Abstract
Context:
Cell lines are a widely used tool in cancer research. However, despite the relatively high incidence of papillary thyroid carcinoma (PTC), there are only four PTC cell lines available for international research audience.
Objective:
The objective of this study was to establish and characterize new PTC cell lines that represent primary tumor biology. Surgical specimens were obtained to generate PTC cell lines. Short tandem repeat profiling was used to confirm the uniqueness of the cell lines against databases of known cell lines and mutations were assessed using Sequenom. The expression of thyroid-specific genes was examined using real-time PCR. Tumorigenicity was determined using an orthotopic thyroid xenograft tumor mouse model.
Results:
Six PTC cell lines (five conventional PTCs and one follicular variant of PTC) were generated and found to be unique when compared by short tandem repeat profiling against databases of all existing cell lines. The five conventional PTC cell lines carry the BRAF V600E mutation and the follicular variant of PTC cell line had an NRAS mutation. Five of the six cell lines had a mutation in the promoter of the human telomerase reverse transcriptase gene. None of the cell lines have RET/PTC rearrangements. Three cell lines were tumorigenic in the orthotopic thyroid xenograft tumor mouse model.
Conclusions:
These five characterized conventional PTC cell lines and the unique follicular variant of PTC cell line should be valuable reagents for thyroid cancer research. The three tumorigenic cell lines can be used for in vivo testing of targeted therapeutic and novel agents.
Thyroid cancer is the most common endocrine cancer with increasing incidence worldwide and an estimated 62, 980 new cases for 2014 (1). Papillary thyroid carcinoma (PTC) is the most common type of thyroid cancer and accounts for 80–85% of primary thyroid malignancies (2).
PTC has an excellent prognosis when given appropriate treatment with surgical intervention and radioactive-iodine therapy (RAI) as necessary followed by long-term TSH suppression in high-risk patients. However, for a small group of patients with locally advanced disease or distant metastasis, especially when the tumor is refractory to RAI, conventional treatment modalities such as cytotoxic chemotherapy and external beam radiation therapy have shown disappointing outcomes.
As with other cancers, many discrete mutations are known to contribute to the development and progression of PTC. Among these, point mutations of BRAF and RET/PTC rearrangement are most common. Novel therapeutic agents have been used to target the abnormalities in their signal transduction pathways. Recently published phase 3 studies of sorafenib showed benefit in progression-free survival for patients with RAI-refractory differentiated thyroid cancer (3). This led to US Food and Drug Administration approval of sorafenib in treatment of RAI-refractory differentiated thyroid cancer patients in November 2013. Many researchers are trying to find effective targeted molecular therapies for unresectable, advanced, and distant metastatic cases of PTC. Clinically relevant, well-established cell lines and xenograft models are absolutely necessary for the preclinical studies of effectiveness and safety, before conducting clinical trials with novel targeted agents. Unfortunately, a PTC cell line is much harder to establish than an aggressive anaplastic thyroid cancer (ATC) cell line, owing to the frequently observed chronic indolent nature of the disease. As a result, few PTC cell lines are available to effectively simulate the various characteristics associated with this disease.
In this study, we established six cell lines from tumor tissues of patients with PTC, successfully authenticated these cell lines by immunohistochemistry and short tandem repeat (STR) profiling, and assessed tumorigenicity in an orthotopic thyroid xenograft tumor mouse model. These cell lines will be a valuable asset for investigating novel therapies on thyroid cancer.
Materials and Methods
Patient history
The MDA-T22 cell line was derived from a primary PTC (conventional) (4) with extrathyroidal extension in a 79-year-old woman and no lymphovascular invasion was detected. MDA-T32 was derived from a primary PTC (conventional) demonstrating extrathyroidal extension, cervical lymph node metastasis, and lymphovascular invasion in a 74-year-old man. MDA-T41 was derived from metastatic lymph nodes in a 74-year-old man with recurrent PTC that exhibited extracapsular extension and lymphovascular invasion. MDA-T68 was derived from a follicular variant of papillary thyroid carcinoma (FVPTC) with extrathyroidal extension and a cervical lymph node metastasis in a 75-year-old man. Lymphovascular invasion was detected. MDA-T85 was derived from a metastatic lymph node in a 61-year-old man with a primary PTC (conventional) demonstrating extrathyroidal extension and lymphovascular invasion. MDA-T120 was derived from a metastatic cervical lymph node in a 72-year-old woman with recurrent PTC (that had an anaplastic component), extracapsular extension, and lymphovascular invasion.
Cell line generation and cell culture condition
All PTC specimens were obtained from patients who underwent surgical treatment of PTC at The University of Texas MD Anderson Cancer Center and provided written informed consent, and the study was approved by the institutional review boards at MD Anderson Cancer Center. The surgical specimens were tested histopathologically to confirm the diagnosis of PTC and single-cell suspension was generated as described previously (5). Cells were then cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2mM L-glutamine, and 2 mg/ml Primocin (InvivoGen) in an incubator supplied with 95% O2 and 5% CO2 at 37°C. Fibroblasts were labeled with antihuman fibroblast antibody attached to microbeads (Miltenyi Biotec) and removed by passing through an LS column under a magnetic field.
K2 (PTC cell line with a BRAF mutation kindly provided by Dr D. Wynford-Thomas from Cardiff University, Cardiff, United Kingdom) and thyroid medullary carcinoma cell line TT were maintained in DMEM/F12 medium (Mediatech) containing 10% fetal bovine serum and 2mM L-glutamine. BCPAP (PTC cell line, DSMZ) and SKOV3 (human ovarian carcinoma cell line) were maintained in RPMI 1640 medium containing 10% fetal bovine serum (Sigma-Aldrich) and 2mM L-glutamine. 8505c (anaplastic thyroid carcinoma cell line) was maintained in MEM media containing 10% fetal bovine serum, nonessential amino acid mixture (Cambrex BioScience), 1mM sodium pyruvate (Cambrex BioScience), and 2mM L-glutamine.
Cytokeratin immunostaining
Trypsinized single cells (3 × 105) were centrifuged to Superfrost/Plus microscope slides (Fisher Scientific) through Cyto-Tek filter paper by centrifugation (Miles Scientific). The cytospin slides were fixed in cold acetone for 10 minutes at −20°C and then rinsed in PBS and air dried before staining with cytokeratin antibodies. Automated immunohistochemical analysis for keratin was performed on cytospins. In brief, the tumor cells were incubated with a keratin antibody cocktail composed of the clones AE1/3 (1:100, Dako), CAM 5.2 (1:100, BD Bioscience), MNF116 (1:100, Dako), and Zym5.2 (1:50, Life Technologies), followed by blocking with 3% hydrogen peroxide and a polymer enhancer. Hematoxylin was used as a counterstain and then aminoethylcarbazole was used for antibody detection.
Real-time PCR
Preparation of total RNA and real-time PCR were described previously (5). Specific primers for TTF-1, paired box transcription factor 8 (PAX8), thyroglobulin (Tg), sodium/iodide symporter (NIS), POU5F1, and TSH receptor (TSHR) were purchased from Applied Biosystems as Assays-on-Demand gene expression products.
Western blot analysis
Protein extracts from patient tumors, PTC cell lines, and mouse tumors were prepared and analyzed as described previously (6). The antibodies for TTF-1 and PAX8 (Cell Signaling Technology) were used at a dilution of 1:1000. Actin (Sigam-Aldrich, 1:4000) or tubulin (Cell Signaling, 1:2000) was used as loading controls.
Soft agar selection
PTC cells underwent soft agar selection following 20 passages and were collected by trypsinization using TrypLE Express (Life Technologies). Two milliliters of 0.65% methylcellulose (Sigma-Aldrich) containing 1 × 106 trypsinized cells were plated in a six-well low-retention plate and maintained in a 37°C incubator supplied with 95% O2 and 5% CO2 for 7–14 days. Colonies were collected and maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2mM L-glutamine, 1mM sodium pyruvate, and 1× nonessential amino acids in a 37°C incubator supplied with 95% O2 and 5% CO2.
Thyrospheroid culture
Generation of thyrospheroids (spheroid cell lines) were described previously (5).
STRs DNA fingerprinting
Genomic DNA was extracted from frozen or paraffin-embedded tissue using the Gentra Puregene kit (QIAGEN). STR analysis for each cell line and its matching tissue was performed at the University of Colorado Cancer Center DNA Sequencing & Analysis Core and the Characterized Cell Line Core Facility at MD Anderson. These STR profiles were then compared with those in the ATCC, DSMZ, JCRB, RIKEN, and MD Anderson databases for possible matches.
Mutational analyses
Mutational analyses using genomic DNA were performed using Sequenom Known Panel in the Characterized Cell Line Core at MD Anderson Cancer Center and at Memorial Sloan Kettering Cancer Center. Detection of specific mutations in BRAF, NRAS, and HRAS was described previously (7). PCR primers for PIK3CA (Integrated DNA Technologies) were 5′-GATTGGTTCTTTCCTGTCTCTG-3′ (sense) and 5′-CCACAAATATCAATTTACAACCATTG-3′ (antisense). For the human telomerase reverse transcriptase (TERT) promoter, PCR was performed with 100 ng of genomic DNA using AccuTaq DNA polymerase in the presence of 4% dimethyl sulfoxide (Sigma-Aldrich). PCR primers (Integrated DNA Technologies) were 5′-TGCACCCTGGGAGCGCGAGCGGC-3′ (sense) and 5′-CTCGGCAGCGGGGAGCGCGCGGCAT-3′ (antisense). For TP53, exons 5–9 were examined using advantage HF-2 (Clontech) or Kapa2G Robust HotStart ReadyMix (Kapa Biosystems). Primers for TP53 were described previously (8). All PCR products were sequenced with both sense and antisense primers. PCR products were treated with ExoSAP-IT to eliminate unincorporated primers and deoxyribonucleotide triphosphate (Affymetrix/USB) before sequencing.
Detection of RET/PTC rearrangements
RET/PTC1 to RET/PTC3 rearrangements were evaluated as described previously (7).
Tumorigenesis analysis in immunodeficient mice using an orthotopic model
The orthotopic thyroid carcinoma model in mice has been described previously (9). The mice were euthanized, and orthotopic thyroid tumors from the neck region along with surrounding tissues, submandibular lymph nodes, and lungs were collected and fixed in 10% neutral buffered formalin. Fixed tissues were processed into 4-μm-thick sections, stained with hematoxylin and eosin, and examined microscopically by a veterinary pathologist using a BX41 Olympus microscope and an Aperio digital image scanner. Morphology, invasion, metastasis, and morphometric analysis of the tumor cells were evaluated.
Results
Generation of PTC cell lines
To generate PTC cell lines, 120 fresh tumor specimens were obtained from operation room after PTC confirmation by Pathology as described in Materials and Methods. Fibroblasts were eliminated and six specimens (MDA-T22, MDA-T32, MDA-T41, MDA-T68, MDA-T85, and MDA-T120) attached to the tissue culture plates and became cell lines after 20 passages. To confirm thyroid origin, cells were stained with a cytokeratin cocktail at passages 3–5 as thyroid follicular cells express cytokeratin. All six cell lines showed different degrees of cytokeratin expression as detected by immunohistochemistry (Figure 1A). All cell lines have been split for more than 40 passages.
Figure 1.
Generation of PTC cell lines. A, single cells from MDA-T22, MDA-T32, MDA-T41, MDA-T68, MDA-T85, and MDA-T120 were collected after trypsinization, centrifugation, and cytokeratin immunostaining. B, total RNA was prepared from patient tumor of MDA-T22, MDA-T32, MDA-T41, MDA-T68, and MDA-T120; MDA-T22, MDA-T32, MDA-T41, MDA-T68, MDA-T85, MDA-T120 cell lines; and orthotopic mouse tumors (two tumors for each cell lines) of MDA-T22, MDA-T32, and MDA-T85. Real-time PCR was used to detect the expression of TTF-1, PAX8, Tg, NIS, and TSHR mRNA. The relative quantity of mRNA was quantified to 18S RNA as described in Materials and Methods. C, protein extracts from patient tumor (MDA-T22, MDA-T32, MDA-T41, MDA-T68, MDA-T120), cell lines (MDA-T22, MDA-T32, MDA-T41, MDA-T68, MDA-T85, and MDA-T120), and two mouse thyroid orthotopic tumors each from MDA-T22, MDA-T32, and MDA-T85 were prepared as described in Materials and Methods. Western blot analysis showed the expression of TTF-1 (top) and PAX8 (bottom). Positive controls for TTF-1 and PAX8 were TT and SKVO3 cells, respectively. Actin or tubulin was used as loading controls. D, real-time PCR was used to detect the expression of POU5F1 mRNA. 8505c was used as ATC control and K2 and BCPAP were used as PTC controls. E, single cells from MDA-T22, MDA-T32, MDA-T41, MDA-T68, MDA-T85, and MDA-T120 were collected after trypsinization, mixed with methylcellulose, and cultured for 7–14 d. Images of the soft agar clones were taken under 100× magnifications before the clones were collected. F, single cells from passage 31 of MDA-T22, passage 24 of MDA-T32, and tissue digestion of MDA-T85 were cultured as described in the Methods. Thyrospheroids formed after 5 wk for MDA-T22 and MDA-T85 and after 6 wk for MDA-T32.
Genotyping of PTC cell lines
To confirm that the PTC cell lines were from the corresponding patients, STR profiling (16 STR sites) was performed on all six cell lines and the tissues from each patient. The resulting profiles from the cell lines and tissue specimens are shown (Supplemental Table 1). In MDA-T22, MDA-T32, MDA-T41, MDA-T68, and MDA-T85, the eight STR sites from the cells completely matched the tissues from the corresponding patients. In MDA-T120, seven of eight STR sites from the cells matched the tissue from the patient, and one STR site was a partial match. The STR profile was also compared with the known STR profiles of existing cell lines from ATCC, DSMZ, JCRB, RIKEN databases and from MD Anderson database. No match was found between any of the six cell lines and any existing cell lines in the databases. These results showed that the cell lines are novel and excluded the possibility of cross contamination.
Expression of thyroid-specific genes
The mRNA expression of thyroid-specific genes (TTF-1, PAX8, Tg, NIS, and TSHR) in patient tumors (when available), cell lines, and orthotopic mouse tumors (two tumors for each cell line where applicable) was examined by real-time PCR (Figure 1B). Various amounts of TTF-1 and PAX8 expressions were detected in all patient tumors (no patient tumor was available for MDA-T85), cell lines, and orthotopic mouse tumors from MDA-T22, MDA-T32, and MDA-85. The highest expression of TTF-1 was observed in patient tumor from MDA-T41 and in patient tumor and cell line from MDA-T68. The highest expression of PAX8 was observed in MDA-T22 cell line. The expression of Tg was detected in patient tumor from MDA-T32, MDA-T41, and MDA-T68; and in all cell lines. The highest expression of Tg was identified in MDA-T41 cell line. Little or no Tg expression was found in all orthotopic mouse tumors, patient tumor from MDA-T22, and in patient tumor and cell line from MDA-T120. The expression of NIS was detected in all patient tumors except in MDA-T68. The highest expression of NIS was found in patient tumor from MDA-T22 and MDA-T120. Little or no expression of NIS was observed in all cell lines and in all orthotopic mouse tumors (where applicable). TSHR was expressed in patient tumor from MDA-T22, MDA-T41, and MDA-T68; and in MDA-T32, MDA-T41, and MDA-T68 cell lines. The highest TSHR expression was observed in patient tumor from MDA-T41. Little to no TSHR was expressed in all orthotopic mouse tumors, patient tumor from MDA-T32 and MDA-T120; and in MDA-T85 and MDA-T120 cell lines. A summary of the thyroid-specific gene expressions (TTF-1, PAX8, Tg, NIS, and TSHR) in patient tumors, cell lines, and orthotopic mouse tumors was provided in Table 1.
Table 1.
Summary of the Thyroid Specific Gene Expression by Real-Time PCR
| Name | Source | TTF-1a | PAX8a | Tga | NISa | TSHRa |
|---|---|---|---|---|---|---|
| Patient tumor | 0.09 | 0.22 | n/a | 0.27 | 0.01 | |
| MDA-T22 | Cell line | 0.07 | 3.88 | 0.07 | n/a | n/a |
| Mouse tumorb | 0.10 | 0.38 | n/a | n/a | n/a | |
| Patient tumor | 0.13 | 0.62 | 0.40 | 0.07 | n/a | |
| MDA-T32 | Cell line | 0.11 | 0.77 | 0.08 | 0.01 | n/a |
| Mouse tumorb | 0.04 | 0.60 | 0.01 | 0.01 | n/a | |
| MDA-T85 | Cell line | 0.04 | 0.20 | 0.08 | n/a | n/a |
| Mouse tumorb | 0.10 | 0.86 | 0.02 | 0.01 | n/a | |
| MDA-T41 | Patient tumor | 0.58 | 0.46 | 0.50 | 0.05 | 1.83 |
| Cell line | 0.02 | 0.39 | 4.22 | n/a | 0.1 | |
| MDA-T68 | Patient tumor | 0.62 | 0.75 | 0.63 | n/a | 0.45 |
| Cell line | 0.56 | 0.12 | 0.31 | n/a | n/a | |
| MDA-T120 | Patient tumor | 0.08 | 0.15 | 0.02 | 0.27 | n/a |
| Cell line | 0.04 | 0.28 | 0.08 | 0.01 | n/a |
Abbreviation: n/a, not detectable.
The value of each gene expression is normalized to that of 18S in each sample.
Average of two orthotopic mouse tumors.
The expressions of TTF-1 and PAX8 were examined further in patient tumors (all except in MDA-T85), cell lines, and orthotopic mouse tumors (where applicable, two tumors per cell line) at the protein level using Western blot analysis (Figure 1C). TTF-1 expression was detected in MDA-T68 and MDA-T120 patient tumors and MDA-T41 and MDA-T68 cell lines. Trace amount of TTF-1 expression was detected in MDA-T85 cell line. No TTF-1 expression was detected in MDA-T22 patient tumor and cell line, MDA-T32 patient tumor and cell line, MDA-T120 cell lines, and all orthotopic thyroid mouse tumors. PAX8 expression was detected in MDA-T41 and MDA-T68 patient tumors; MDA-T22, MDA-T32, MDA-T41, MDA-T85, and MDA-T120 cell lines; and all orthotopic mouse tumors. A small amount of PAX8 was detected in MDA-T32 patient tumor. No PAX8 expression was detected in MDA-T22 patient tumor and MDA-T68 cell line. Although PAX8 was not detected in MDA-T120 patient tumor by Western blot analysis, it was detected by immunohistochemistry (data not shown). These data suggested that the PTC cell lines retained some properties of thyroid follicular cells.
Detection of mutations
To further characterize our newly generated PTC cell lines, known mutations in PTC as reported by others were examined using Sequenom Known Panel, which included 179 sites from 37 genes (Supplemental Figure 1). The BRAF mutation (V600E) was found in all of the PTC cell lines except MDA-T68 (Table 2). In addition, a PIK3CA mutation (E545K) was found in MDA-T22 and an HRAS mutation (Q61K) was detected in MDA-T85. An NRAS mutation (Q61K) was found in MDA-T68. All these mutations were further verified and confirmed by Sanger-based DNA sequencing.
Table 2.
Mutation Status of Cell Lines
| Gene | MDA-T22 | MDA-T32 | MDA-T41 | MDA-T68 | MDA-T85 | MDA-T120 |
|---|---|---|---|---|---|---|
| BRAF | V600E | V600E | V600E | WT | V600E | V600E |
| TERTa | −124 C→T | −124 C→T | WT | −124 C→T | −124 C→T | −124 C→T |
| NRAS | WT | WT | WT | Q61K | WT | WT |
| HRAS | WT | WT | WT | WT | Q61K | WT |
| PIK3CA | E545K | WT | WT | WT | WT | WT |
| TP53b | WT | WT | WT | WT | WT | R280T |
Abbreviation: WT, wild type.
in promoter region
Exons 5–9 were examined.
Recent reports of mutations in the TERT promoter led us to examine the sequence in this region (10, 11). PCR products were sequenced, and five of the six cell lines (MDA-T22, MDA-T32, MDA-T68, MDA-T85, and MDA-T120) carried a mutation in the TERT promoter of C to T at −124 (counting from the translation start site; Table 2). MDA-T41 was wild type at −124.
Although TP53 mutations were rare in PTC, TP53 mutation has been reported in aggressive variants of PTC (12). We examined the sequence in the DNA binding domain of TP53, exons 5–9. A single nucleotide change from G to C was detected in exon 8 of MDA-T120 and resulted in amino acid No. 280 changing from Arginine to Threonine (Table 2). A single nucleotide change from C to T in exon 7 was found in MDA-T41 but did not result in amino acid change. No other TP53 mutation was detected in PTC cell lines.
No RET/PTC1 to RET/PTC3 rearrangements were detected by RT-PCR in any of the six cell lines (data not shown).
Expression of a stem cell marker
To identify whether MDA-T120 contains property of a PTC or ATC, the expression of a stem cell marker POU5F1 was examined by real-time PCR in all PTC cell lines. The expression of POU5F1 in MDA-T120 was similar to other PTC cell lines including two known PTC cell lines, K2 and BCPAP (Figure 1D). We did not observe a great increase in the expression of POU5F1 in MDA-T120 comparing to the known ATC control 8505c cell line. This data suggested that MDA-T120 may still reflect a PTC phenotype.
Tumorigenicity of cell lines
After 20 passages, all six cell lines were tested for tumorigenicity in immunodeficient athymic nu/nu mice. To increase the likelihood of tumorigenesis, some cells in each cell line underwent soft agar selection and some were cultured as thyrospheroids as a subcloning selection process. Soft agar clones were generated for all six cell lines (Figure 1E). Although five cell lines were tested for thyrospheroid formation (MDA-T120 did not have enough cells for a spheroid attempt) and only MDA-T22, MDA-T32, and MDA-T85 formed thyrospheroids (Figure 1F). The cells used to test thyrospheroid formation in MDA-T22, MDA-T32, and MDA-T68 cell lines had undergone more than 20 passages. For MDA-T85, thyrospheroids formed using single cells from fresh tissue. No thyrospheroids were formed in MDA-T41 and MDA-T68.
To test tumorigenicity, original cells and soft agar–selected cells from all six cell lines and thyrospheroids from MDA-T22, MDA-T32, and MDA-T85 were inoculated into the thyroid of athymic nu/nu mice (Supplemental Table 2). Thyroid tumors were detected in mice inoculated with soft agar–selected cells from MDA-T22 (four of five mice in 3 months), soft agar–selected cells (one of eight mice in 15 months) and thyrospheroids (eight of nine mice in 2 months) from MDA-T32, and original cells from MDA-T85 (three of 10 mice in 1 month) cell lines. Mouse tumors from these three cell lines (soft agar–selected cells from MDA-T22, thyrospheroids from MDA-T32, and original cells from MDA-T85) were collected, put back in tissue culture to expand, and infected with a retrovirus expressing luciferase, and again inoculated into the thyroids of athymic nu/nu mice. All mice for each cell line grew orthotopic thyroid tumors in 1–2 weeks as detected by Xenogen bioimaging (Figure 2).
Figure 2.
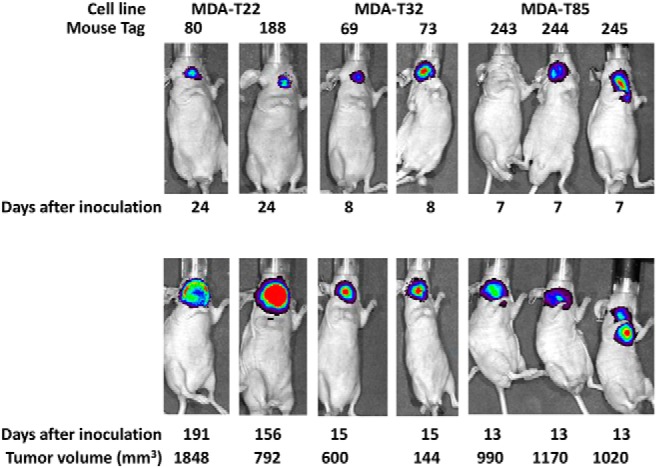
Tumorigenicity of PTC cell lines. Cells (1 × 106) from all cell lines were inoculated into the thyroids of athymic NCrnu/nu mice and tumors were collected from mice that were inoculated with soft agar-selected cells from MDA-T22, thyrospheroids from MDA-T32, and original cells from MDA-T85 (first round of in vivo testing). Mice tumor cells were infected with a retrovirus expressing luciferase and reinoculated into the thyroids of athymic NCr-nu/nu mice (second round of in vivo testing). The expression of luciferase was detected by bioimaging at beginning of inoculation (top) for MDA-T22 (mouse tag numbers 80 and 188); MDA-T32 (mouse tag numbers 69 and 73); and MDA-T85 (mouse tag numbers 243, 244, and 245) and at the day when mouse was sacrificed (bottom). Tumor volume was measured only when mouse was sarcificed and calculated by the formula (length × width × deep).
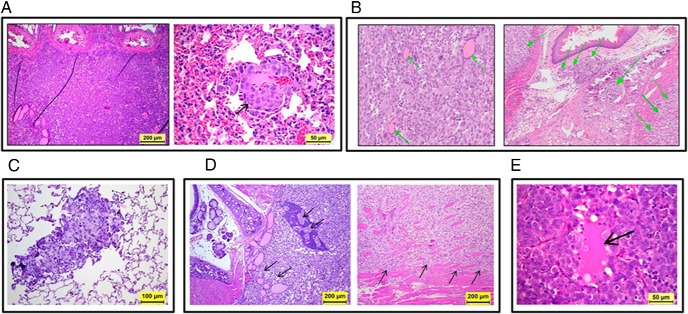
Orthotopic thyroid tumors of these mice were examined histologically. We found that tumors from these three cell lines grew well inside the mouse thyroid glands. In addition, tumor from soft agar–selected MDA-T22 cells infiltrated surrounding tissue (including trachea) and metastasized to the lung (Figure 3A). Tumor cells from MDA-T32 thyrospheroids infiltrated the surrounding skeletal muscle, the cartilage and submucosa of the larynx, and the lymphatic and/or blood vessels (Figure 3B). Tumor from soft agar–selected MDA-T32 metastasized to lung (Figure 3C). Tumors from the original MDA-T85 cells invaded the parathyroid glands and surrounding cervical skeletal muscles (Figure 3D). These tumors are densely cellular and are composed of markedly pleomorphic neoplastic epithelial cells consistent with pleomorphic PTC cells. Tumor cells have abundant eosinophilic cytoplasm and pleomorphic nuclei with hyperchromatic, coarse, clumped, or dust-like chromatin, and marked anisokaryosis. Although no classic papillary structures were identified in these tumor sections, the thyroid tumor cells formed solid islands and trabecular structures, which occasionally delineated small lakes/accumulations of intensely eosinophilic Tg (thyroid colloid) secreted by the tumor cells (Figure 3E).
Figure 3.
Histology of orthotopic PTC in mice by hematoxylin and eosin staining. A, MDA-T22 tumor cells (second round of in vivo testing) infiltrated the thyroid gland and surrounding neck tissues near the trachea (left panel, magnification 100×) and metastasized to the alveoli of the lung (arrow on the right panel, magnification 400×). B, MDA-T32 tumor cells (inoculated with thyrospheroids, second round of in vivo testing) infiltrated the thyroid gland, surrounding neck tissues, and lungs. Pleomorphic tumor cells infiltrated the thyroid gland effacing and displacing the thyroid follicles (arrows on the left panel of B, magnification 200×), and invaded the surrounding skeletal muscle, the laryngeal cartilage and submucosa, and the lymphatic or blood vessels (arrows on the right panel of B, magnification 100×). C, MDA-T32 tumor cells (inoculated with soft agar–selected cells, first round of in vivo testing) metastasized to the lung (magnification 200×). D, MDA-T85 tumor cells (second round of in vivo testing) infiltrated the thyroid and parathyroid glands (arrows on the left panel, magnification 100×) and aggressively invaded the surrounding skeletal muscle (arrows on the right panel, magnification 100×). E, orthotopic thyroid carcinoma with Tg secretion and follicle-like structure (arrow on panel, magnification 400×).
Discussion
Although selected clones or subclones do not fully represent the characteristics of primary tumors, it is obvious that cancer cell lines are a well-defined tool to obtain reproducible data for both in vitro and in vivo models in thyroid cancer research.
Despite recent advances in molecular biology techniques and the high incidence of thyroid cancer, the number of available cell lines in thyroid cancer research has remained very limited. Only two human thyroid cancer cell lines are currently available from the ATCC (medullary thyroid cancer and squamous cell carcinoma), whereas the ATCC contains 40 lung cell lines and 48 breast cell lines.
Recent reports have found that most cell lines being used in cancer research have been misidentified or cross contaminated and that their genetic profiles changed over time. Schweppe et al (13) analyzed 14 PTC cell lines and reported that all but four PTC cell lines were redundant or cross contaminated. These four are the BCPAP and KTC-1 cell lines, which have a BRAF mutation; the TPC-1 cell line, which has the RET/PTC1 rearrangement; and the K1 and K2 cell lines (same STR profiles), which carry BRAF and PIK3CA mutations. Zhao et al (14) reported that four of seven PTC cell lines have one or more problems in their STR profiles. The three unique PTC cell lines from Zhao's study are BCPAP, TPC-1, and K2. These two large-scale validation studies provided a foundation for our efforts to develop new PTC cell lines.
Although we attempted to culture and establish cell lines in over 100 PTC patient samples, only six cell lines were actually established. Of note, all of the newly established cell lines were from older patients with more advanced disease. Advanced age, soft tissue extension, lymph node metastases, and extracapsular extension are factors associated with PTCs that do not have the chronic indolent and often biologically benign PTCs occurring in younger patients. To attempt to create cell lines of the “favorable” PTCs, we pursued exhaustive efforts including varying culture techniques and mediums and attempting primary in vivo cultures, but, we remained unsuccessful in this regard.
All six cell lines generated in this study, five conventional PTCs and one follicular variant of PTC, are all unique and uncontaminated. The STR profile of each cell line completely matches the tissue from the same patients and no matches were found against existing cell lines. In addition, three of six cell lines are tumorigenic. These cell lines will be a significant addition to the thyroid cancer research armamentarium, especially for the study of FVPTC. We intend to distribute this information and these cell lines to the thyroid cancer research community to promote further advances in this field.
The expression of thyroid specific genes (TTF-1, PAX8, Tg, NIS, and TSHR) varied in all six cell lines, suggesting that these genes are not uniformly maintained in PTC. Indeed, the most frequently used PTC cell lines, TPC-1 and K2, have no expression of TTF-1 (13), and the most frequently used BRAF-mutated PTC cell lines, BCPAP and K2, have little to no detectable expression of Tg (data not shown). TTF-1 and Tg are widely considered diagnostic markers for PTC in well-differentiated thyroid cancers. We have detected higher amounts of TTF-1 mRNA expression in all patient tumors than in their corresponding cell lines. The expression of TTF-1 protein was detected in some patient tumors (MDA-T68 and MDA-T120) and in some cell lines (MDA-T41, MDA-T68, and MDA-T85). The absence of TTF-1 protein expression may be due to sensitivity of Western blot analysis vs real-time PCR or different controls at translation vs transcription. The expression of PAX8 mRNA was higher in MDA-T22, MDA-T32, and MDA-T120 cells than in corresponding patient tumors and these correlated well in the PAX8 protein expression. The expression of PAX8 protein was detected in all orthotopic mouse tumors of MDA-T22, MDA-T32, and MDA-T85, but the expression of PAX8 mRNA in these tumors was limited. This data again suggested that different controls were observed at transcription and at translation and some thyroid follicular cell functions may be retained in these cells at both mRNA and protein levels.
In PTC, clinical manifestations and prognosis vary with the status of the two most common mutations, RET/PTC rearrangements and BRAF mutation. We showed previously that dual BRAF and RET/PTC mutations were detected in recurrent PTC patients (7). Five of six cell lines we generated carried the BRAF mutation, but no RET/PTC1–3 rearrangements were detected in any of the cell lines. Not all of the BRAF-mutated PTC cell lines were tumorigenic. Two of the tumorigenic cell lines carried mutations in addition to the BRAF mutation. Genetic abnormalities have been reported in PTC other than BRAF mutation and RET/PTC rearrangement, such as RAS and PIK3CA mutations (15–18). MDA-T22 cells have a PIK3CA mutation (E545K) similar to K1 cells (19) and MDA-T85 cells carry an HRAS mutation (Q61K) in addition to the BRAF mutation. Tumorigenic K1 cells carry three mutations: a BRAF mutation and PIK3CA mutations at E542K and E545K (18, 19). Nikiforova et al (17) screened 27 PTC samples and found that the presence of more than one mutation was associated with metastasis or recurrence, such as lung metastasis with BRAF and TP53 mutations and local recurrence with BRAF, TP53, and PIK3CA mutations. These results may suggest that BRAF-mutated PTC cells may be easier to grow in vitro and adapt to tissue culture conditions than PTC cells with wild-type BRAF. Whether additional mutations contributed to tumorigenesis in mice remain unclear.
TP53 mutations were common in many types of tumors (20). However, it is uncommon in PTC and was only found in ATC (70–80%) and some aggressive variants of PTC (1%) (12, 21, 22). The only TP53 mutation we found was a single nucleotide change in exon 8 of MDA-T120 and resulted in amino acid change at 280 from Arginine (R) to Threonine (T). The R280T mutation in TP53 has been reported in human glioma and is involved in promoting cell proliferation (23). MDA-T120 was derived from a patient who had PTC with an anaplastic component and the anaplastic component may contribute to this TP53 mutation. Additional assay was performed in an effort to distinguish whether MDA-T120 is a representative of PTC or ATC (24). A stem cell marker POU5F1 was examined based on reports from others that ATC may express stem cell markers (25, 26). The expression of stem cell marker POU5F1 mRNA in MDA-T120 was similar to the expression of POU5F1 in other PTC cell lines. We did not observe a great increase in the expression of POU5F1 in MDA-T120 comparing to the known ATC control 8505c cells. Given that the TP53 mutation in MDA-T120 implies an aggressive type of PTC or ATC genotype and the lower levels of stem cell marker POU5F1 expression suggest that MDA-T120 may still maintain a PTC phenotype, this unique cell line allows us to study this critical biologic transition point for the dedifferentiation potential of PTC to ATC in future.
MDA-T68, a FVPTC, carries an NRAS mutation (Q61K). This mutation has been reported in FVPTC by others (16, 17) and RAS mutations are considered the second highest mutation in PTC after the BRAF mutation (16). The RAS family of genes are membrane-bound GTPase that regulate the activation of MAPK, PIK3CA/AKT, and other signaling transduction pathways for cell differentiation, proliferation, and survival (27, 28). Three subtypes of RAS are found in humans: NRAS, KRAS, and HRAS. Mutations in RAS are frequently found at position 12, 13, or 61 and result in constitutive activation of RAS. NRAS mutations have been detected in FVPTC more frequently than KRAS and HRAS (16). Lee et al (29) have suggested that the RAS mutation is a better diagnostic marker than the BRAF mutation in FVPTC. Limited studies have been done in FVPTC, possibly owing to the lack of human cell lines for this PTC variant.
Increased telomerase activity has been reported in cancer and mutations in the TERT promoter may be responsible for this increased activity (10). Mutations in the TERT promoter have been reported recently in melanoma (30), bladder cancer (10), and thyroid cancer (10, 11). The most frequent mutation sites are −124 and −146 before the translation start site. In thyroid, the C to T mutation at −124 in the TERT promoter was recently reported in 11% of PTC and has been associated with large, malignant tumors with lymph nodes metastasis (10, 11). This mutation generates a putative consensus binding site (GGAA in reverse complement) for E-twenty-six (ETS) transcription factors (31). Five of six PTC cell lines reported here carry this mutation and four of those five also carry the BRAF (V600E) mutation. Liu et al (11) have suggested that PTC carrying both BRAF and TERT promoter mutations is more clinically aggressive. Mutation at −146 was found to be rare in PTC (11); indeed, no mutation at −146 was found in any of our cell lines.
Tumorigenic PTC cell lines provide opportunities for in vivo study of inhibitors and drugs. All six of our cell lines were tested for tumorigenesis using original cells, soft agar–selected cells, and thyrospheroids (where applicable). Subcloning approaches (soft agar and spheroid selection) were used to improve tumorigenicity in immunodeficient mice and were successful for MDA-T22 and MDA-T32 cells where the original cells did not produce tumors. The tumors generated from the three cell lines in mice mimic aggressive PTC in human where the tumor was found to extend outside of the thyroid gland and invade surrounding tissues. Metastastic PTC to the lung was observed in two cell lines (MDA-T22 and MDA-T32).
All cells except MDA-T120 (which did not have enough cells for a spheroid attempt) were tested for spheroid formation, but only three cell lines (MDA-T22, MDA-T32, and MDA-T85) formed thyrospheroids. Of these three thyrospheroids, only MDA-T32 generated in vivo tumors in mice. Our unsuccessful attempts may be attributed to a multitude of issues, including lack of sufficient cell numbers required for generating tumors in these mice, a slow spheroid proliferation rate (as observed in MDA-T22), and insufficient purity of the spheroids (MDA-T85 used cells from fresh tissue before the cell line was generated). Nevertheless, three tumorigenic PTC cell lines have been generated and this is a significant addition to the three existing tumorigenic PTC cell lines (TPC-1, K2, and BCPAP). The pathology of these new tumorigenic cell lines showed similar tumor growth in mouse thyroids and metastases, which mimics the growth of aggressive PTC in humans.
In summary, six unique PTC cell lines including five conventional PTCs and one follicular variant of PTC have been generated, and three of which are tumorigenic in nude mice. We anticipate that these well-characterized cell lines will have great utility for both in vitro and in vivo studies by the thyroid cancer research community.
Acknowledgments
We thank Jim Fagin and Jeffrey Knauf from Memorial Sloan Kettering Cancer Center for providing part of Sequenom analysis; Xuesong Li, Vivian Gabisi, and Keri Sherman for providing part of STR profiling and Sequenom analyses; Viju Varghese for Sanger-based DNA sequencing; Ge Zhou for a retrovirus expressing luciferase; Sarah Bronson for text editing; and Rafael Toro-Serra, Cynthia Steward, Stella Njoku, Deborah Rodriguez, Karla Valencia, Mercilla Mangol, and Samar Jasser for technical support.
This work was partly supported by donation from Marty Schaffel, the Alando J. Ballantyne Distinguished Chair Fund, and National Cancer Institute Cancer Center Support (CORE) Grant CA16672 for media production, DNA sequencing, and cell line authentication in MDA. S.Y.L. was supported in part by a National Institutes of Health Mentored Career Development Award K08 DE018061. R.E.S. and B.R.H. were supported by RC1 CA147371, National Institutes of Health Challenge Grant 15-CA-103 (Thyroid Cancer Cell Line Project), and National Cancer Institute Cancer Center Support Grant P30 CA046934 for University of Colorado Cancer Center Gene Expression Core and DNA Sequencing and Analysis Core.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ATC
- anaplastic thyroid cancer
- FVPTC
- follicular variant of papillary thyroid carcinoma
- NIS
- sodium/iodide symporter
- PTC
- papillary thyroid carcinoma
- RAI
- radioactive-iodine therapy
- STR
- short tandem repeat
- Tg
- thyroglobulin.
References
- 1. Cancer Facts, Figures 2014. American Cancer Society. http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf.
- 2. LiVolsi VA. Papillary thyroid carcinoma: An update. Mod Pathol. 2011;24:S1–S9. [DOI] [PubMed] [Google Scholar]
- 3. Brose MS, Nutting C, Jarzab B, et al. Sorafenib in locally advanced or metastatic patients with radioactive iodine-refractory differentiated thyroid cancer: The phase III DECISION trial. Proc 31st Annual Meeting of the American Society of Clinical Oncology, (June 20 Suppl), 2013. [Google Scholar]
- 4. Lloyd R, Buehler D, Khanafshar E. Papillary thyroid carcinoma variants. Head and Neck Pathol. 2011;5:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahn SH, Henderson YC, Williams MD, Lai SY, Clayman GL. Detection of thyroid cancer stem cells in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2014;99:536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Henderson YC, Chen Y, Frederick MJ, Lai SY, Clayman GL. MEK Inhibitor PD0325901 significantly reduces the growth of papillary thyroid carcinoma cells in vitro and in vivo. Mol Cancer Ther. 2010;9:1968–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Henderson YC, Shellenberger TD, Williams MD, et al. High rate of BRAF and RET/PTC dual mutations associated with recurrent papillary thyroid carcinoma. Clin Cancer Res. 2009;15:485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lehman TA, Bennett WP, Metcalf RA, et al. p53 Mutations, ras Mutations, and p53-Heat Shock 70 protein complexes in human lung carcinoma cell lines. Cancer Res. 1991;51:4090–4096. [PubMed] [Google Scholar]
- 9. Ahn SH, Henderson Y, Kang Y, et al. An orthotopic model of papillary thyroid carcinoma in athymic nude mice. Arch Otolaryngol Head Neck Surg. 2008;134:190–197. [DOI] [PubMed] [Google Scholar]
- 10. Vinagre J, Almeida A, Pópulo H, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4:2185. [DOI] [PubMed] [Google Scholar]
- 11. Liu X, Bishop J, Shan Y, et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer. 2013;20:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McFadden DG, Vernon A, Santiago PM, et al. p53 constrains progression to anaplastic thyroid carcinoma in a Braf-mutant mouse model of papillary thyroid cancer. Proc Natl Acad Sci U S A. 2014;111:E1600–E1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schweppe RE, Klopper JP, Korch C, et al. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab. 2008;93:4331–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao M, Sano D, Pickering CR, et al. Assembly and initial characterization of a panel of 85 genomically validated cell lines from diverse head and neck tumor sites. Clin Cancer Res. 2011;17:7248–7264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park JY, Kim WY, Hwang TS, et al. BRAF and RAS mutations in follicular variants of papillary thyroid carcinoma. Endocr Pathol. 2013;24:69–76. [DOI] [PubMed] [Google Scholar]
- 16. Gupta N, Dasyam AK, Carty SE, et al. RAS mutations in thyroid fna specimens are highly predictive of predominantly low-risk follicular-pattern cancers. J Clin Endocrinol Metab. 2013;98:E914–E922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nikiforova MN, Wald AI, Roy S, Durso MB, Nikiforov YE. Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. J Clin Endocrinol Metab. 2013;98:E1852–E1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meireles AM, Preto A, Rocha AS. Molecular and genotypic characterization of human thyroid follicular cell carcinoma-derived cell lines. Thyroid. 2007;17:707–715. [DOI] [PubMed] [Google Scholar]
- 19. Ricarte-Filho JC, Ryder M, Chitale DA, et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009;69:4885–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leroy B, Anderson M, Soussi T. TP53 Mutations in human cancer: Database reassessment and prospects for the next decade. Hum Mutat. 2014;35:672–688. [DOI] [PubMed] [Google Scholar]
- 21. Charles RP, Silva J, Iezza G, Phillips WA, McMahon M. Activating BRAF and PIK3CA mutations cooperate to promote anaplastic thyroid carcinogenesis. Mol Cancer Res. 2014;12:979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simon D, Goretzki PE, Gorelev V, et al. Significance of P53 in human thyroid tumors. World J Surg. 1994;18:535–540; discussion 540–541. [DOI] [PubMed] [Google Scholar]
- 23. Lin C, Liang Y, Zhu H, Zhang J, Zhong X. R280T mutation of p53 gene promotes proliferation of human glioma cells through GSK-3β/PTEN pathway. Neurosci Lett. 2012;529:60–65. [DOI] [PubMed] [Google Scholar]
- 24. Marlow LA, D'Innocenzi J, Zhang Y, et al. Detailed molecular fingerprinting of four new anaplastic thyroid carcinoma cell lines and their use for verification of RhoB as a molecular therapeutic target. J Clin Endocrinol, Metab. 2010;95:5338–5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yun JY, Kim YA, Choe JY, et al. Expression of cancer stem cell markers is more frequent in anaplastic thyroid carcinoma compared to papillary thyroid carcinoma and is related to adverse clinical outcome. J Clin Pathol. 2014;67:125–133. [DOI] [PubMed] [Google Scholar]
- 26. Carina V, Zito G, Pizzolanti G, et al. Multiple pluripotent stem cell markers in human anaplastic thyroid cancer: The putative upstream role of SOX2. Thyroid. 2013;23:829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: Weaving a tumorigenic web. Nat Rev Cancer. 2011;11:761–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karnoub AE, Weinberg RA. Ras oncogenes: Split personalities. Nat Rev Mol Cell Biol. 2008;9:517–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee SR, Jung CK, Kim TE. Molecular genotyping of follicular variant of papillary thyroid carcinoma correlates with diagnostic category of fine-needle aspiration cytology: values of RAS mutation testing. Thyroid. 2013;23:1416–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Horn S, Figl A, Rachakonda PS, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–961. [DOI] [PubMed] [Google Scholar]
- 31. Hollenhorst PC, McIntosh LP, Graves BJ. Genomic and biochemical insights into the specificity of ETS transcription factors. Annu Rev Biochem. 2011;80:437–471. [DOI] [PMC free article] [PubMed] [Google Scholar]