Abstract
Context:
Thyroid hormone production is dependent on adequate iodine intake. Excess iodine is generally well-tolerated, but thyroid dysfunction can occur in susceptible individuals after excess iodine exposure. Radiological iodinated contrast media represent an increasingly common source of excess iodine.
Objective:
This review will discuss the thyroidal response after acute exposure to excess iodine; contrast iodine-induced thyroid dysfunction; risks of iodine-induced thyroid dysfunction in vulnerable populations, such as the fetus, neonate, and patients with impaired renal function; and recommendations for the assessment and treatment of contrast iodine-induced thyroid dysfunction.
Methods:
Data for this review were identified by searching PubMed, Google Scholar, and references from relevant articles from 1948 to 2014.
Conclusions:
With the increase in the use of computed tomography scans in the United States, there is increasing risk of contrast-induced thyroid dysfunction. Patients at risk of developing iodine-induced thyroid dysfunction should be closely monitored after receiving iodinated contrast media and should be treated as needed.
Iodine is actively transported into the thyroid gland by the sodium-iodine symporter (NIS) (1). NIS gene expression and membrane localization are stimulated by TSH (2). Once in thyroid follicles, iodine is used for the synthesis of the thyroid hormones, T4 and T3.
In susceptible individuals, exposure to supraphysiological amounts of iodine can result in thyroid dysfunction. Sources of iodine excess include radiographic iodinated contrast media (ICM), medications, diet, skin cleansers, and nutritional supplements. With increased use of computed tomography (CT) scans in the United States over the past 10 years (3), approximately 70 million scans annually as of 2007 (4), ICM is an increasingly common source of excess iodine. The iodine content in ICM (320 to 370 mg/mL) is much higher than the US recommended daily allowance of 150 μg for adults and 220–290 μg for pregnant and lactating women (5, 6). The tolerable upper limit (the approximate threshold below which significant adverse effects are unlikely to occur in a healthy population) for iodine is 1100 μg/d in adults (7).
Materials and Methods
Data for this review were identified by searching PubMed, Google Scholar, and references from relevant articles using the search terms “Wolff-Chaikoff,” “Jöd-Basedow,” “iodine” and “hyperthyroid,” “iodine” and “hypothyroid,” “iodine” and “thyrotoxicosis,” “contrast-induced hyperthyroidism,” “contrast-induced hypothyroidism,” “contrast-induced thyrotoxicosis,” “iodine-induced thyroid dysfunction,” “thyroid dysfunction after CT scan,” “thyroid dysfunction after iodinated contrast,” and “iodine content in radiographic contrast.” Articles published between 1948 and 2014 were included.
Overview of Radiographic Contrast Media and Its Iodine Content
Compared to high osmolar contrast media, low osmolar contrast media have fewer toxic side effects, such as sudden changes in hemodynamic parameters from introduction of hyperosmolar solutions into the circulation (8). Low osmolar (600–1000 mOsm/kg) and iso-osmolar (280–290 mOsm/kg) contrast agents are preferentially used in patients with chronic kidney disease (CKD) to reduce the risk of contrast-induced nephropathy (9). However, they have a higher ratio of iodine atoms to the number of contrast particles in solution as compared with high osmolar agents and hence have a greater concentration of iodine than the high osmolar (1500–2000 mOsm/kg) agents (9, 10). In both high and low osmolar agents, the iodine content is far greater than the recommended daily allowance (Table 1). Patients generally receive between 50 and 100 mL of contrast per CT scan. Higher doses may be required for invasive procedures such as cardiac catheterization. Typical doses for CT scans provide 2500 to 5000 μg of bioavailable free iodine and 15 to 37 g of total iodine (11). Nonbioavailable iodine may be liberated to free iodide, particularly with increased circulating times in the body (ie, impaired kidney function) (11, 12).
Table 1.
Commonly Used Radiographic Contrast Media With Iodine Contents and Osmolarity
| Name (Trade Name) | Iodine Content, mg I/mL | Osmolarity |
|---|---|---|
| Ionic contrast media | ||
| Diatrizoate (Hypaque 50) | 300 | High |
| Ioxaglate (Isopaque 370) | 370 | Low |
| Iothalamate (Conray) | 320 | Low |
| Nonionic contrast media | ||
| Iopamidol (Isovue 370) | 370 | Low |
| Iohexol (Omnipaque 350) | 350 | Low |
| Ioxilan (Oxilan 350) | 370 | Low |
| Iodixanol (Visipaque 320) | 320 | Low |
| Ioversol (Optiray 350) | 350 | Low |
After ICM administration, iodine stores remain elevated for up to 4–8 weeks in patients with intact thyroids (13). In euthyroid, healthy US adults with intact thyroid and renal function, median urinary iodine concentrations increased by 300% from baseline to peak levels, and they did not return to baseline until a median of 43 days after ICM administration (14). In a study of athyreotic patients in Brazil, urinary iodine levels did not normalize until 1 month after undergoing a single iodinated CT scan (15).
Thyroidal Response to Acute Exposure to Excess Iodine
Wolff-Chaikoff effect
The acute thyroid response to excess iodine exposure, known as the acute Wolff-Chaikoff effect, was first described in 1948 (16). In rats exposed to high amounts of iodide, transient reduction in thyroid hormone synthesis was observed. The mechanism for this acute Wolff-Chaikoff effect is partially explained by generation of substances such as iodolactones, iodoaldehydes, and/or iodolipids, that inhibit thyroid peroxidase (TPO) activity, which is necessary for thyroid hormone synthesis (17–19). A decrease in the hydrolysis of thyroglobulin and subsequent decrease in thyroid hormone secretion have also been suggested as contributing mechanisms (20–22).
The transient decrease in thyroid hormone synthesis of the acute Wolff-Chaikoff effect does not normally lead to hypothyroidism. In rats, normal thyroid function resumes within 24–48 hours, despite continued excess iodine exposure, a phenomenon known as the escape from the acute Wolff-Chaikoff effect (23). Early studies of the escape phenomenon suggested that it was due to a reduction in the active transport of iodide into the thyroid (24). In 1999, Eng et al (25) reported a striking reduction in the NIS by 24 hours after excess iodine exposure as the mechanism of the escape. The subsequent decrease in the active transport of iodide into the thyroid leads to the resumption of normal hormone synthesis in both rats and humans (25, 26) (Figure 1). A recent study showed that acute iodide exposure activates the phosphatidylinositol-3-kinase/protein kinase B pathway and generates reactive oxygen species, leading to NIS down-regulation (27).
Figure 1.
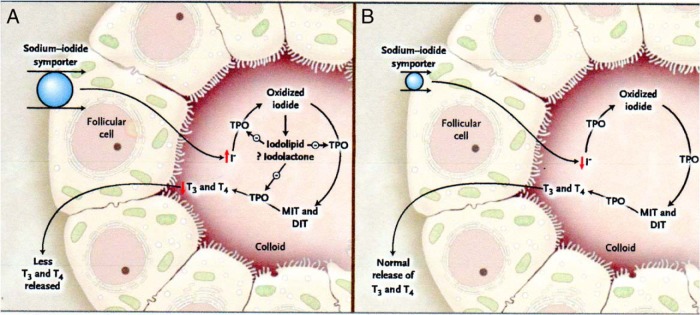
The Wolff-Chaikoff Effect.
Panel A shows a propsed mechanism of the acute Wolff-Chaikoff effect. During the the first day of iodine exposure, the sodium-iodide symporter transports the excess iodine into the thyroid, resulting in transient inhibition of thyroid peroxidase (TPO) and a decrease in thyroid hormone synthesis. Panel B shows the mechanism that turns off the acute Wolff-Chaikoff effect: a dramatic decrease in sodium-iodide symporter expression results in decreased iodine transport and the subsequent resumption of thyroid hormone synthesis. DIT denotes diiodotyrosine, I iodide, MIT monolodotyrosline, T3 triiodothyronine, and T4 thyroxine. Reprinted from P. Pramyothin et al: Clinical problem-solving. A hidden solution. N Engl J Med. 2011;365:2123–2127 (26), with permission. © Massachusetts Medical Society.
Iodine-induced hyperthyroidism
When the normal response to excess iodine, the acute Wolff-Chaikoff effect, is impaired, thyroid dysfunction can occur. Excess iodine intake can result in transient or permanent hyperthyroidism in susceptible individuals (26, 28, 29). Iodine-induced hyperthyroidism, known as the Jöd-Basedow phenomenon, was first described in 1821 (30). The hyperthyroidism may be due to quiescent nodules that become hyperfunctioning when exposed to excess iodine. Risk factors include nontoxic diffuse or nodular goiter, latent Graves' disease, and long-standing iodine deficiency (31, 32).
Iodine-induced hypothyroidism
Iodine-induced hypothyroidism occurs when the thyroid fails to escape from the acute Wolff-Chaikoff effect. It occurs in euthyroid patients with a wide variety of underlying thyroid abnormalities, including pre-existing thyroid disease, such as Hashimoto's thyroiditis, previously treated euthyroid Graves' disease, and a history of thyroid lobectomy, postpartum lymphocytic thyroiditis, interferon-α therapy, or type 2 amiodarone-induced thyrotoxicosis (19, 33–35).
Contrast-Induced Thyroid Dysfunction
Contrast-induced hyperthyroidism
Thyroid dysfunction may arise after ICM use, even in individuals without prior thyroid dysfunction and with normal thyroid glands. ICM exposure in 39 previously euthyroid German patients was associated with significantly increased serum T3 levels and suppressed TSH levels (36). Conn et al (37) reported a trend toward hyperthyroidism after nonionic contrast radiography in a cohort of 73 patients (mean age, 65.7 y), a result similar to that reported by Nygaard et al (38), whose study included some patients with pre-existing thyroid disease. In a Turkish study of 101 patients who underwent coronary angiography, 6% developed subclinical hyperthyroidism at 8 weeks after iodine exposure (39). Case reports include a 53-year-old woman who developed thyroid storm and cardiopulmonary arrest immediately after an iodinated radiological study (38) and a 76-year-old man with prior amiodarone use who developed new-onset atrial fibrillation after eight iodinated CT scans over 10 months (40).
A recent retrospective case-control study by Rhee et al (41) examined the association between ICM use and thyroid dysfunction at two tertiary US hospitals over 20 years. Patients without pre-existing thyroid dysfunction who received a single ICM dose had a 2- to 3-fold increased risk of developing either incident hyperthyroidism or overt hypothyroidism at a median of 9 months after exposure, compared to unexposed patients. Subsequently, Rhee et al (42) studied a large, community-based cohort of ambulatory patients. In patients without pre-existing thyroid disease, those with ICM exposure had 1.6–2 times the odds of developing incident hyperthyroidism and incident overt hypothyroidism compared to unexposed patients.
In contrast, an Italian study of over 1700 patients reported a low incidence of hyperthyroidism of 1.9% after coronary angiography (43). Similarly, hyperthyroidism was not seen in a US study of 56 patients who underwent coronary angiography (44). The low rate of contrast-induced hyperthyroidism in the Italian study, conducted in an iodine-deficient region, was likely due to the use of antithyroid medications and/or radioactive iodine ablation before contrast exposure.
Contrast-induced hypothyroidism
Studies examining hypothyroidism after ICM exposure have reported conflicting data. Frequently, thyroid function remains within the normal range, and clinical symptoms of hypothyroidism are rare (38, 45–47). In a study of patients with no underlying thyroid dysfunction and no palpable goiter, iodine from CT or angiography resulted in significant increases in serum TSH concentrations up to 6.4 mIU/L in 18% of patients within 3–5 days. Serum free T4 and free T3 concentrations remained unchanged, although the study was underpowered to assess small changes in thyroid hormone levels (48). Another recent study demonstrated an association between ICM exposure and the development of overt hypothyroidism (41). One limitation was that this was a retrospective study with variable duration between ICM exposure and the development of incident hypothyroidism (median, 158 d; interquartile range, 50–294 d).
Some patients with transient iodine-induced hypothyroidism later develop permanent hypothyroidism (33), which suggests that the failure to escape from the acute Wolff-Chaikoff effect may be related to thyroid autoimmunity or underlying structural abnormalities of the thyroid gland (Table 2). The presence of TPO antibodies, a marker of thyroid autoimmunity, may indicate a predisposition to iodine-induced thyroid dysfunction. A decrease in mRNA levels of TPO has been shown in animal studies of iodine-induced hypothyroidism (19), as well as decreased organification and increased iodide dischargeability in patients who developed iodide-induced myxedema (49, 50). Therefore, underlying subtle defects in the organification of iodide may be another mechanism of iodine-induced hypothyroidism (31). Susceptible individuals may also have underlying NIS dysfunction (25). Perhaps the failure to decrease NIS expression in the presence of large quantities of plasma iodine is related to underlying autoimmune disease, given a higher incidence of iodine-induced hypothyroidism in patients with a history of autoimmune thyroid disease (28).
Table 2.
Risk Factors for Iodine-Induced Thyroid Dysfunction
| Iodine-induced hyperthyroidism |
| Nontoxic nodular goiter |
| Latent Graves' disease |
| Long-standing iodine deficiency |
| Iodine-induced hypothyroidism |
| Hashimoto's thyroiditis |
| Euthyroid Graves' disease previously treated with surgery, radioactive iodine, or antithyroid drug therapy |
| History of partial thyroidectomy |
| History of postpartum lymphocytic thyroiditis or subacute thyroiditis |
| History of interferon-α therapy |
| History of type 2 amiodarone-induced thyrotoxicosis |
| Fetus or neonates |
Thyroid Dysfunction After Excess Iodine Exposure in Susceptible Populations
ICM exposure in utero and during neonatal life and the risk of fetal hypothyroidism
The fetus is at particular risk for developing hypothyroidism after in utero exposure to radiographic ICM. Fetal TSH secretion begins at approximately 12 weeks gestation, but fetal iodine uptake and thyroid hormone production do not occur until approximately 18–20 weeks gestation. The fetal thyroid is particularly sensitive to iodine overload because the ability to fully escape from the acute Wolff-Chaikoff effect does not mature until approximately 36 weeks (31, 51). In rats, exposure to excess iodine during pregnancy and lactation results in fetal and neonatal hypothyroidism in the pups, and escape from the acute Wolff-Chaikoff effect in pups occurs at age 21 days postpartum, which corresponds to term gestation in humans (52). Iodine easily crosses the placenta, and maternal exposure to iodide or iodine-containing medications may result in transient hypothyroidism and goiter in infants (19, 35, 53). However, because current ICM are water-soluble and readily cleared from the body, fetal exposure to high iodine loads from ICM is transient. One case series of in utero contrast exposure in pregnancy demonstrated no significant neonatal hypothyroidism (51), and several small studies have shown that administration of ICM during pregnancy does not result in a significant increase in fetal thyroid dysfunction (51, 54–56). However, high-osmolar lipid-soluble ICM, with their delayed excretion, confer a greater risk of fetal hypothyroidism, and these should be used with caution in pregnancy.
A literature review regarding ICM exposure in utero and via breast milk showed that there were no associated teratogenic or mutagenic effects in animals (53). Although iodine is concentrated at 20–50 times higher amounts in breast milk compared to plasma, due to NIS expression in lactating mammary glands (19, 57), the amount of ICM excreted in breast milk is low, with only 0.02–0.5% of the water-soluble iodinated contrast dose given to mothers excreted in breast milk (53). The recommended adequate intake for iodine is 110 μg/d for infants aged 0–6 months and 130 μg/d for infants aged 7–12 months. The tolerable upper limit is not defined in neonates/infants because of a lack of sufficient data; the current threshold for children aged 1–3 years is 200 μg/d (6). Although the amount of iodine excreted in breast milk after maternal ICM exposure is higher than the adequate intake, the duration of iodine exposure to infants is brief.
Although exposure to ICM during pregnancy does not appear to be associated with subsequent fetal or neonatal hypothyroidism, excess iodine exposure both during pregnancy and in the first few weeks of life may cause hypothyroidism in newborns. Cases of neonatal iodine-induced hypothyroidism have been recently reported after maternal exposure to iodine-rich seaweed soup or ingestion of Iodoral (a high-potency over-the-counter iodine supplement) (58, 59) and neonatal exposure to ICM at 1–2 weeks of life in the management of congenital cardiac disease (60, 61). Prematurity appears to increase the risk of contrast-induced hypothyroidism in neonates; a systematic review by Ahmet et al (62) showed that 8.3% of term infants and 18.3% of premature infants studied developed hypothyroidism after ICM exposure. Because even transient fetal and neonatal hypothyroidism can be detrimental to neurodevelopment, serum TSH levels should be followed closely during the first few weeks of life after maternal or neonatal contrast exposure, and thyroid function should be monitored in nursing infants whose mothers receive iodinated contrast agents.
Contrast-induced thyroid dysfunction in patients with impaired renal function
Epidemiological data suggest that predialysis CKD and end-stage renal disease (ESRD) patients are at increased risk for thyroid disease, such as hypothyroidism and goiter (63–69). Although the mechanism for this is unclear, iodine excess may be a risk factor for thyroid dysfunction in CKD and ESRD (63, 64). Iodine is renally excreted, and several case series have reported iodine-induced hypothyroidism among adult and pediatric dialysis patients with high dietary iodine intake (70, 71) or exposure to povidone-iodine cleansing agents (72).
In patients with normal renal function, ICM is rapidly excreted (t1/2, ∼2 h) by glomerular filtration. However, in moderate to severe renal dysfunction, contrast elimination is delayed (t1/2, ∼30 h) (73), which may result in greater cumulative iodide exposure (74). Given their frequent need for interventional procedures employing contrast (eg, fistulograms, cardiac catheterizations) and perceived invulnerability to contrast-induced nephropathy, ESRD patients may be disproportionately exposed to contrast, compared to general and predialysis CKD populations. Moisey et al (12) described a hemodialysis patient who developed post-angiography thyrotoxicosis in the context of significantly elevated plasma iodine levels. However, it has yet to be determined whether there is a stronger association between contrast exposure and thyroid dysfunction in patients with impaired renal function. In the study by Rhee et al (41), a differential association between contrast agents and hyperthyroidism according to the level of renal function was not observed. However, the low prevalence of renal failure in this cohort may have limited the ability to detect effect modification by estimated glomerular filtration rate.
Assessment and Treatment of Contrast Iodine-Induced Thyroid Dysfunction
Guidelines of the Contrast Media Safety Committee of the European Society of Urogenital Radiology recommend that high-risk patients should be monitored for thyroid dysfunction after ICM exposure (11). There are currently no US guidelines for screening or following at-risk patients receiving iodinated contrast.
Diagnosis and management of contrast-induced hyperthyroidism
The development of hyperthyroidism may occur up to several weeks after ICM exposure (14, 15). Symptoms of hyperthyroidism include palpitations, weight loss, tremor, insomnia, anxiety, diarrhea, hair loss, and heat intolerance. Serum TSH concentrations will be suppressed, and T4, free T4 and/or total T3 concentrations may be elevated. The serum TSH is the most useful initial test for screening. Thyroid nuclear imaging can help determine the etiology of hyperthyroidism. Low radioiodine uptake is seen in iodine-induced hyperthyroidism because the radioiodine uptake is inversely related to plasma iodine concentrations. A spot urinary iodine concentration may be helpful to support iodine excess as the cause of hyperthyroidism and to monitor continued iodine exposure over time (26, 75). A European study showed a positive correlation between low uptake in thyroid scintigraphy and low risk of development of hyperthyroidism after coronary angiography (76); however, this may not be cost-effective as a screening test and may not be applicable in areas of sufficient iodine intake, such as the United States.
Once contrast-induced hyperthyroidism is diagnosed, further excess iodine exposure should be avoided. Patients should be monitored after subsequent ICM exposure. Although contrast-induced hyperthyroidism is generally transient and resolves after withdrawal of iodine, symptomatic patients may be treated with a β-blocker and/or methimazole. In selected patients at high risk of developing contrast-induced hyperthyroidism or in patients with underlying cardiac disease such as atrial fibrillation, clinicians may consider prophylactic antithyroid drug therapy with methimazole or perchlorate, a competitive inhibitor of NIS that blocks excess iodine transport into the thyroid, before ICM administration. Although not available for medical use in the United States, 200 mg of reagent-grade sodium or potassium perchlorate can be formulated in capsules and given twice or three times daily. A prospective randomized study by Nolte et al (77) showed that prophylaxis with thiamazole 20 mg/d or sodium perchlorate 900 mg/d, starting on the day of coronary angiography and continuing for 14 days, resulted in protection against TSH suppression.
Diagnosis and management of contrast-induced hypothyroidism
There is no indication for routine universal screening for hypothyroidism in patients after ICM because this would not be cost-effective. Rather, we recommend monitoring only in patients with known underlying thyroid disease and in those who develop signs and symptoms of hypothyroidism (Table 2). Common signs and symptoms of hypothyroidism include fatigue, cold intolerance, constipation, weight gain, delayed relaxation of deep tendon reflexes, bradycardia, and periorbital edema (78). Symptoms should be carefully evaluated, especially in elderly patients, who are at increased risk for severe hypothyroidism (ie, myxedema coma) when there are additional insults, such as superimposed infection or the administration of sedatives.
If hypothyroidism is suspected, a serum TSH level should be measured. TPO antibody titers may be checked to assess for underlying thyroid autoimmunity. If the serum TSH is ≥ 10 mIU/L, treatment with levothyroxine is warranted. When the serum TSH is 5–10 mIU/L, treatment can be considered if the patient is symptomatic. Thyroid function should be carefully followed in untreated patients. After starting levothyroxine, serum TSH should be measured every 6–12 weeks, with dose titration until a stable dose of levothyroxine is established. Because ICM-induced hypothyroidism is frequently transient, levothyroxine may be tapered after a few months of treatment. However, even after the resolution of contrast-induced hypothyroidism, we recommend continued monitoring, given the risk of developing permanent hypothyroidism.
Summary
The incidence of contrast-induced thyroid dysfunction in the United States is unknown, but risk is increasing with the increased use of CT scans. Although most cases of contrast-induced thyroid dysfunction are transient, there is a risk for serious complications, such as atrial fibrillation with hyperthyroidism and myxedema coma with hypothyroidism, especially in elderly patients. The fetus and neonate are susceptible to developing iodine-induced hypothyroidism after maternal or neonatal ICM exposure. Patients with impaired renal function, underlying thyroid disease, or a history of thyroid dysfunction are also at higher risk of contrast-induced thyroid dysfunction. These patients should be closely monitored for thyroid dysfunction after receiving ICM and should be treated as needed. Patients who develop contrast-induced hypothyroidism should be monitored for symptoms of hypothyroidism, even after initial resolution, because they remain at risk for developing permanent hypothyroidism.
Acknowledgments
This work was supported in part by National Institutes of Health Grants 7K23HD068552 (to A.M.L.) and 1K23DK102903 (to C.M.R.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CKD
- chronic kidney disease
- CT
- computed tomography
- ESRD
- end-stage renal disease
- ICM
- iodinated contrast media
- NIS
- sodium-iodine symporter
- TPO
- thyroid peroxidase.
References
- 1. Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379(6564):458–460. [DOI] [PubMed] [Google Scholar]
- 2. Bizhanova A, Kopp P. Minireview: the sodium-iodide symporter NIS and pendrin in iodide homeostasis of the thyroid. Endocrinology. 2009;150(3):1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Medicare Payment Advisory Commission. A Data Book: Health Care Spending and the Medicare Program, June 2013. Available at: http://medpac.gov/documents/June13DataBookEntireReport.pdfAccessed September 19, 2013.
- 4. Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169:2078–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. 3rd ed 2007. http://whqlibdoc.who.int/publications/2007/9789241595827_eng.pdf Accessed September 19, 2013.
- 6. National Academy of Sciences, Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academy Press; 2001. http://www.nap.edu/catalog.php?record_id=10026 Accessed September 23, 2013. [Google Scholar]
- 7. Otten JJ, Hellwig JP, Meyers LD. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 8. McClennan BL, Preston M. Hickey memorial lecture. Ionic and nonionic iodinated contrast media: evolution and strategies for use. AJR Am J Roentgenol. 1990;155(2):225–233. [DOI] [PubMed] [Google Scholar]
- 9. Solomon R, Briguori C, Bettmann M. Selection of contrast media. Kidney Int Suppl. 2006;100:S39–S45. [DOI] [PubMed] [Google Scholar]
- 10. Katzberg RW, Haller C. Contrast-induced nephrotoxicity: clinical landscape. Kidney Int Suppl. 2006;100:S3–S7. [DOI] [PubMed] [Google Scholar]
- 11. van der Molen AJ, Thomsen HS, Morcos SK. Effect of iodinated contrast media on thyroid function in adults. Eur Radiol. 2004;14(5):902–907. [DOI] [PubMed] [Google Scholar]
- 12. Moisey RS, McPherson S, Wright M, Orme SM. Thyroiditis and iodide mumps following an angioplasty. Nephrol Dial Transplant. 2007;22(4):1250–1252. [DOI] [PubMed] [Google Scholar]
- 13. Lee SY, Chang DLF, He X, Pearce EN, Braverman LE, Leung AM. Urinary iodine excretion and changes in thyroid hormone levels after computer tomography scan with iodinated contrast administration. Poster presented at: 84th Annual Meeting of American Thyroid Association; October 29–November 2, 2014; Coronado, CA. [Google Scholar]
- 14. Nimmons GL, Funk GF, Graham MM, Pagedar NA. Urinary iodine excretion after contrast computed tomography scan: implications for radioactive iodine use. JAMA Otolaryngol Head Neck Surg. 2013;139(5):479–482. [DOI] [PubMed] [Google Scholar]
- 15. Padovani RP, Kasamatsu TS, Nakabashi CC, et al. One month is sufficient for urinary iodine to return to its baseline value after the use of water-soluble iodinated contrast agents in post-thyroidectomy patients requiring radioiodine therapy. Thyroid. 2012;22(9):926–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wolff J, Chaikoff IL. Plasma inorganic iodide as a homeostatic regulator of thyroid function. J Biol Chem. 1948;174(2):555–564. [PubMed] [Google Scholar]
- 17. Wolff J, Chaikoff IL. The inhibitory action of iodide upon organic binding of iodine by the normal thyroid gland. J Biol Chem. 1948;172(2):855. [PubMed] [Google Scholar]
- 18. Raben MS. The paradoxical effects of thiocyanate and of thyrotropin on the organic binding of iodine by the thyroid in the presence of large amounts of iodide. Endocrinology. 1949;45(3):296–304. [DOI] [PubMed] [Google Scholar]
- 19. Markou K, Georgopoulos N, Kyriazopoulou V, Vagenakis AG. Iodine-induced hypothyroidism. Thyroid. 2001;11(5):501–510. [DOI] [PubMed] [Google Scholar]
- 20. Lamas L, Ingbar SH. The effect of varying iodine content on the susceptibility of thyroglobulin to hydrolysis by thyroid acid protease. Endocrinology. 1978;102(1):188–197. [DOI] [PubMed] [Google Scholar]
- 21. Bagchi N, Brown T, Shivers B, Mack RE. Effect of inorganic iodide on thyroglobulin hydrolysis in cultured thyroid glands. Endocrinology. 1977;100(4):1002–1007. [DOI] [PubMed] [Google Scholar]
- 22. Becks GP, Eggo MC, Burrow GN. Regulation of differentiated thyroid function by iodide: preferential inhibitory effect of excess iodide on thyroid hormone secretion in sheep thyroid cell cultures. Endocrinology. 1987;120(6):2569–2575. [DOI] [PubMed] [Google Scholar]
- 23. Wolff J, Chaikoff IL. The temporary nature of the inhibitory action of excess iodine on organic iodine synthesis in the normal thyroid. Endocrinology. 1949;45(5):504–513, illust. [DOI] [PubMed] [Google Scholar]
- 24. Braverman LE, Ingbar SH. Changes in thyroidal function during adaptation to large doses of iodide. J Clin Invest. 1963;42:1216–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eng PH, Cardona GR, Fang SL, et al. Escape from the acute Wolff-Chaikoff effect is associated with a decrease in thyroid sodium/iodide symporter messenger ribonucleic acid and protein. Endocrinology. 1999;140(8):3404–3410. [DOI] [PubMed] [Google Scholar]
- 26. Pramyothin P, Leung AM, Pearce EN, Malabanan AO, Braverman LE. Clinical problem-solving. A hidden solution. N Engl J Med. 2011;365(22):2123–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Serrano-Nascimento C, da Silva Teixeira S, Nicola JP, Nachbar RT, Masini-Repiso AM, Nunes MT. The acute inhibitory effect of iodide excess on sodium/iodide symporter expression and activity involves the PI3K/Akt signaling pathway. Endocrinology. 2014;155(3):1145–1156. [DOI] [PubMed] [Google Scholar]
- 28. Braverman LE. Iodine and the thyroid: 33 years of study. Thyroid. 1994;4(3):351–356. [DOI] [PubMed] [Google Scholar]
- 29. Leung AM, Braverman LE. Iodine-induced thyroid dysfunction. Curr Opin Endocrinol Diabetes Obes. 2012;19(5):414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coindet J. Nouvelles recherches sur les effets de l'iode, et sur les precautions a suivre dans le traitement de goitre par le nouveau remede. Bibl Univ Sci Belle-Lettres Arts. 1821;16(140). [Google Scholar]
- 31. Braverman LE. Effects of iodine on thyroid function in man. Trans Am Clin Climatol Assoc. 1991;102:143–151; discussion 151–152. [PMC free article] [PubMed] [Google Scholar]
- 32. Roti E, Uberti ED. Iodine excess and hyperthyroidism. Thyroid. 2001;11(5):493–500. [DOI] [PubMed] [Google Scholar]
- 33. Braverman LE, Woeber KA, Ingbar SH. Induction of myxedema by iodide in patients euthyroid after radioiodine or surgical treatment of diffuse toxic goiter. N Engl J Med. 1969;281(15):816–821. [DOI] [PubMed] [Google Scholar]
- 34. Braverman LE, Ingbar SH, Vagenakis AG, Adams L, Maloof F. Enhanced susceptibility to iodide myxedema in patients with Hashimoto's disease. J Clin Endocrinol Metab. 1971;32(4):515–521. [DOI] [PubMed] [Google Scholar]
- 35. Roti E, Vagenakis AG. Effect of excess iodide: clinical aspects. In: Braverman LE, Cooper DS, eds. Werner & Ingbar's The Thyroid: A Fundamental and Clinical Text. 10th ed Philadelphia, PA: Lippincott, Williams & Wilkins; 2012:242–256. [Google Scholar]
- 36. Breuel HP, Breuel C, Emrich D, Fischer P, Gilak A, Winkler C. Changes in thyroid function after application of iodinated contrast media to normal subjects [in German]. Med Klin. 1979;74(41):1492–1496. [PubMed] [Google Scholar]
- 37. Conn JJ, Sebastian MJ, Deam D, Tam M, Martin FI. A prospective study of the effect of nonionic contrast media on thyroid function. Thyroid. 1996;6(2):107–110. [DOI] [PubMed] [Google Scholar]
- 38. Nygaard B, Nygaard T, Jensen LI, et al. Iohexol: effects on uptake of radioactive iodine in the thyroid and on thyroid function. Acad Radiol. 1998;5(6):409–414. [DOI] [PubMed] [Google Scholar]
- 39. Özkan S, Oysu AS, Kayata K, et al. Thyroid functions after contrast agent administration for coronary angiography: a prospective observational study in euthyroid patients. Anadolu Kardiyol Derg. 2013;13(4):363–369. [DOI] [PubMed] [Google Scholar]
- 40. Mushtaq U, Price T, Laddipeerla N, Townsend A, Broadbridge V. Contrast induced hyperthyroidism due to iodine excess. BMJ Case Rep. 2009;2009:pii:bcr06.2009.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rhee CM, Bhan I, Alexander EK, Brunelli SM. Association between iodinated contrast media exposure and incident hyperthyroidism and hypothyroidism. Arch Intern Med. 2012;172(2):153–159. [DOI] [PubMed] [Google Scholar]
- 42. Rhee CM, Lynch KE, Zandi-Nejad K, Pearce EN, Alexander EK, Brunelli SM. Iodinated contrast media exposure and incident hyperthyroidism and hypothyroidism in a community-based cohort [published online October 2, 2013]. Endocrinol Stud. doi.org/10.4081/es.2013.e8. [Google Scholar]
- 43. Marraccini P, Bianchi M, Bottoni A, et al. Prevalence of thyroid dysfunction and effect of contrast medium on thyroid metabolism in cardiac patients undergoing coronary angiography. Acta Radiol. 2013;54(1):42–47. [DOI] [PubMed] [Google Scholar]
- 44. Koroscil TM, Pelletier PR, Slauson JW, Hennessey J. Short-term effects of coronary angiographic contrast agents on thyroid function. Endocr Pract. 1997;3(4):219–221. [DOI] [PubMed] [Google Scholar]
- 45. Jaffiol C, Baldet L, Bada M, Vierne Y. The influence on thyroid function of two iodine-containing radiological contrast media. Br J Radiol. 1982;55(652):263–265. [DOI] [PubMed] [Google Scholar]
- 46. Bürgi H, Wimpfheimer C, Burger A, Zaunbauer W, Rösler H, Lemarchand-Béraud T. Changes of circulating thyroxine, triiodothyronine and reverse triiodothyronine after radiographic contrast agents. J Clin Endocrinol Metab. 1976;43(6):1203–1210. [DOI] [PubMed] [Google Scholar]
- 47. Wu SY, Chopra IJ, Solomon DH, Bennett LR. Changes in circulating iodothyronines in euthyroid and hyperthyroid subjects given ipodate (Oragrafin), an agent for oral cholecystography. J Clin Endocrinol Metab. 1978;46(4):691–697. [DOI] [PubMed] [Google Scholar]
- 48. Gartner W, Weissel M. Do iodine-containing contrast media induce clinically relevant changes in thyroid function parameters of euthyroid patients within the first week? Thyroid. 2004;14(7):521–524. [DOI] [PubMed] [Google Scholar]
- 49. Oppenheimer JH, McPherson HT. The syndrome of iodide-induced goiter and myxedema. Am J Med. 1961;30:281–288. [DOI] [PubMed] [Google Scholar]
- 50. Paley KR, Sobel ES, Yalow RS. Some aspects of thyroidal iodine metabolism in a case of iodine-induced hypothyroidism. J Clin Endocrinol Metab. 1958;18(1):79–90. [DOI] [PubMed] [Google Scholar]
- 51. Atwell TD, Lteif AN, Brown DL, McCann M, Townsend JE, Leroy AJ. Neonatal thyroid function after administration of IV iodinated contrast agent to 21 pregnant patients. AJR Am J Roentgenol. 2008;191(1):268–271. [DOI] [PubMed] [Google Scholar]
- 52. Theodoropoulos T, Braverman LE, Vagenakis AG. Iodide-induced hypothyroidism: a potential hazard during perinatal life. Science. 1979;205(4405):502–503. [DOI] [PubMed] [Google Scholar]
- 53. Webb JA, Thomsen HS, Morcos SK. The use of iodinated and gadolinium contrast media during pregnancy and lactation. Eur Radiol. 2005;15(6):1234–1240. [DOI] [PubMed] [Google Scholar]
- 54. Kochi MH, Kaloudis EV, Ahmed W, Moore WH. Effect of in utero exposure of iodinated intravenous contrast on neonatal thyroid function. J Comput Assist Tomogr. 2012;36(2):165–169. [DOI] [PubMed] [Google Scholar]
- 55. Rajaram S, Exley CE, Fairlie F, Matthews S. Effect of antenatal iodinated contrast agent on neonatal thyroid function. Br J Radiol. 2012;85(1015):e238–e242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bourjeily G, Chalhoub M, Phornphutkul C, Alleyne TC, Woodfield CA, Chen KK. Neonatal thyroid function: effect of a single exposure to iodinated contrast medium in utero. Radiology. 2010;256(3):744–750. [DOI] [PubMed] [Google Scholar]
- 57. Semba RD, Delange F. Iodine in human milk: perspectives for infant health. Nutr Rev. 2001;59(8):269–278. [DOI] [PubMed] [Google Scholar]
- 58. Shumer DE, Mehringer JE, Braverman LE, Dauber A. Acquired hypothyroidism in an infant related to excessive maternal iodine intake: food for thought. Endocr Pract. 2013;19(4):729–731. [DOI] [PubMed] [Google Scholar]
- 59. Connelly KJ, Boston BA, Pearce EN, et al. Congenital hypothyroidism caused by excess prenatal maternal iodine ingestion. J Pediatr. 2012;161(4):760–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Thaker VV, Leung AM, Braverman LE, Brown RS, Levine B. Iodine-induced hypothyroidism in full-term infants with congenital heart disease: more common than currently appreciated? J Clin Endocrinol Metab. 2014;99(10):3521–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. del Cerro Marín M, Fernández Ruiz A, García-Guereta L, et al. Thyroid function alterations in children with congenital cardiac disease after catheterization with iodinated contrast agents [in Spanish]. Rev Esp Cardiol. 2000;53(4):517–524. [PubMed] [Google Scholar]
- 62. Ahmet A, Lawson ML, Babyn P, Tricco AC. Hypothyroidism in neonates post-iodinated contrast media: a systematic review. Acta Pædiatrica. 2009;98(10):1568–1574. [DOI] [PubMed] [Google Scholar]
- 63. Iglesias P, Díez JJ. Thyroid dysfunction and kidney disease. Eur J Endocrinol. 2009;160(4):503–515. [DOI] [PubMed] [Google Scholar]
- 64. Lo JC, Chertow GM, Go AS, Hsu CY. Increased prevalence of subclinical and clinical hypothyroidism in persons with chronic kidney disease. Kidney Int. 2005;67(3):1047–1052. [DOI] [PubMed] [Google Scholar]
- 65. Chonchol M, Lippi G, Salvagno G, Zoppini G, Muggeo M, Targher G. Prevalence of subclinical hypothyroidism in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2008;3(5):1296–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kaptein EM. Thyroid hormone metabolism and thyroid diseases in chronic renal failure. Endocr Rev. 1996;17(1):45–63. [DOI] [PubMed] [Google Scholar]
- 67. Kutlay S, Atli T, Koseogullari O, Nergizoglu G, Duman N, Gullu S. Thyroid disorders in hemodialysis patients in an iodine-deficient community. Artif Organs. 2005;29(4):329–332. [DOI] [PubMed] [Google Scholar]
- 68. Lin CC, Chen TW, Ng YY, Chou YH, Yang WC. Thyroid dysfunction and nodular goiter in hemodialysis and peritoneal dialysis patients. Perit Dial Int. 1998;18(5):516–521. [PubMed] [Google Scholar]
- 69. Ng YY, Wu SC, Lin HD, et al. Prevalence of clinical and subclinical thyroid disease in a peritoneal dialysis population. Perit Dial Int. 2012;32(1):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sanai T, Inoue T, Okamura K, et al. Reversible primary hypothyroidism in Japanese patients undergoing maintenance hemodialysis. Clin Nephrol. 2008;69(2):107–113. [DOI] [PubMed] [Google Scholar]
- 71. Takeda S, Michigishi T, Takazakura E. Iodine-induced hypothyroidism in patients on regular dialysis treatment. Nephron. 1993;65(1):51–55. [DOI] [PubMed] [Google Scholar]
- 72. Brough R, Jones C. Iatrogenic iodine as a cause of hypothyroidism in infants with end-stage renal failure. Pediatr Nephrol. 2006;21(3):400–402. [DOI] [PubMed] [Google Scholar]
- 73. Morcos SK, Thomsen HS, Webb JA. Dialysis and contrast media. Eur Radiol. 2002;12(12):3026–3030. [DOI] [PubMed] [Google Scholar]
- 74. Rendl J, Saller B. Schilddrüse und Röntgenkontrastmittel: Pathophysiologie, Häufigkeit und Prophylaxe der jodinduzierten Hyperthyreose. Dtsch Arztebl. 2001;98:A-402/B-339/C-316. [Google Scholar]
- 75. Arum SM, He X, Braverman LE. Excess iodine from an unexpected source. N Engl J Med. 2009;360(4):424–426. [DOI] [PubMed] [Google Scholar]
- 76. Fricke E, Fricke H, Esdorn E, et al. Scintigraphy for risk stratification of iodine-induced thyrotoxicosis in patients receiving contrast agent for coronary angiography: a prospective study of patients with low thyrotropin. J Clin Endocrinol Metab. 2004;89(12):6092–6096. [DOI] [PubMed] [Google Scholar]
- 77. Nolte, Muller R, Siggelkow H, Emrich D, Hufner M. Prophylactic application of thyrostatic drugs during excessive iodine exposure in euthyroid patients with thyroid autonomy: a randomized study. Eur J Endocrinol. 1996;134(3):337–341. [DOI] [PubMed] [Google Scholar]
- 78. Canaris GJ, Manowitz NR, Mayor G, Ridgway E. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526–534. [DOI] [PubMed] [Google Scholar]


