primarily because of the small number of samples. Thus, further investigations in the independent, large, consecutive series of patients are needed to solve this problem.
In summary, the current study showed that miR-219–5p dramatically inhibited PTC cell growth and migration by targeting ERα, which is a novel function of miR-219–5p. The model offers new insight into the pathological occurrence of PTC and helps us better understand the regulation of ERα.
Acknowledgments
We thank Dr Robert Gagel (MD Anderson Cancer Center, University of Texas, Houston, Texas) for the gift of the PTC cell line W3 and Drs Congfeng Xu, Hongzhu Yan, and Yanan Wang for their kind assistance.
This work was supported by the National Natural Science Foundation of China (Grants 81202113, 81370889, and 81171117), the Anhui Province Natural Science Foundation (Grants 1208085QH157 and KJ2013A188), the Anhui Key Technologies R&D Program (Grant 12010402127), and the Shanghai First People's Hospital Foundation (Grant 12B05).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- EGFR
- endothelial growth factor receptor
- ER
- estrogen receptor
- FBS
- fetal bovine serum
- FITC
- fluorescein isothiocyanate
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- miRNA
- micro-RNA
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- p
- phospho
- PI
- propidium iodide
- PTC
- papillary thyroid carcinoma
- qPCR
- quantitative PCR
- RQ
- relative quantification
- UTR
- untranslated region
- WT
- wild type.
References
- 1. Bhaijee F, Nikiforov YE. Molecular analysis of thyroid tumors. Endocr Pathol. 2011;22:126–133. [DOI] [PubMed] [Google Scholar]
- 2. Massoni F, Simeone C, Ricci P, Onofri E, Ricci S. Papillary thyroid carcinoma and medicolegal considerations. Minerva Med. 2013;104:493–494. [PubMed] [Google Scholar]
- 3. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. [DOI] [PubMed] [Google Scholar]
- 4. LiVolsi VA. Papillary thyroid carcinoma: an update. Mod Pathol. 2011;24(suppl 2):S1–S9. [DOI] [PubMed] [Google Scholar]
- 5. Mazzaferri EL, Massoll N. Management of papillary and follicular (differentiated) thyroid cancer: new paradigms using recombinant human thyrotropin. Endocr Relat Cancer. 2002;227–247. [DOI] [PubMed] [Google Scholar]
- 6. Ciampi R, Nikiforov YE. RET/PTC rearrangements and BRAF mutations in thyroid tumorigenesis. Endocrinology. 2007;148:936–941. [DOI] [PubMed] [Google Scholar]
- 7. Soares P, Trovisco V, Rocha AS, et al. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene. 2003;22:4578–4580. [DOI] [PubMed] [Google Scholar]
- 8. Mazzaferri EL. Long-term outcome of patients with differentiated thyroid carcinoma: effect of therapy. Endocr Pract. 2000;6:469–476. [DOI] [PubMed] [Google Scholar]
- 9. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. [DOI] [PubMed] [Google Scholar]
- 10. Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. [DOI] [PubMed] [Google Scholar]
- 11. Rhoades MW, Reinhart BJ, Lim LP, Burge CB, Bartel B, Bartel DP. Prediction of plant microRNA targets. Cell. 2002;110:513–520. [DOI] [PubMed] [Google Scholar]
- 12. He H, Jazdzewski K, Li W, et al. The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci USA. 2005;102:19075–19080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pallante P, Visone R, Ferracin M, et al. MicroRNA deregulation in human thyroid papillary carcinomas. Endocr Relat Cancer. 2006;13:497–508. [DOI] [PubMed] [Google Scholar]
- 14. Lv M, Zhang X, Li M, et al. miR-26a and its target CKS2 modulate cell growth and tumorigenesis of papillary thyroid carcinoma. PLoS One. 2013;8:e67591. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15. Zhang X, Li D, Li M, et al. MicroRNA-146a targets PRKCE to modulate papillary thyroid tumor development. Int J Cancer. 2014;134:257–267. [DOI] [PubMed] [Google Scholar]
- 16. Visone R, Russo L, Pallante P, et al. MicroRNAs (miR)-221 and miR-222, both overexpressed in human thyroid papillary carcinomas, regulate p27Kip1 protein levels and cell cycle. Endocr Relat Cancer. 2007;14:791–798. [DOI] [PubMed] [Google Scholar]
- 17. Huang N, Lin J, Ruan J, et al. MiR-219–5p inhibits hepatocellular carcinoma cell proliferation by targeting glypican-3. FEBS Lett. 2012;586:884–891. [DOI] [PubMed] [Google Scholar]
- 18. Izzotti A, Calin GA, Arrigo P, Steele VE, Croce CM, De Flora S. Downregulation of microRNA expression in the lungs of rats exposed to cigarette smoke. FASEB J. 2009;23:806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rao SA, Arimappamagan A, Pandey P, et al. miR-219–5p inhibits receptor tyrosine kinase pathway by targeting EGFR in glioblastoma. PLoS One. 2013;8:e63164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gong L, Chen P, Liu X, et al. Expressions of D2–40, CK19, galectin-3, VEGF and EGFR in papillary thyroid carcinoma. Gland Surg. 2012;1:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blander CL. Estrogens and breast cancer. N Engl J Med. 2006;354:1647–1648; author reply 1647–1648. [DOI] [PubMed] [Google Scholar]
- 22. Klinge CM. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001;29:2905–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chu R, van Hasselt A, Vlantis AC, et al. The cross-talk between estrogen receptor and peroxisome proliferator-activated receptor gamma in thyroid cancer. Cancer. 2014;120:142–153. [DOI] [PubMed] [Google Scholar]
- 24. Cho MA, Lee MK, Nam KH, et al. Expression and role of estrogen receptor alpha and beta in medullary thyroid carcinoma: different roles in cancer growth and apoptosis. J Endocrinol. 2007;195:255–263. [DOI] [PubMed] [Google Scholar]
- 25. Clark OH, Gerend PL, Davis M, Goretzki PE, Hoffman PG., Jr Estrogen and thyroid-stimulating hormone (TSH) receptors in neoplastic and nonneoplastic human thyroid tissue. J Surg Res. 1985;38:89–96. [DOI] [PubMed] [Google Scholar]
- 26. Lloyd RV, Buehler D, Khanafshar E. Papillary thyroid carcinoma variants. Head Neck Pathol. 2011;5:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]